Abstract
The incidence of diabetes mellitus (DM) is increasing globally, and it is a major source of concern. This study was undertaken to assess the antidiabetic effect of the aqueous extract of Moringa oleifera, Ocimum gratissimum and Vernonia amygdalina. Sixty adult Wistar rats with body weight of 120-150 g were randomly assigned to groups of five rats each (n=12). Group 1 served as normal control; Groups 2-5 were diabetic groups; group 2 served as negative control; group 3-5 received 100, 200 and 400 mg/kg of triherbal formula, respectively. The body weight (BW) and fasting blood glucose level (FBSL) of the rats were monitored weekly. At the end of the experiment, all the rats were anaesthetized intraperitoneally (I.P) and blood samples were collected by cardiac puncture for biochemical analysis. There was an increase in the BW of the control group and varying doses of tri-herbal formulation. It caused 88% decrease in FBSL; 371.7%, 386.6% and 296.0% with respect to 100, 200 and 400 mg/kg. Sub-chronic study of the effect of the extract showed a significant increase (P<0.05) in packed cell volume (PCV), white blood counts in rat induced diabetes. The histological studies showed that the diabetic rats with the architecture of the pancreas distorted, was restored to normal by the extract. Its LD50 was found to be greater than 1000 mg/kg indicating its safety in rats. This study has shown that triherbal formula has hypoglycemic and hematological effects.
Keywords
Antidiabetic, Moringa oleifera, Ocimum gratissimum, Vernonia amygdalina, Alloxan-induced
Introduction
Diabetes mellitus (DM) is characterized by absolute or relative deficiencies in insulin secretion and insulin action associated with chronic hyperglycemia and disturbances of carbohydrate, lipid and protein metabolism [1-3]. It results from either defect in insulin secretion by the pancreas or inability of the cells to utilize the insulin produced [4-5]. Insulin is secreted by the beta-cells of the islet of Langerhans in the pancreas. Diabetes is a prime risk factor for cardiovascular disease and also affects the heart muscle causing both systolic and diastolic heart failure and also includes polyuria, polyphagia, polydipsia, and ketosis [5].
DM is a global burden, associated with life-threatening complications including stroke, renal failure, and cardiac attack. Life for a person with diabetes mellitus means constant awareness of the illness, one or two insulin shots a day, frequent finger punctures to monitor blood glucose level, a restrictive diet, and concern over complications. Global diabetes prevalence has more than doubled over the last three decades, with prevalence rates far exceeding modeled projections; even after allowing for improved surveillance. Nearly 1 in 10 adults worldwide are now affected with diabetes [6-8].
The World Health Organization estimated that over 86% of the people in developing countries rely on traditional remedies such as herbs for their daily needs and about 855 traditional medicines include crude plant extracts [6]. DM is one of the world’s leading causes of death, with over 150 million diabetic cases worldwide [9]. More than 1.70 million Nigerians above 15 years old are diabetic, with about 70,000 children less than 15 years developing Type 1 diabetes annually. Diabetes is prevalently rising globally as a result of obesity, population growth and sedentary lifestyles, and it is projected to be over 360 million cases by 2030 [10].
Over 50% of plants serve in traditional medicine for their health benefits in combating certain ailments affecting humans such as dysentery, diarrhea, toothache, skin infections and diabetes. Many conventional drugs have been derived from prototypic molecules in medicinal plants. To date, over 400 traditional plant treatments for diabetes have been reported, although only few of the plants have received scientific and medical evaluation to assess their efficacy [11]. The attributed hypoglycemic effects of these plants is due to their ability to restore the function of pancreatic tissues by causing an increase in insulin output or inhibit the intestinal absorption of glucose or by the facilitation of metabolites in insulin dependent processes. Hence, treatment with herbal drugs has an effect on protecting β-cells and smoothing out fluctuation in glucose levels. Most of these plants have been found to have chemical constituents like glycosides, alkaloids, phenols, terpenoid, and flavonoids that are frequently connected as having antidiabetic effects [12].
Moringa oleifera is the most widely cultivated species of a monogeneric family, the Moringaceae. Native to the sub- Himalayan tracts, it is now widely cultivated and has become naturalized in many locations in the tropics [13]. The relative ease with which it propagates through both sexual and asexual means and its low demand for soil nutrients and water after being planted makes its production and management easy [14]. Moringa preparations have been cited in the scientific literature as having nutritional, antimicrobial, hypotensive, antispasmodic, antiulcer, antiinflammatory, hypo-cholesterolemic, and hypoglycemic activities, as well as having considerable efficacy in water purification by flocculation, sedimentation, antibiosis and even reduction of Schistosome cercariae titer [15]. The plant has also been used extensively for treating inflammation, cardiovascular disease, liver disease and hematological, hepatic and renal function [16]. Moringa leaf extracts have also been shown to have ameliorating effect on chromium-induced testicular toxicity in rats [17] and to enhance sexual activity in mice [18].
Ocimum gratissimum, African sweet basil, is a plant belonging to the Lamiceae family. The Ocimum species are widely found in tropical and subtropical regions and commonly used as food spice and traditional herb. The herb has been recommended for the treatment of various diseases [19]. Its hypoglycemic efficacy has been reported [20,21] as it has therapeutic potentials on hepatic disorders [22].
Vernonia amygdalina, a member of Asteracea, found throughout the tropical Africa. It is commonly known as bitter leaf. The parts of the plants mostly used are the leaves, usually used in the treatment of diarrhea, headache, wart and kidney function [23].
Materials and Methods
Collection of plant materials
Fresh matured Moringa oleifera, Ocimum gratissimum and Vernonia amygdalina leaves were harvested from the Botanical Garden, University of Lagos, Nigeria and were authenticated at the Department of Botany, University of Lagos, Nigeria. Voucher specimens’ were deposited in the Department of Botany herbarium, University of Lagos. The leaves were rinsed severally with clean tap water to remove dust particles and debris and thereafter allowed to dry completely. About 2 kg portion of each leaf was then taken for preparation of the plant extracts.
Preparation of plant extract
Two kilograms (2 kg) each of M. oleifera, O. gratissimum and V. amygdalina was separately homogenized with an electric blender in 4.0 liters of 80% (v/v) ethanol. The blends were allowed for 48 hours in a refrigerator at 4ºC to allow for thorough extraction of the plants’ active components. These were then filtered with a cheese cloth and later with Whatman No. 1 filter paper to obtain a homogenous filtrate. The filtrates were then concentrated in vacuo at low temperatures (37-40ºC) to about one tenth of the original volume using a rotary evaporator. The concentrates were allowed open in a water bath at 40oC to allow for complete dryness yielding 80 g (4.0%), 75.5 g (3.8%) and 75. 5 g of greenish brown substance obtained.
Acute toxicity test
The acute toxicity and lethality test of the aqueous plant extracts in rats was estimated using the method of Lorke as described by (Okoli et al. [7]), and the animals were observed continuously after every 2 hrs. Under the following profiles:
Behavioral Profile: Alertness, restlessness
Neurological Profile: Pain response, touch response, gait
Autonomic Profile: Defecation and urination
After a period of 24 hrs., the animals were observed for any lethality or death.
Experimental animals
Sixty adult male albino Wistar rats with a body weight of 120-180 g obtained from the animal house of the Department of Biological Sciences of Federal University of Agriculture, Abeokuta, Nigeria were used for this study. The animals were placed in standard cages maintained in 12-hour light: dark cycle under standard conditions (temperature 25+5ºC, relative humidity 50+5%). All animals received standard laboratory animal’s chow and water ad libitum during the whole period of experiment.
Experimental induction of diabetes
Diabetes was induced according to the principle of Aruna et al., as described by (Okoli et al. [7]) with slight modification. A single intraperitoneal injection of alloxan monohydrate (Parchem Company 3237-50-1) (150 mg/kg b.w.) was given and after day 5, blood was drawn from tail vein of the rat by snipping to determine blood glucose level with an automated glucose analyzed device (One Touch Glucometer, Lifecan, California, USA). Sensitivity and specificity of capillary blood glucose measurement by glucometer were 83.5% and 97.5% respectively (ppv=80%), (npv=98%). Animals with blood glucose level ≥ 225 mg/dl were considered diabetic and used for the study.
Experimental design
Sixty rats were divided into five different experimental groups of 12 animals each. Two groups, normal control group (NC) and diabetic control group (DC) received placebo, 0.5ml DMSO; the other three diabetic groups, the treated groups, received respectively Insulin (administered at a dose of 5Ukg-1b.w.s.c once per day to simulate human regimens), 100 mg/kg, 200 mg/kg and 400 mg/kg of a combination of M. oleifera, O. gratissimum, and V. amygdalina. The plant extracts were administered orally. The dosage of the extract was determined from preliminary studies in our laboratory. The duration of treatment was 28 days after induction of diabetes. At the end of the experimental period, the animals were fasted for 12h, then anaesthetized under chloroform vapor and dissected. Whole blood was obtained by cardiac puncture into sterile plain tubes for the hematological assays. The organs were also surgically removed for histological study.
Statistical analysis
Results were analyzed using Statistical Package for Social Sciences SPSS version 20.0. All data were reported as mean ± SEM. For the evaluation of significant differences, the comparison of means between any two experimental groups was done by GraphPad Prism 7.02 for student-t test (paired t-test). Differences were considered to be statistically significant if P = 0.5.
Results
Body weight
The extract showed a significant increase (P<0.05) in body weight when compared with pre-diabetic values (Table 1).
| Treatment | Pre-treatment | Day 7 | Day 14 | Day 28 |
| BLOOD GLUCOSE LEVEL (mg/dl) | ||||
| Control | 87.8 | 103.4* | 122.6* | 156.6* |
| Negative | 90.4 | 106.4* | 107.2* | 132.8* |
| 100 mg/kg | 74.6 | 81.4* | 98.1* | 109.6* |
| 200 mg/kg | 77.6 | 82.6* | 99.4* | 107.8* |
| 400 mg/kg | 79.5 | 83.9* | 89.8* | 104.6* |
| Value express as mean. Values with superscripts* represent increase in body weight relative to pre-treatment | ||||
Anti-Diabetic effect of Moringa oleifera, Ocimum gratissimum and Vernonia amygdalina combination
The extract showed a significant reduction (P<0.05) in blood glucose levels following sub-chronic administration when compared with the diabetic pre-treatment values. At day 28, the extract reduced the blood glucose level (Table 2).
| Treatment | Pre-treatment | Day 7 | Day 14 | Day 28 |
| BLOOD GLUCOSE LEVEL (mg/dl) | ||||
| Control | 87.8 | 88.6 | 86.4 | 88.0 |
| Negative | 90.4 | 326.9* | 266.2* | 106.2* |
| 100 mg/kg | 74.6 | 371.6* | 202.2* | 103.5* |
| 200 mg/kg | 85.6 | 386.7* | 101.0* | 106.2* |
| 400 mg/kg | 405.6 | 296.0* | 305.6* | 308.2* |
| Value express as mean. Values with superscripts* represent reduction in blood glucose level in rats | ||||
Hematological Parameters
The extract showed a significant reduction (P<0.05) in blood hematological parameters when compared with the diabetic pre-treatment values (Table 3).
| Group | Days | PVC (%) | WBC | NEU (%) | EOSIN (%) | LYMPH (%) |
| Control | Pre | 27 | 5.1* | 57* | 1.0* | 32* |
| 7-days | 35* | 3.0 | 60* | 1.7* | 40* | |
| 14-days | 34* | 7.9* | 60* | 0.0* | 40* | |
| 28-days | 31* | 6.2* | 68* | 0.0* | 38* | |
| Negative | Pre | 30 | 3.6 | 57 | 1.0* | 37 |
| 7-days | 46* | 5.2* | 62* | 1.0* | 40* | |
| 14-days | 40* | 5.3* | 61* | 1.0* | 41* | |
| 28-days | 34* | 4.2* | 60* | 1.0* | 40* | |
| 100 mg/kg | Pre | 29 | 3.3 | 54 | 1.0* | 30 |
| 7-days | 42* | 4.3* | 60* | 0.0* | 37* | |
| 14-days | 32* | 4.3* | 62* | 0.0* | 37* | |
| 28-days | 35* | 7.1* | 63* | 1.0* | 35* | |
| 200 mg/kg | Pre | 32* | 4.8 | 54* | 1.0* | 30 |
| 7-days | 40* | 5.9* | 60* | 1.0* | 40* | |
| 14-days | 35* | 6.6* | 60* | 1.0* | 40* | |
| 28-days | 30 | 5.8* | 64* | 1.0* | 43* | |
| 400 mg/kg | Pre | 32 | 4.8 | 42 | 1.0* | 30 |
| 7-days | 52* | 5.0* | 60* | 1.0* | 39* | |
| 14-days | 33* | 5,6* | 62* | 1.0* | 40* | |
| 28-days | 34* | 6.4* | 60* | 1.0* | 37* | |
| Value express as mean. Values with superscripts* represent reduction in hematological parameters | ||||||
Histological studies
The effects of the extract on pancreatic tissues of diabetic-treated were shown in plates 1-5.
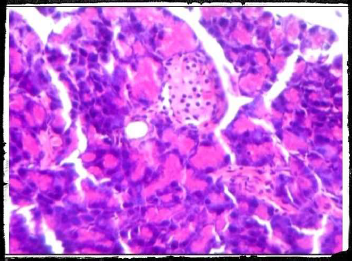
Plate 1. Photomicrography of pancreatic tissue of control (H and E x400).
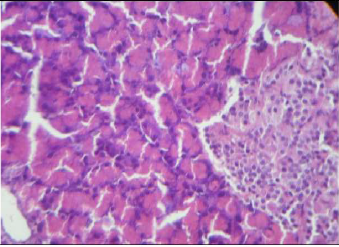
Plate 2. Photomicrography of pancreatic tissue of control (H and E x400). There was no visible change in the structure of the pancreas of animals administered when compared with non-diabetic group.
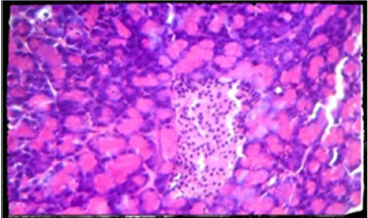
Plate 3. Photomicrography of pancreatic tissue of control (H and E x400). There was no visible change in the pancreas of animals administered with 100 mg/kg compare to control.
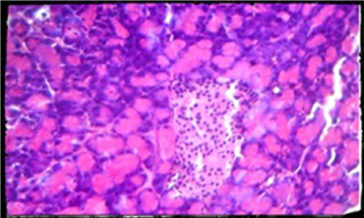
Plate 4. Photomicrography of pancreatic tissue of control (H and E x400). There was no visible change in the pancreas of animals administered with 200 mg/kg compare to control.
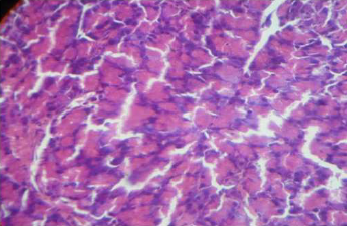
Plate 5. Photomicrography of pancreatic tissue of control (H and E x400). There was no visible change in the pancreas of animals administered with 400 mg/kg compare to control
Discussion
DM poses a substantial threat to the health and quality of life of an individual. Due to increaseed complications and mortality in DM, adequate glycemic controls might be useful in its management. In this study, anti-diabetic potential of aqueous extract of Moringa oleifera, Ocimum gratissimum, and Vernonia amygdalina in alloxan-induced diabetic rats showed that a single oral administration of the extract reduced the fasting blood glucose levels as well as suppressed the rise in blood glucose of normal rats after a heavy glucose meal. A number of investigators have shown that coumarin, flavonoid, terpenoid and a host of other secondary plant metabolites including arginine and glutamic acids possess’ hypoglycemic effects in various experimental animal models [11]. However, in line with the hypothesis of Marles and Farnsworth [5], which stipulates that plant which contains terpenoid possesses hypoglycemic activities in diabetic and normal mammal, then it could be thus concluded that aqueous extract of Moringa oleifera, Ocimum gratissimum and Vernonia amygdalina possess hypoglycemic activity.
In the anti-diabetic activity studies, daily oral administration of the extract for 28 days revealed a gradual but sustained reduction in blood glucose levels in diabetic rats. According to Oyedepo et al. [9], aqueous extract of Moringa oleifera, Ocimum gratissimum, and Vernonia amygdalina combination has been shown to normalize the high blood glucose level in diabetic rats by the 28th day of the experiment. Treatment with the extract also reduced mortality of diabetic rats from hyperglycemia and prolonged their survival. In this study, the control animals all died on day 14 post-induction of diabetes (data not shown) whereas the extract-treated group survived beyond the period of the experiment. Since a better activity has been achieved in severely diabetic rats with damaged islet, it is possible that aqueous extract of Moringa oleifera, Ocimum gratissimum, and Vernonia amygdalina combination has some direct effect. The increase in the total cholesterol and TAG and the decrease in HDL levels of diabetic rats observed in this study are in accordance with earlier report in diabetic subjects [5]. Diabetes-induced hyperlipidemia has been attributed to excess mobilization of fat from the adipose due to underutilization of glucose [9]. Chronic oral administration of the extract also revealed a significant reduction in cholesterol levels as well as the increase in HDL levels of the diabetic-treated rats when compared with the control rats supports the findings of Coon and Ernst [8] who stated that most hypoglycemic plants have potentials of reducing blood glucose level.
Also, significant advancement in the hematological parameters on a long term treatment with extract for 28 days suggests the favorable effects of the aqueous extract of Moringa oleifera, Ocimum gratissimum, and Vernonia amygdalina combination. Sub-chronic administration of the extract also showed weight gain in diabetic-treated compared to control rats.
Histopathological analysis showed that after the long administration of the extract at 100, 200 and 400 mg/kg body weight, the extract was able to restore the structure of the pancreas damaged by alloxan. (Plates 1-5). Alloxan owes its diabetogenic potential to destruction of β -cells of the islet [12,13] which consequently impairs insulin secretion and leads to hyperglycemia. Extract treatment may have restored the integrity and possibly, functions of the damaged pancreatic tissues. The precise mechanism of this tissue repair is yet to be known. However, due to the tremendous implication of oxidative stress [12,13] which leads to the damage to the pancreas, it seems reasonable to suggest that the antioxidant [27] and radical scavenging effects [14] of Moringa oleifera leaf may play a significant role in protecting pancreatic tissues from oxidants including those generated by alloxan. Alloxan destroys insulin-producing pancreatic β-cells through the production of reactive oxygen species which then cause tissue damage [16]. The hypoglycemic effect of the extract may be responsible for protection confer on the pancreas from the deleterious effect of chronic hyperglycemia. Rather than reflecting a direct tissue repair effect, it is likely that the extract, through antioxidative and hypoglycemic effects, protected the already compromised pancreas from further assault or pancreatic damage which may allow the natural repair processes to proceed and restore the tissues. However, it is unknown if the repaired tissues also had their functions fully or partially restored since the blood glucose level of the animals did not return to normalcy or pre-treatment levels as at the end of the experiment. A return to pre-treatment levels might indicate a full restoration of insulin secretion by the repaired pancreatic tissues [20].
The combination has hypolipidemic effect due to its chemical composition, which reveals the presence of alkaloids, flavonoids, saponin, and cardiac glycosides. All these components are known to reduce serum lipid level in animals [24,25]. Saponins may lower cholesterol by binding with cholesterol in the intestinal lumen, preventing its absorption, or by binding with bile acids, causing a reduction in the enterohepatic circulation of bile acids and increase in its fecal excretion [26]. The increased bile acid excretion might have been offset by enhanced bile acid synthesis from cholesterol in the liver and consequent lowering of the plasma cholesterol. Although the precise mechanism through which the leaf extract exerted its hypolipidemic effect is neither clearly known nor studied, it is suggested from the findings that the control of glycaemia might have been contributory since control group is a major determinant of total cholesterol and triglyceride [27].
Ethical Approval
All authors hereby declare that the research has been determined exempt from review by the university animal research and ethics review committee and that the principles of the laboratory animal care were followed.
Acknowledgements
This work was supported by students provided by Department of cell biology and Genetics. The authors are also grateful to Lagos State University Teaching Hospital for the histological analyses.
Conflict of Interest Declaration
All authors declare that there is no conflict of interest in this work.
References
2. Agrawal RP, Sharma P, Pal M, Kochar A, Kochar DK. Magnitude of dyslipedemia and its association with micro and macro vascular complications in type 2 diabetes: a hospital based study from Bikaner (Northwest India). Diabetes Research and Clinical Practice. 2006 Aug 1;73(2):211-4.
3. Scott AI, Clarke BE, Healy H, D’Emden M, Bell SC. Microvascular complications in cystic fibrosis-related diabetes mellitus: a case report. JOP. Journal of the Pancreas. 2000;1(4):208-10.
4. Gordon L, Ragoobirsingh D, St Errol YA, Choo-Kang E, McGrowder D, Martorell E, et al. Lipid profile of type 2 diabetic and hypertensive patients in the Jamaican population. Journal of Laboratory Physicians. 2010 Jan;2(1):25-30.
5. Marles RJ, Farnsworth NR. Antidiabetic plants and their active constituents. Phytomedicine. 1995 Oct 1;2(2):137-89.
6. Nimenibo-Uadia R. Effect of aqueous extract of Canavalia ensiformis seeds on hyperlipidaemia and hyperketonaemia in alloxan-induced diabetic rats. Biokemistri. 2003;15(1):7-15.
7. Okoli CO, Ibiam AF, Ezike AC, Akah PA, Okoye TC. Evaluation of antidiabetic potentials of Phyllanthus niruri in alloxan diabetic rats. African Journal of Biotechnology. 2010;9(2):248-259.
8. Owulade MO, Eghianruwa KI, Daramola FO. Effects of aqueous extracts of Hibiscus sabdariffa calyces and ocimun gratissimumleaves on interstinal transit in rats. African Journal of Biomedical Research. 2004;7(1):31-34.
9. Oyedepo TA, Babarinde SO, Ajayeoba TA. Evaluation of anti-hyperlipidemic effect of aqueous leaves extract of Moringa oleifera in alloxan induced diabetic rats. International Journal of Biochemistry Research & Review. 2013 Jul 1;3(3):162-170.
10. Rang HP, Dale MM, Ritter JM, Moore PK. Rang and Dale’s Pharmacology. 5th Edition, Churchill Livingstone London. 2005; pp: 385-388.
11. Rockwood JL, Anderson BG, Casamatta DA. Potential uses of Moringa oleifera and an examination of antibiotic efficacy conferred by M. oleifera seed and leaf extracts using crude extraction techniques available to underserved indigenous populations. International Journal of Phytotherapy Research. 2013 Jan;3(2):61-71.
12. Rotimi SO, Omotosho OE, Rotimi OA. Persistence of acidosis in alloxan-induced diabetic rats treated with the juice of Asystasia gangetica leaves. Pharmacognosy Magazine. 2011 Jan;7(25):25-30.
13. Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiological Research. 2001 Jan 1;50(6):536-46.
14. Tasaduq SA, Singh K, Sethi S, Sharma SC, Bedi KL, Singh J, et al. Hepatocurative and antioxidant profile of HP-1, a polyherbal phytomedicine. Human & Experimental Toxicology. 2003 Dec;22(12):639-45.
15. Thompson CJ, Ernst E. Herbs for serum cholesterol reduction: a systematic view. The Journal of Family Practice. 2003 Jun;52(6):468-78.
16. Duncan DB. Multiple range and multiple F tests. Biometrics. 1955 Mar 1;11(1):1-42.
17. Fröde TS, Medeiros YS. Animal models to test drugs with potential antidiabetic activity. Journal of Ethnopharmacology. 2008 Jan 17;115(2):173-83.
18. Goji AD, Dikko AA, Bakari AG, Mohammed A, Ezekiel I, Tanko Y, et al. Effect of aqueous-ethanolic stem bark extract of commiphora africana on blood glucose levels on normoglycemic wistar rats. International Journal of Animal and Veterinary Advances. 2009 Jul 1;1(1):22-4.
19. Hayden MR, Tyagi SC, Kerklo MM, Nicolls MR. Type 2 diabetes mellitus as a conformational disease. Jop. 2005 Jul 8;6(4):287-302.
20. Jagetia GC, Baliga MS. The evaluation of nitric oxide scavenging activity of certain Indian medicinal plants in vitro: a preliminary study. Journal of Medicinal Food. 2004 Sep 1;7(3):343-8.
21. Jisieike-Onuigbo NN, Unuigbe EI, Kalu OA, Oguejiofor CO, Onuigbo PC. Prevalence of dyslipidemia among adult diabetic patients with overt diabetic nephropathy in Anambra state South-East Nigeria. Nigerian Journal of Clinical Practice. 2011;14(2):171-5.
22. Lee JH, Park JW, Kim JS, Park BH, Rho HW. Protective effect of Amomi semen extract on alloxaninduced pancreatic ß-cell damage. Phytotherapy Research: An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives. 2008 Jan;22(1):86-90.
23. Leung KK, Leung PS. Effects of hyperglycemia on angiotensin II receptor type 1 expression and insulin secretion in an INS-1E pancreatic beta-cell line. Jop. 2008 May 8;9(3):290-9.
24. Priadarshini A, Pankaj PP, Varma MC, Kumar K. Evaluation of the antibacterial potential of Moringa oleifera and Azadirachta indica against some pathogenic microbes: A comparative study. Int. J. Drug Dev. & Res. 2013 Jan;5(1):214-8.
25. Ayinla MT, Dada SO, Shittu ST, Olayaki LA, Akiode AO, Ojulari SL, et al. Anti-hyperlipidemic effect of aqueous leaf extract of Ocimum gratissimum in alloxan induced diabetic rats. International Journal of Medicine and Medical Sciences. 2011 Nov 21;3(12):360-3.
26. Babu R, Chaudhuri M. Home water treatment by direct filtration with natural coagulant. Journal of Water and Health. 2005 Mar;3(1):27-30.
27. Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. Jama. 2002 May 15;287(19):2570-81.
