Abstract
Since the discovery of the Hepatitis B Virus (HBV) by Blumberg et al. in 1965, significant progress has been made in understanding the pathogenesis of HBV. The nucleos(t)ide analogues (NAs) have succeeded in decreasing the viral loads to undetectable levels, and reduced the incidence of HCC significantly. However, risk of HCC persists due to the inability of eradicating the covalently closed circular DNA (cccDNA) in the hepatocyte nucleus that causes persistent HBV infection. Recently, a number of different drug targets are being identified that intervene on the viral replication cycle or the host immune system. In this review, we aim to discuss the immunopathogenesis of the virus, the effectiveness of NA’s, and recent therapeutic developments.
Keywords
hepatitis B virus, HBV, Hepatocellular Carcinoma, Carcinoma, HCC
Introduction
Since the discovery of the hepatitis B virus (HBV) by Blumberg et al., great progress has been made in understanding the pathogenesis of the virus and its role in hepatocellular carcinoma (HCC). It is estimated that hepatitis B is responsible for about 50% of the HCC cases worldwide [1,2]. Because of geographic variations in HBV incidence, the burden of HBV-related HCC (HBV-HCC) is highest in endemic areas such as Asian-Pacific and sub- Saharan Africa and lowest in the United States and the West [3]. The hepatitis B vaccines, developed in the 1980s, transformed the evolution of hepatitis B in the modern era. This was followed by high effective anti-viral that reduced HBV infections and HBV-HCC.
The goal of HBV treatment was to decrease HBV viral load and to ultimately prevent hepatocarcinogenesis. The first treatment was interferon, but this injectable was soon replaced with Lamivudine, an oral antiviral with a more tolerable profile [4]. Lamivudine was the first of a number of other nucleos(t)ide analogues that control viral replication through the inhibition of reverse transcriptase and interruption of the HBV DNA formation [5]. Currently, tenofovir disoproxil fumarate, tenofovir alafenamide and entecavir are the most popular antivirals due to their potency and low risk of resistance [6].
Despite major achievements, HBV remains a major public health issues and is responsible for over 780,000 deaths worldwide [7]. Many people are living with chronic hepatitis B who have not receive vaccination after instituting of universal vaccination by the World Health Organization in 1997 [8]. Vertical transmission, an extremely effective method for the transmission of HBV combined with lack of adequate screening in developing countries, continues to be a major driver for the large disease burden. Additionally, the treatments for hepatitis B have not shown to cure the virus. The viral therapies help slow or prevent progression of disease, but they do not eliminate covalently-closed-circular DNA (cccDNA) which persists in the hepatocytes. Therefore, the current therapies are not able to cure HBV. Multiple studies have demonstrated a persistent risk for HCC even with the use of antivirals and successful viral suppression for a number of years [9-14].
Decreased Incidence of HCC Treated with Antiviral Therapy
It is currently known that nucleos(t)ide analogues decrease the incidence of HCC and prevent the recurrence of HCC [15]. The discovery of the first oral antiviral, Lamivudine, was the result of persistent efforts aimed at treating HIV in the 1980s. It was shown to decrease hepatic decompensation, progression to cirrhosis, and the risk of developing HCC. Liaw et al. demonstrated that patients treated with lamivudine compared to controls had a lower Child–Pugh scores (hazard ratio, 0.45; P=0.02) and fewer cases of HCC (hazard ratio, 0.49; P=0.047) after a median duration of 32.4 months [16]. Similar findings were shown with the newer antivirals, entecavir and tenofovir disoproxil fumarate. In one study, Hosaka et al., demonstrated that HCC suppression was greater in an entecavir-treated group compared to a no-treatment group with incidence rates of 3.7% and 13.7%, respectively, after 5 years of treatment (P<0.002) [17]. Kim et al. Showed a decreased incidence of HCC with tenofovir disoproxil fumarate compared the incidence predicted by the “REACH-B risk calculator” [18].
Development of New HCC on Antiviral Therapy
Although the incidence of HCC with antiviral therapy has decreased, HBV positive individuals are still at risk for the development of HCC despite adequate viral suppression. Cases of HBV-HCC are usually reported to occur within 5 years of starting antiviral therapy [9,10], but data on the long-term risk of HCC on antiviral therapy is lacking [19]. However, recent observations at the Liver Disease Prevention Center, Division of Gastroenterology and Hepatology at Thomas Jefferson University Hospital have demonstrated that HBV positive individuals may develop HCC even after 10 or more years of successful viral suppression [13,14]. In Table 1, we have included a new and more comprehensive list of patients on antiviral therapy for HBV (treated for 9-19 years) that later developed HCC.
| Pt | Date StartTx | Date HCC Dx | Yrs on anti-HBV Tx at HCC Dx | Yrs with HBV DNA(-) | Age (yr) at HCC Dx | Tumor size (cm) | HBV DNA at HCC Dx |
Anti-HBV Tx |
|---|---|---|---|---|---|---|---|---|
| 1 | 4/1998 | 7/2007 | 9 | 3 | 53 | 1.1 Junction | UD | LAM + TDF |
| 2 | 1/1998 | 3/2008 | 10 | 8 | 68 | 2.8 Rt | UD | LAM + TDF |
| 3 | 5/1998 | 2/2008 | 10 | 7 | 76 | 1.8 Lt | UD | LAM + TDF |
| 4 | 7/2001 | 9/2010 | 9 | 4 | 54 | 2.8 Rt | UD | LAM + TDF |
| 5 | 8/2004 | 11/2010 | 16 | 4 | 53 | 3.9 Rt | UD | LAM + TDF |
| 6 | 7/2001 | 1/2011 | 10 | 5 | 55 | 2.8 Rt | UD | LAM + TDF |
| 7 | 2/2004 | 6/2013 | 9 | 8 | 57 | 2.5 Lt med | UD | TDF |
| 8 | 2/1996 | 7/2013 | 17 | 10 | 73 | 1.6 Rt | UD | TDF |
| 9 | 8/1997 | 6/2014 | 17 | 6 | 54 | 2.2 Lt lat | UD | ETV |
| 10 | 3/2004 | 6/2013 | 9 | 7 | 57 | 2.5 Lt | UD | TDF |
| 11 | 7/2001 | 6/2014 | 13 | 7 | 54 | 2.2 Lt | UD | TDF |
| 12 | 5/1996 | 10/2014 | 18 | 10 | 74 | 3.4 Rt | UD | LAM + TDF |
| 13 | 2/2000 | 10/2014 | 14 | 12 | 62 | 3.8 Rt | UD | ETV + TDF |
| 14 | 2/2000 | 4/2015 | 15 | 12 | 62 | 3.4 Rt | UD | TDF |
| 15 | 2/2000 | 5/2015 | 15 | 12 | 65 | 3.8 Rt | UD | TDF |
| 16 | 12/1998 | 8/2017 | 19 | 8 | 64 | 2.0 Rt | UD | LAM + TDF |
| 17 | 2/2008 | 6/2019 | 11 | 10 | 57 | 2.2 Rt | UD | ETV + TDF |
| ETV: Entecavir, LAM: Lamivudine, Lt: Left, Pt: Patient, Rt: Right, TDF: Tenofovir Disoproxil Fumarate, Tx: Treatment, UD: Undetectable | ||||||||
Development of Subsequent New and Recurrent HCC
Patients who undergo resection or ablation of the initial HCC still have a risk for the development of recurrent HCC. Studies have reported different outcomes in the incidence of recurrence and survival after tumor resection followed by antiviral therapy maintenance. In two randomized control trials, Huang et al. demonstrated that antiviral therapy reduces the risk of late HCC recurrence (> 2 years) post hepatectomy in HBV-related HCC patients with both high [20] and low [21] preoperative serum HBV-DNA levels. It has been postulated that early recurrence is associated with oncogenic properties of the tumor and late recurrence is related to the underlying HBV replication. Antivirals can limit chronic inflammation that occur in the late recurrence phase and prevent development of a second primary tumor [22]. Kuzuya et al. demonstrated that recurrence rates of HCC were not different in those treated with lamivudine vs. the no treatment group in patients who had undergone hepatic resection or radiofrequency ablation [23].
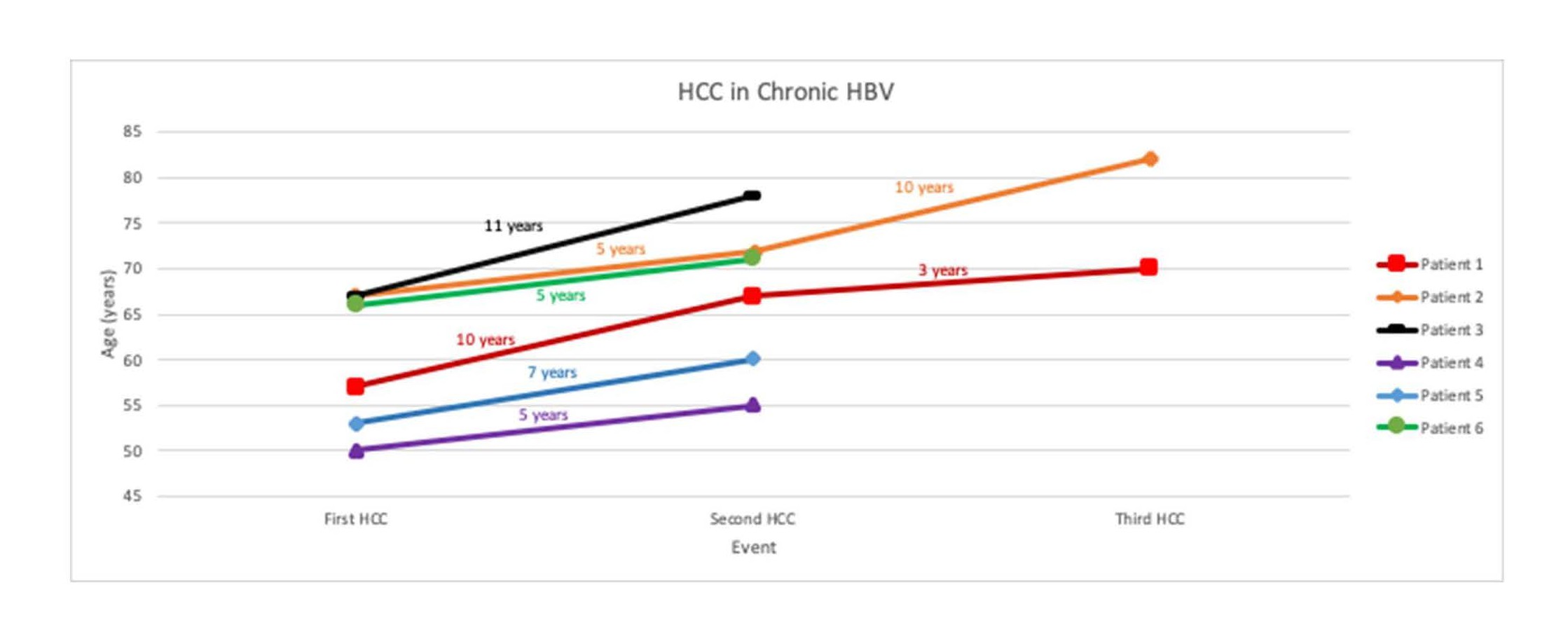
At our center we have observed the case of six patients who achieved successful HBV suppression for 5-11 years with antivirals after undergoing curative therapy for HCC but yet developed subsequent new or recurrent HCC (Figure 1). Not much is known about the efficacy of antivirals against HCC recurrence and further studies with longer follow-up periods are necessary to fully understand their role in preventing recurrence.
Figure 1. Graph of 6 patients demonstrating long duration time between initial and subsequent HCC diagnoses.
Need for HBV Cure
Chronic HBV infection is maintained by cccDNA accumulation in the hepatocyte nucleus. With this intracellular replication intermediate, HBV continues to carry the potential of carcinogenesis. Unfortunately, current antiviral therapies are not able to eliminate cccDNA. Given our understanding of recurrent HCC despite the use of antivirals, it is crucial to find alternative mechanisms for targeting HBV clearance. There are several steps in the HBV replication cycle that have been identified as targets for new drugs. These new therapeutics are currently in different stages of clinical trials.
HBV Replication Cycle and New Therapeutics for HBV
HBV is an enveloped, partially double-stranded relaxed circular DNA (rcDNA) with a genome of about 3200 base pairs in length. The virus enters hepatocytes via a cell surface receptor, sodium taurocholate cotransporting polypeptide (NCTP) [24]. Once in the cell, the virus delivers the nucleocapsid to a nuclear-pore complex and releases the rcDNA into the nucleus. The rcDNA is then converted to cccDNA which remains in the nucleus of the infected hepatocyte and is used as a template for the transcription of four viral mRNA intermediates. The largest of these intermediates, pregenomic RNA (pgRNA), undergoes reverse transcription and is used as a template for the new copies of rcDNA, the capsid core protein and the viral RNAdependent- DNA-polymerase. These components assemble together to form the DNA- containing nucleocapsids. It then gets coated with envelope proteins before being released from the hepatocyte.
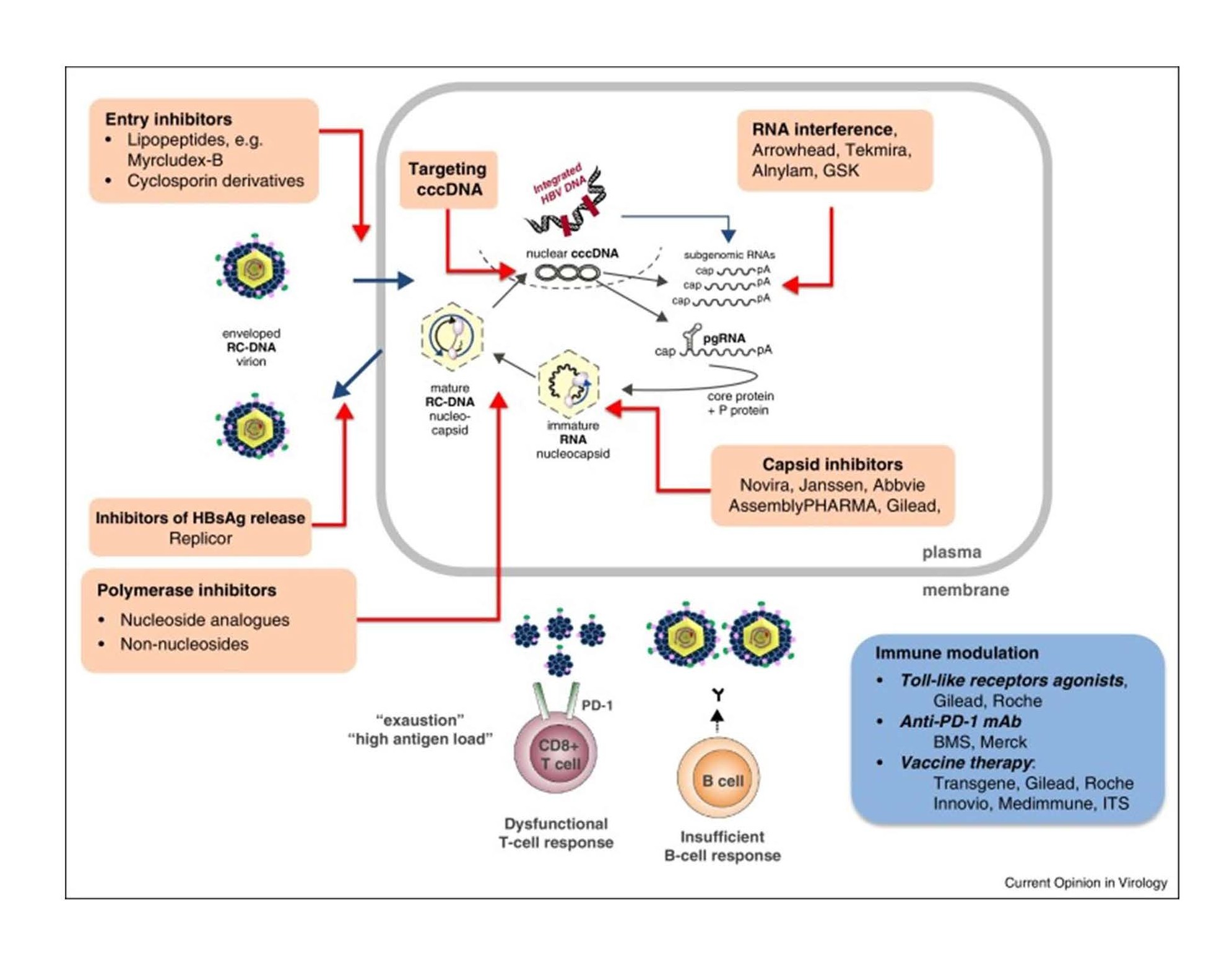
A number of therapeutics targeting different stages of the replication cycle are currently under investigation. Each drug targets a specific point of the viral replication cycle or acts indirectly on the host immune functions. Targets can interfere with replication through the following mechanisms: 1) inhibition of viral entry, 2) blocking rcDNA entry into the hepatic nucleus, 3) prevention of cccDNA formation, 4) prevention of mRNA transcription, 5) inhibition of capsid formation, 6) inhibition of reverse transcription and 7) inhibition of HBV release into circulation [25] (Figure 2).
Figure 2. Hepatitis B Replication Cycle and Potential Drug targets [25].
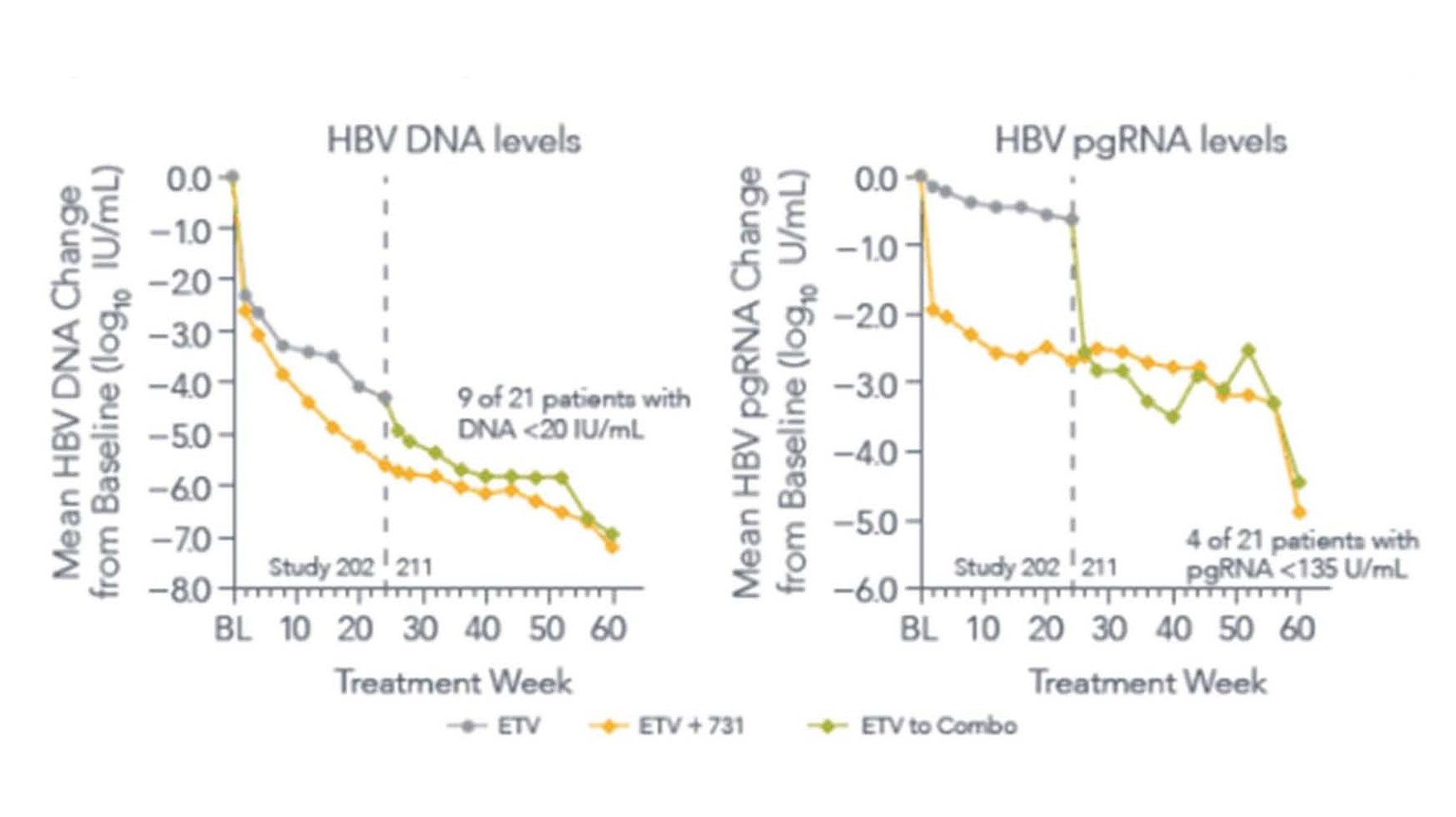
Entry inhibitors consist of a category of drugs such as Bulevirtide (formally known as Myrcludex B) that block HBV entry into the hepatocyte by targeting the sodium taurocholate cotransporting polypeptide (NTCP) receptor [26]. Other therapeutics under investigation work through RNA interference and gene silencing. Small interfering RNA’s exhibit their effect by binding and interfering with mRNAs transcripts. These mRNA intermediates can be used to reduce the expression of expression of viral proteins (HBsAg and HBeAg) [27]. Capsid inhibitors disrupt the HBV lifecycle by the destabilization of the nucleocapsid, production of defective capsids or production of capsid that lack genetic material [28]. Interestingly, Capsid inhibitors are the only drugs that have been shown to reduce levels cccDNA. A recent phase 2 study that was mentioned in an earlier paper [29] demonstrated that ABI-H0731, a core inhibitor, not only interfered with viral capsid formation and entry of rcDNA into the nucleus, but also decreased the levels of pgRNA [30]. In the study, the combination of the ABI-H0731 and Entecavir demonstrated greater reductions of viral DNA load and pgRNA levels by week 24 in treatment naïve, HBeAg+, individuals compared to entecavir alone(p=0.0452). After switching patient from entecavir to the combination of both, they saw immediate decline in HBV DNA and pgRNA levels (Figure 3) [30]. By week 48, the mean HBV DNA and pgRNA levels declined 6.3 logs and 3.0 logs from baseline, respectively. This decline was thought to be attributed to the reduction of cccDNA, the only source of pgRNA.
Figure 3. Study 202 and 211 results of Entecavir alone, Entecavir+ABI-H0731 (Core Protein Inhibitor), and switch from entecavir to combo on HBV DNA levels and pgRNA levels [30].
Conclusion
Currently there is no curative treatment for HBV, despite the use of our current antivirals that reduce viral loads to undetectable levels. The ultimate goal of the new therapies will be to eradicate cccDNA from hepatocytes and to prevent hepatocarcinogenesis. The cure for the virus will likely rely on the combination of multiple therapies, targeting different aspects of the replication cycle. With the recent advances in drug therapy, a complete cure for HBV may be on the horizon.
Conflict of Interest
Hann receives clinical research grants from Gilead Sciences and Assembly Biosciences and serves National Advisory Board of Gilead Sciences.
The rest of the authors have no conflict of Interest.
References
2. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: A Cancer Journal for Clinicians. 2011 Mar;61(2):69-90.
3. Zamor PJ, Delemos AS, Russo MW. Viral hepatitis and hepatocellular carcinoma: etiology and managsement.Journal of Gastrointestinal Oncology. 2017 Apr;8(2):229-42.
4. Maddrey WC. Hepatitis B--an important public health issue. Clinical laboratory. 2001;47(1-2):51-5.
5. Lai CL, Shouval D, Lok AS, Chang TT, Cheinquer H, Goodman Z. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. New England Journal of Medicine. 2006 Mar 9;354(10):1011-20.
6. Tang LS, Covert E, Wilson E, Kottilil S. Chronic hepatitis B infection: a review. JAMA. 2018 May 1;319(17):1802-13.
7. Jefferies M, Rauff B, Rashid H, Lam T, Rafiq S. Update on global epidemiology of viral hepatitis and preventive strategies. World Journal of Clinical Cases. 2018 Nov 6;6(13):589-99.
8. Komatsu H. Hepatitis B virus: where do we stand and what is the next step for eradication?. World Journal of Gastroenterology: WJG. 2014 Jul 21;20(27):8998-9016.
9. Papatheodoridis GV, Manolakopoulos S, Touloumi G, Vourli G, Raptopoulou-Gigi M, Vafiadis-Zoumbouli I, et al. Virological suppression does not prevent the development of hepatocellular carcinoma in HBeAg-negative chronic hepatitis B patients with cirrhosis receiving oral antiviral (s) starting with lamivudine monotherapy: results of the nationwide HEPNET. Greece Cohort Study. Gut. 2011 Aug 1;60(8):1109-16.
10. Papatheodoridis GV, Lampertico P, Manolakopoulos S, Lok A. Incidence of hepatocellular carcinoma in chronic hepatitis B patients receiving nucleos (t) ide therapy: a systematic review. Journal of Hepatology. 2010 Aug 1;53(2):348-56.
11. Vlachogiannakos J, Papatheodoridis G. Hepatocellular carcinoma in chronic hepatitis B patients under antiviral therapy. World Journal of Gastroenterology: WJG. 2013 Dec 21;19(47):8822-30.
12. Dargan A, Wong SY, Coben R, Conn M, Dimarino AJ, Hann HW. Persistent risk for hepatocellular carcinoma after more than a decade of successful hepatitis B virus suppression. Minerva Gastroenterologica Dietologica. 2017 Mar;63(1):74-6.
13. Yoo J, Hann HW, Coben R, Conn M, DiMarino AJ. Update treatment for HBV infection and persistent risk for hepatocellular carcinoma: prospect for an HBV cure. Diseases. 2018 Jun;6(2):27.
14. Shinn BJ, Martin A, Coben RM, Conn MI, Prieto J, Kroop H, et al. Persistent risk for new, subsequent new and recurrent hepatocellular carcinoma despite successful anti-hepatitis B virus therapy and tumor ablation: The need for hepatitis B virus cure. World Journal of Hepatology. 2019 Jan 27;11(1):65-73.
15. Michailidis E, Kirby KA, Hachiya A, Yoo W, Hong SP, Kim SO, et al. Antiviral therapies: focus on hepatitis B reverse transcriptase. The International Journal of Biochemistry & Cell Biology. 2012 Jul 1;44(7):1060-71.
16. Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. New England Journal of Medicine. 2004 Oct 7;351(15):1521-31.
17. Hosaka T, Suzuki F, Kobayashi M, Seko Y, Kawamura Y, Sezaki H, et al. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology. 2013 Jul;58(1):98- 107.
18. Kim WR, Berg T, Loomba R, Schall RA, Dinh P, Yee LJ, et al. 43 long term tenofovir disoproxil fumarate (tdf) therapy and the risk of hepatocellular carcinoma. Journal of Hepatology. 2013 Apr 1;58:S19.
19. Kodali S, Singal AK. Potent suppression of hepatitis B virus and hepatocellular carcinoma: how long is good enough?. Hepatobiliary Surgery and Nutrition. 2018 Jun;7(3):212-3.
20. Huang G, Lau WY, Wang ZG, Pan ZY, Yuan SX, Shen F, Zhou WP, Wu MC. Antiviral therapy improves postoperative survival in patients with hepatocellular carcinoma: a randomized controlled trial. Annals of surgery. 2015 Jan 1;261(1):56-66.
21. Huang G, Li PP, Lau WY, Pan ZY, Zhao LH, Wang ZG, et al. Antiviral therapy reduces hepatocellular carcinoma recurrence in patients with low HBV-DNA levels: a randomized controlled trial. Annals of Surgery. 2018 Dec 1;268(6):943-54.
22. Akateh C, Pawlik TM, Cloyd JM. Adjuvant antiviral therapy for the prevention of hepatocellular carcinoma recurrence after liver resection: indicated for all patients with chronic hepatitis B?. Annals of Translational Medicine. 2018 Oct;6(20):397.
23. Kuzuya T, Katano Y, Kumada T, Toyoda H, Nakano I, Hirooka Y, et al. Efficacy of antiviral therapy with lamivudine after initial treatment for hepatitis B virus-related hepatocellular carcinoma. Journal of Gastroenterology and Hepatology. 2007 Nov;22(11):1929- 35.
24. Yan H, Li W. Sodium taurocholate cotransporting polypeptide acts as a receptor for hepatitis B and D virus. Digestive Diseases. 2015;33(3):388-96.
25. Levrero M, Testoni B, Zoulim F. HBV cure: why, how, when?. Current Opinion in Virology. 2016 Jun 1;18:135- 43.
26. Kang C, Syed YY. Bulevirtide: First Approval. Drugs. 2020 Sep 14:1-5.
27. Gish RG, Given BD, Lai CL, Locarnini SA, Lau JY, Lewis DL, et al. Chronic hepatitis B: virology, natural history, current management and a glimpse at future opportunities. Antiviral Research. 2015 Sep 1;121:47-58.
28. Brahmania M, Feld J, Arif A, Janssen HL. New therapeutic agents for chronic hepatitis B. The Lancet Infectious Diseases. 2016 Feb 1;16(2):e10-e21.
29. Cao C, Shinn B, Halegoua-DeMarzio D, Hann HW. Are we close to achieving HBV cure? Risk for Hepatocellular Carcinoma persists despite successful suppression of Hepatitis B virus for over a decade. Japanese Journal of Gastroenterology and Hepatology. 2020;3(2):1-5.
30. Sulkowski M, Agarwal K, Fung S, Yuen M-F, Ma X, Lalezari J, et al. Continued therapy with ABI-H0731+ NrtI results in sequential reduction/loss of HBV DNA, HBV RNA, HBeAg, HBcrAg and HBsAg in HBeAg-positive patients. Hepatology. 2019;70(6):1486A-7A.