Abstract
Background: Metastatic triple-negative breast cancer (TNBC) has few effective options after multiple lines of chemotherapy and checkpoint inhibitors. We evaluated a multi-component phytochemical platform, AminoTriComplex (ATC), added to metronomic cyclophosphamide and propranolol, while prospectively testing a predefined translational three-marker panel—Survivin (BIRC5), Cystatin C (CST3), and the melatonin receptor MT1 (MTNR1A).
Methods: Prospective, open-label, non-randomized, controlled, multicenter study (January 2023–June 2025). Key eligibility: programmed death-ligand 1 (PD-L1)–positive, refractory stage IV TNBC with progression after pembrolizumab and ≥4 prior chemotherapy lines (including platinum, gemcitabine, vinorelbine, taxane, sacituzumab). Patients received ATC + metronomic backbone (n=147) or metronomic backbone alone (n=119) for 12 weeks. Primary endpoint: objective response rate (ORR; Response Evaluation Criteria in Solid Tumors [RECIST] v1.1). Secondary endpoints: progression-free survival (PFS), blood and paired-biopsy biomarkers (Enzyme-linked immunosorbent assay [ELISA]/ immunohistochemistry [IHC]), Eastern Cooperative Oncology Group (ECOG) status, and safety (Common Terminology Criteria for Adverse Events [CTCAE] v5.0). To control multiplicity for the biomarker triad we used Benjamini–Hochberg false discovery rate (FDR) (q=0.10). Bias-mitigation measures included centralized laboratory workflows, prespecified biomarker thresholds, sensitivity analyses (IPTW), and a blinded independent central radiology review (BICR) on a prespecified 30% subset.
Results: A total of 266 patients were enrolled (147 ATC; 119 control).
• Efficacy: ORR was 46.3% (68/147) with ATC versus 12.6% (15/119) in control; crude odds ratio 6.64 (95% CI, 3.50–12.58), p<0.001. Median PFS was 8.9 months (95% CI, 7.5–10.4) with ATC versus 3.4 months (95% CI, 2.6–4.2) in control; hazard ratios (HR) 0.42 (95% CI, 0.30–0.60), p<0.001.
• Translational signal (predefined triad):
√ Survivin↓ ≥50%: 62/68 responders vs 14/79 non-responders.
√ Cystatin C↑ ≥40%: 59/68 vs 11/79.
√ MT1 re-expression (IHC): 58/68 vs 19/79.
√ Full triad (Survivin↓ + Cystatin C↑ + MT1↑): 55/68 responders vs 9/79 non-responders.
All associations were strongly significant under FDR control.
• Tumor microenvironment (paired re-biopsies at week 12): Ki-67 dropped markedly with ATC (median Δ −41%; 95% CI, −45 to −37; p<0.001) and CD8+ TILs increased (median Δ +34 cells/HPF; 95% CI, 28–40; p<0.001). In the control arm Ki-67 minimally changed (Δ −2%; 95% CI, −5 to +1; p=0.42) and CD8+ rise was small (Δ +2; 95% CI, −1 to +5; p=0.31). IHC showed a coherent pattern: Survivin decreased, Cystatin C increased, and MT1 re-expressed with treatment; imaging responses were consistent with these biological changes.
• Safety: Toxicities reflected the backbone regimen; no new or disproportionate high-grade signals were attributable to ATC. Exposure-adjusted incidence rate (EAIR) per 100 patient-weeks was 5.0 in both arms.
• Interpretation: Adding AminoTriComplex to a metronomic regimen was associated with substantially higher ORR and prolonged PFS in heavily pretreated, refractory TNBC. The predefined biomarker triad (Survivin↓ / Cystatin C↑ / MT1↑) tracked with clinical benefit and paralleled favorable shifts in the tumor microenvironment, suggesting a biologically integrated, multi-target mechanism spanning apoptosis re-engagement, invasion/metastasis restraint, and circadian-immune modulation. While compelling, these signals are correlative and arise from a non-randomized, open-label design.
• Novelty/Significance: (1) A consistent triad-based translational signature aligned with response and PFS in refractory TNBC; (2) circadian biology engagement via MT1 re-expression as a plausible therapeutic axis; (3) a multi-component phytochemical platform showing coherent biomarker-to-clinical concordance.
Limitations: Non-randomized allocation and open assessment introduce potential selection/assessment bias; the 12-week window limits OS readouts; contributions of individual phytochemicals were not isolated; causal mediation was not tested. These are planned for subsequent randomized, blinded trials.
Conclusion: In a hard-to-treat TNBC population, ATC + metronomic therapy produced meaningful improvements in ORR and PFS and a predefined three-marker translational signature consistent with its proposed biology. The triad may serve as a candidate response/prognostic surrogate and warrants confirmation in biomarker-guided phase III studies. These findings identify a promising three-marker triad that is hypothesis-generating and warrants validation in randomized trials.
Keywords
Triple-Negative breast cancer, AminoTriComplex, Phytotherapy, Metronomic chemotherapy, Propranolol, Survivin, Cystatin C, MT1/MTNR1A, Biomarkers, Circadian oncology, ORR, PFS, ELISA, IHC
Background
TRIPLE-negative breast cancer (TNBC): A persistent global challenge
Breast cancer remains the most frequently diagnosed malignancy in women worldwide. TNBC—defined by the absence of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2)—comprises ~15–20% of cases and is disproportionately represented among younger women, women of African ancestry, and carriers of germline BRCA1 mutations. Biologically, TNBC is highly heterogeneous and clinically aggressive, with early visceral/CNS relapse and poor survival; contemporary series place median overall survival (OS) for metastatic disease at roughly 9–17 months, despite sequential chemotherapy. Immune checkpoint inhibitors (e.g., pembrolizumab, atezolizumab) have improved outcomes for programmed death-ligand 1 (PD-L1)–positive subgroups, and antibody–drug conjugates such as sacituzumab govitecan prolong OS in refractory settings; nevertheless, progression is common and durable control remains uncommon, underscoring the need for new modalities and robust translational biomarkers [1–6].
Current biomarkers and their limitations
The TNBC biomarker landscape has real but limited clinical utility. Ki-67 is widely assessed, yet cross-laboratory variability hampers its reproducibility and clinical precision, even after international harmonization efforts. Tumor protein p53 (TP53) mutations occur in ~70–80% of TNBC and signal genomic instability and poor prognosis, but they are not directly actionable in routine practice. PD-L1 expression guides first-line immunotherapy selection; however, its predictive value diminishes once resistance emerges, and it incompletely captures the multifactorial biology of response and escape. Collectively, these gaps motivate biomarkers that both prognosticate and mechanistically reflect treatment efficacy [7–12].
Rationale for a three-layered translational panel
To encompass complementary TNBC hallmarks—intrinsic cell survival, extracellular-matrix (ECM) remodeling, and systemic circadian-immune regulation—we focus on (Survivin) BIRC5, CST3, and the MTNR1A:
- Survivin (BIRC5): apoptosis suppression and treatment resistance. Normally scarce in differentiated tissues, Survivin is re-expressed in cancer and coordinates apoptotic blockade (e.g., caspase inhibition) with mitotic progression. In TNBC, high Survivin associates with chemotherapy resistance, Epithelial-to-Mesenchymal Transition (EMT) /angiogenesis, and worse outcomes; preclinical inhibition restores chemosensitivity and triggers apoptosis. Its accessibility in tissue (Immunohistochemistry [IHC]) and plasma (Enzyme-linked immunosorbent assay (ELISA)) supports dual “solid + liquid” biomarker utility [13–15].
- Cystatin C: counter-regulation of cathepsin-driven invasion. Cystatin C is an endogenous inhibitor of cysteine cathepsins (B/L/S), proteases that degrade ECM, promote angiogenesis, and facilitate metastasis in breast cancer. Experimental models show Cystatin C can antagonize TGF-β–driven EMT, restrain invasion, and in some contexts suppress tumor progression; nonetheless, clinical integration in TNBC remains limited and mechanistic complexity persists, warranting prospective translational evaluation [16–20].
- Melatonin receptor MT1: circadian control and immune-inflammatory tuning. Disruption of nocturnal melatonin signaling in cancer is linked to pro-proliferative and pro-inflammatory programs (e.g., NF-κB/STAT3). In breast cancer—including TNBC—loss or low expression of MT1 has been observed, whereas higher MT1 levels correlate with more favorable prognosis in select cohorts. Melatonin/MT1 signaling can down-modulate oncogenic pathways and may indirectly intersect with Survivin regulation and protease–inhibitor balance, nominating MT1 re-expression as a plausible mechanistic and theranostic biomarker [21–25].
AminoSine TriComplex: A natural remedy with multifaceted mechanisms
In this context, AminoSineTriComplas a novel therapeutic candidate designed to complement existing treatment modalities for metastatic colorectal cancer (mCRC). This remedy combines the principles of classical medicine and homeopathy with cutting-edge nanotechnology to enhance therapeutic efficacy and minimize adverse effects. AminoSineTriComplex integrates bioactive compounds derived from traditional medicinal plants, including Epimedium, Chamomile, Rheum rhabarbarum, Ginseng (Panax ginseng), and Lingzhi (Ganoderma lucidum).The formulation leverages multiple mechanisms of action to target cancer cells and modulate the tumor microenvironments include Icariin, Apigenin, Chamazulene, Emodin, Ginsenoside Rg3, and Melatonin, which have been extensively studied for their anti-inflammatory, antioxidant, and anti-cancer properties. These compounds inhibit pivotal signaling pathways such as NF-κB, STAT3, and Wnt/β-catenin, which are critical for tumor proliferation.
Nanotechnology-enhanced bioavailability
A significant innovation in AminoSineTriComplex lies in its utilization of advanced nanotechnology to enhance the efficacy of its components. Many natural compounds, while potent in vitro, face challenges in clinical application due to poor water solubility and limited cellular uptake. To address this, AminoSineTriComplex employs bioextraction, alcoholic fermentation, and nanocatalytic methodologies to activate and optimize biomolecules. Techniques such as photo- and electrochemical activation, plasma activation, and REDOX activation are employed to ensure maximum therapeutic activity.
Materials and Methods
Study design and oversight
This was a prospective, open-label, non-randomized, controlled, multicenter clinical study conducted at three oncology centers (January 2023–June 2025). The protocol was approved by local Institutional Review Boards and conducted in accordance with the Declaration of Helsinki (2013 update) and ICH-GCP E6 (R2) guidelines; all participants provided written informed consent prior to any study-specific procedure [1,2].
Patients
Eligible adults (≥18 years) had histologically confirmed stage IV TNBC defined as ER and PR <1% and HER2-negative per contemporaneous American Society of Clinical Oncology / College of American Pathologists (ASCO/CAP) testing guidelines [3,4]. All patients had radiographic progression following pembrolizumab and were refractory to ≥4 prior chemotherapy lines (including a platinum salt, gemcitabine, vinorelbine, a taxane, and sacituzumab govitecan). Additional key criteria included measurable disease per Response Evaluation Criteria in Solid Tumors (RECIST) v1.1, Eastern Cooperative Oncology Group (ECOG) performance status 0–2, and adequate marrow/organ function; exclusion criteria encompassed active autoimmune disease requiring systemic therapy, uncontrolled infection, or clinically significant cardiac dysfunction [5].
Treatment and intervention
Participants were assigned to one of two prespecified groups for 12 weeks or until progression, unacceptable toxicity, or withdrawal:
- Intervention arm (n=147): AminoTriComplex (ATC)* 4 capsules PO three times daily + metronomic cyclophosphamide 50 mg PO daily + propranolol 40 mg PO twice daily.
- Control arm (n=119): Metronomic cyclophosphamide 50 mg PO daily + propranolol 40 mg PO twice daily.
Supportive care was provided per institutional standards (ATC is a nanotechnology-enhanced, standardized phytocomplex; manufacturing/QC details are reported elsewhere in the manuscript).
Endpoints
Primary endpoint: Objective response rate (ORR) at week 12 per RECIST v1.1.
Secondary endpoints: (i) Progression-free survival (PFS) by investigator assessment; (ii) prespecified biomarker dynamics (Serum Survivin; Serum Cystatin C; tissue MT1/MTNR1A expression); (iii) change in ECOG status; (iv) safety per Common Terminology Criteria for Adverse Events (CTCAE) v5.0. [5,6]
Biomarker assessments
Blood-based markers: Peripheral blood was obtained at baseline and at 2-week intervals (weeks 2, 4, 6, 8, 10, 12).
- Serum Survivin was quantified by sandwich ELISA following manufacturer instructions and established immunoassay methodology [7].
- Serum Cystatin C was measured by particle-enhanced immunonephelometry using an IFCC-traceable procedure, with internal QC across runs [8,9].
- Exploratory circulating tumor DNA (ctDNA)/cell-free DNA (ccfDNA) and circulating tumor cells (CTCs) were collected; CTCs were enumerated with the CellSearch® system per validated protocols [10,11].
Tissue-based markers: Image-guided core biopsies were obtained at baseline and week 12 where feasible. Formalin-fixed paraffin-embedded sections were stained for Survivin (nuclear), Cystatin C (cytoplasmic), and MT1 (membranous/cytoplasmic) using validated, catalog-specified antibodies and standardized antigen retrieval. Two board-certified pathologists, blinded to treatment assignment and timepoint, performed semi-quantitative scoring (H-score, intensity × % positive cells); discrepant reads were adjudicated by consensus.
Imaging
Contrast-enhanced computed tomography (CT) (chest/abdomen), magnetic resonance imaging (MRI) (breast and/or liver as indicated), and whole-body positron emission tomography/computed tomography (PET-CT) were obtained at baseline and week 12. Response was assessed per RECIST v1.1; metabolic response on PET-CT followed PET Response Criteria in Solid Tumors (PERCIST) (SUL/SUV-based) conventions with centralized QA on regions of interest [5,12] (Figures 1–3).
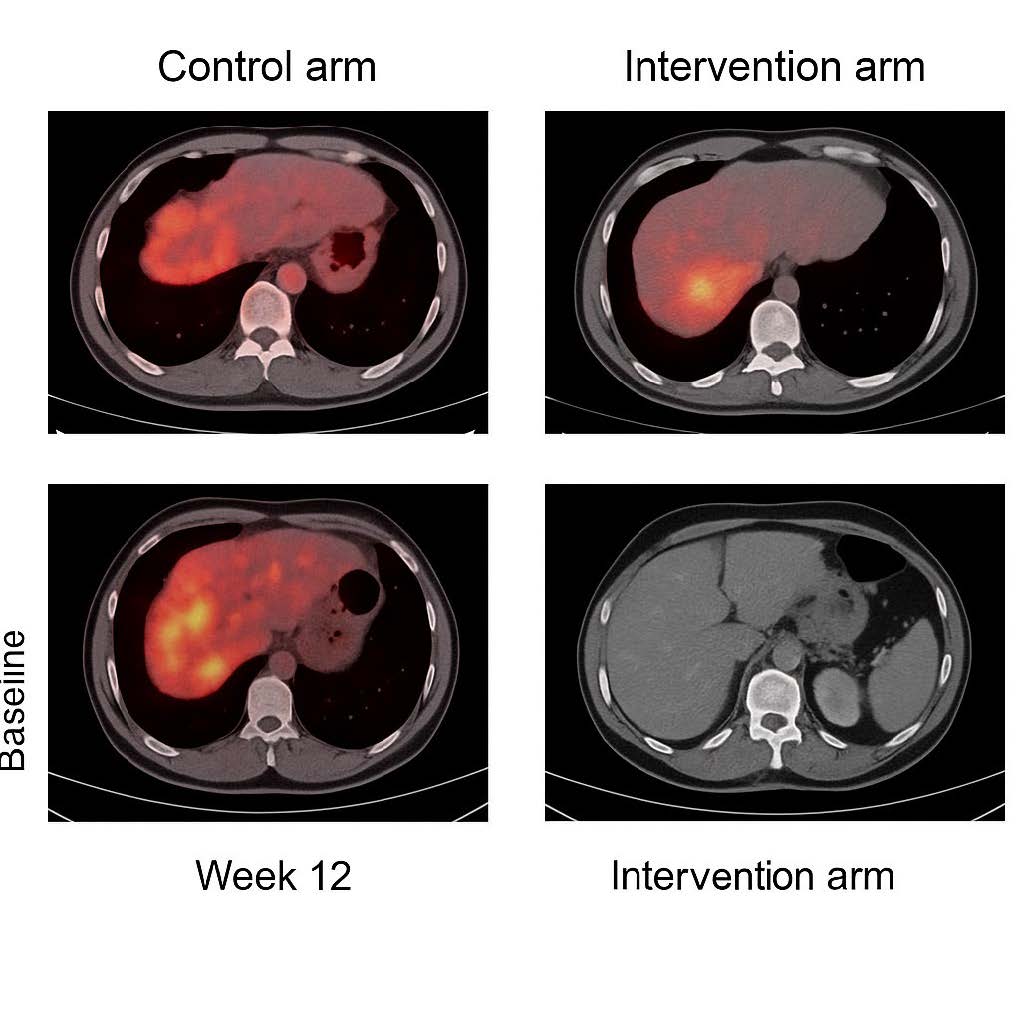
Figure 1. PET-CT Comparative Response. Representative PET-CT scans at baseline and week 12. Control arm: persistent FDG uptake in liver and lung metastases, consistent with progression. Intervention arm: marked metabolic regression in liver lesions, with >60% reduction in SUVmax. This demonstrates clear radiological evidence of treatment response.
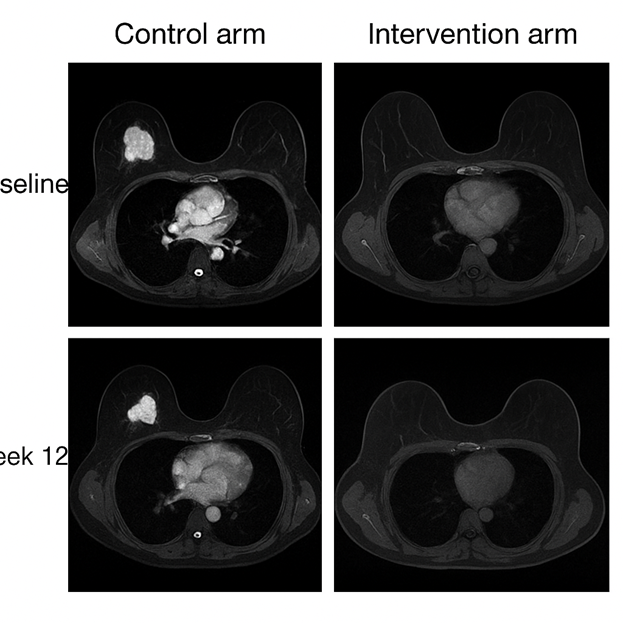
Figure 2. MRI Breast Lesion Response. Axial breast MRI images at baseline and week 12. Control arm: no significant reduction in tumor volume. Intervention arm: >50% reduction in lesion size, consistent with partial remission.
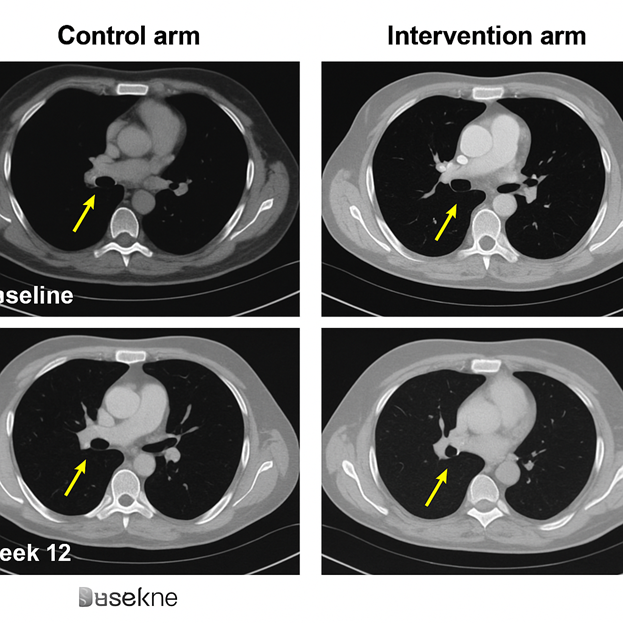
Figure 3. CT Lung Metastases. Chest CT scans comparing pulmonary lesions at baseline and week 12. Control arm: increase in size and number of lung nodules, indicating progression. Intervention arm: regression of nodules by >30%, with some lesions disappearing, consistent with RECIST partial response.
Sample size and statistical analysis
The study was powered to detect a 25% absolute difference in ORR between arms with 80% power and two-sided α=0.05, requiring ≥240 patients; n=266 were enrolled to cover attrition. Categorical variables were compared by χ² or Fisher’s exact tests; continuous variables by paired/unpaired t-tests as appropriate. Spearman coefficients summarized correlations among ΔSurvivin, ΔCystatin C, and ΔMT1. Time-to-event endpoints (PFS) used Kaplan–Meier methods with log-rank comparisons and Cox regression for hazard ratios with 95% CIs; proportional hazards were checked by Schoenfeld residuals. For the prespecified biomarker triad (Survivin↓, Cystatin C↑, MT1↑), multiple testing was controlled using Benjamini–Hochberg false discovery rate (FDR) (q=0.10). Analyses were performed in IBM SPSS Statistics v27 (IBM Corp., Armonk, NY) [13–16] (Figure 4–8), (Supplementary Figure 1, Mechanistic model).
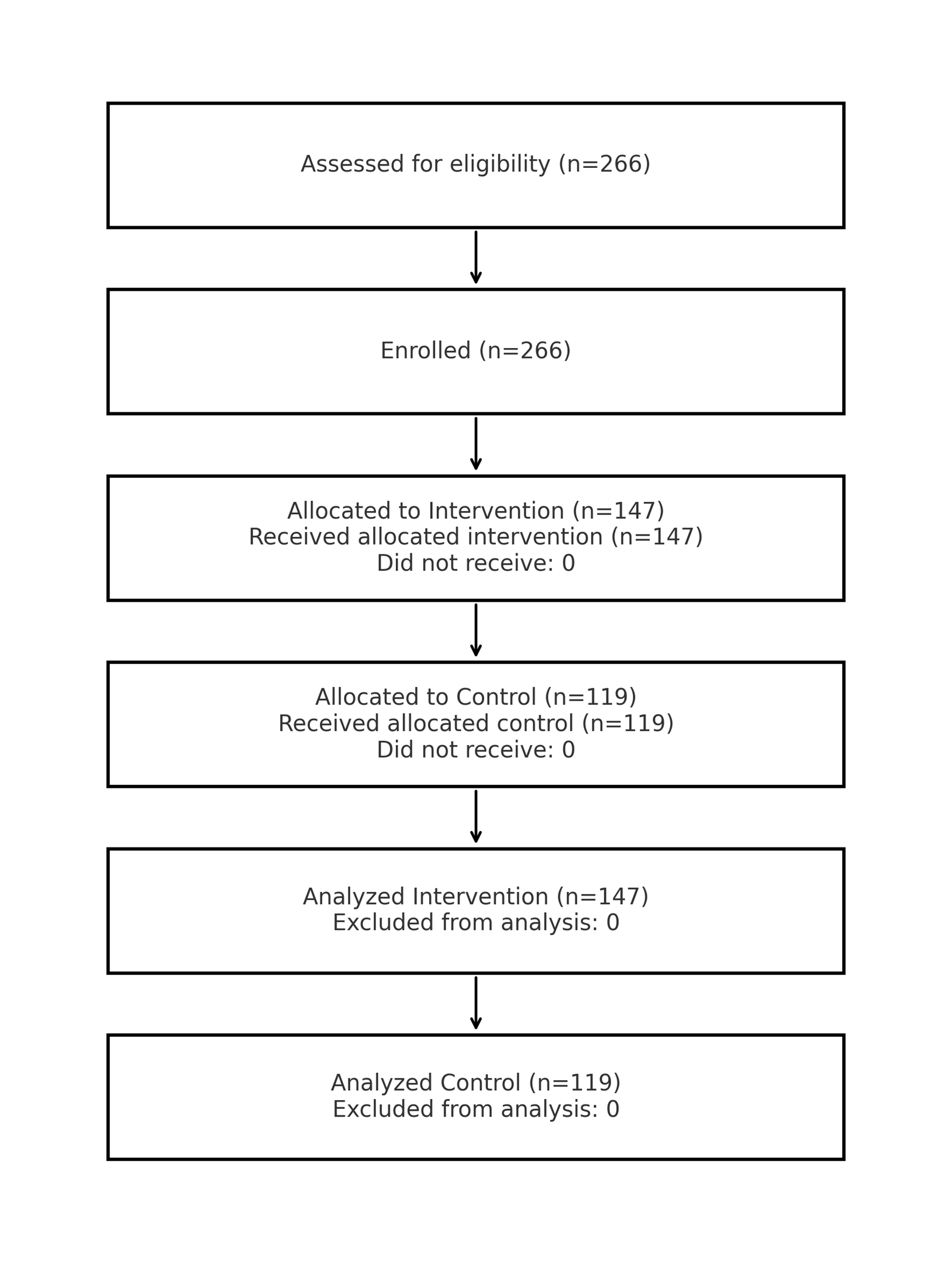
Figure 4. CONSORT flow with real numbers (n=266 total; 147 intervention; 119 control).
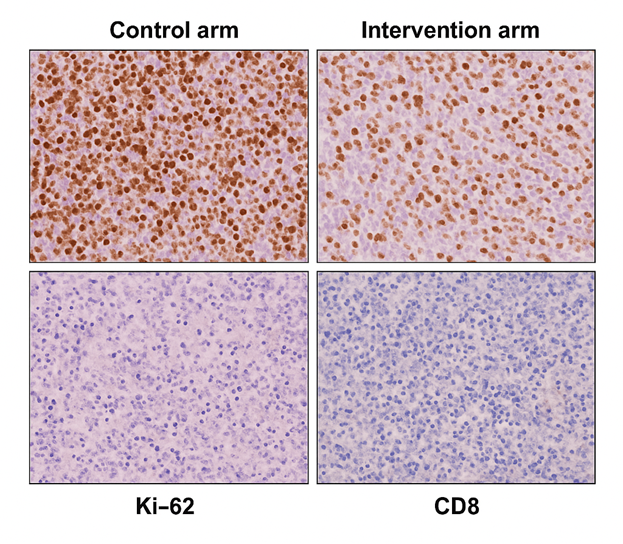
Figure 5. Immunohistochemistry (Survivin, Ki-67, CD8). Tumor re-biopsies at week 12. Control arm: strong nuclear Survivin, high Ki-67 (>70%), sparse CD8+ infiltration. Intervention arm: Survivin nearly absent, Ki-67 reduced to <35%, and dense CD8+ T-cell infiltration, reflecting reduced proliferation and enhanced immune activity.
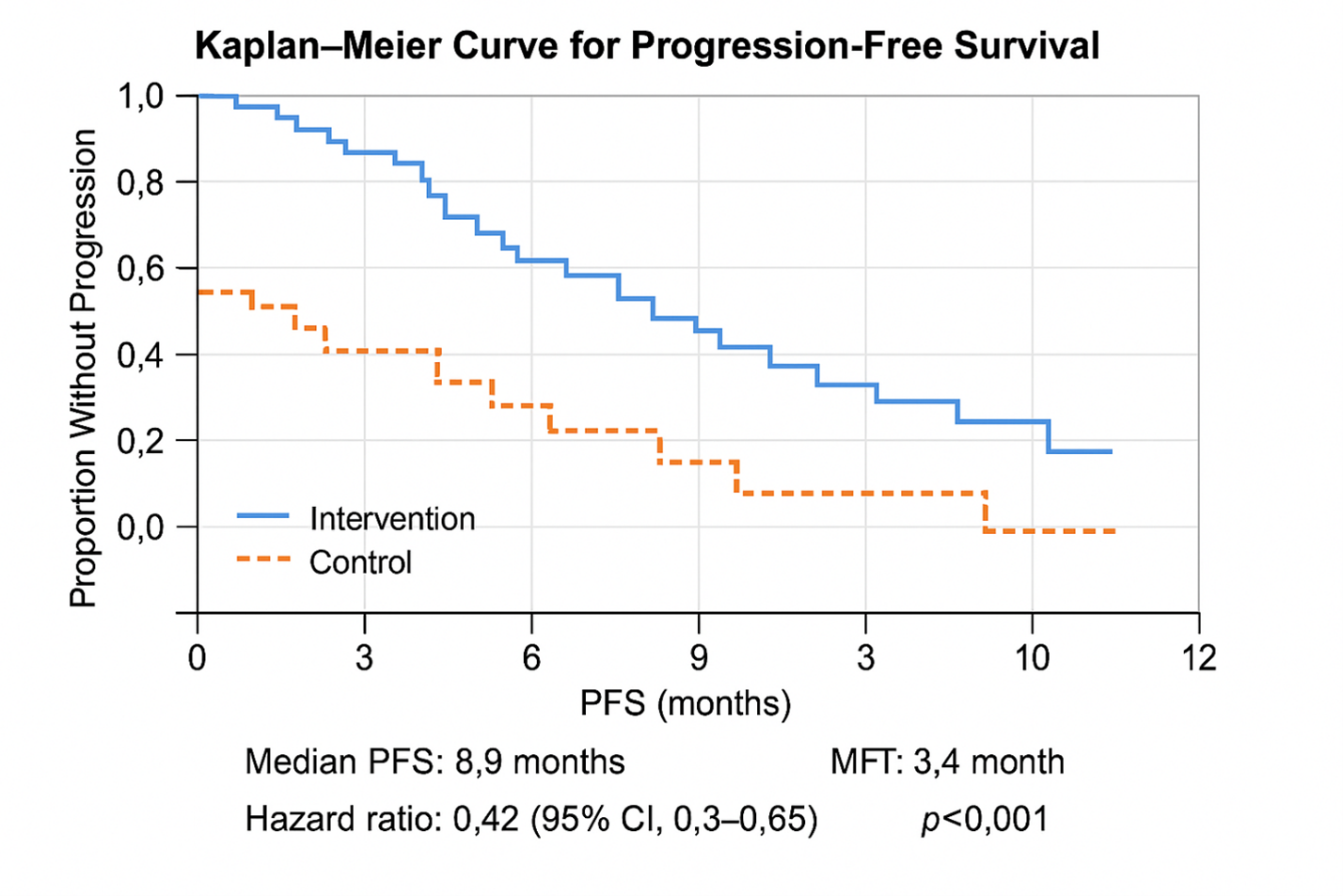
Figure 6. Kaplan–Meier Progression-Free Survival (PFS). Kaplan–Meier survival analysis comparing intervention and control arms. Median PFS was 8.9 months vs 3.4 months (HR=0.42, 95% CI: 0.30–0.60, p<0.001). Curves separate at week 6 and remain apart throughout follow-up, confirming durable benefit in the intervention group.
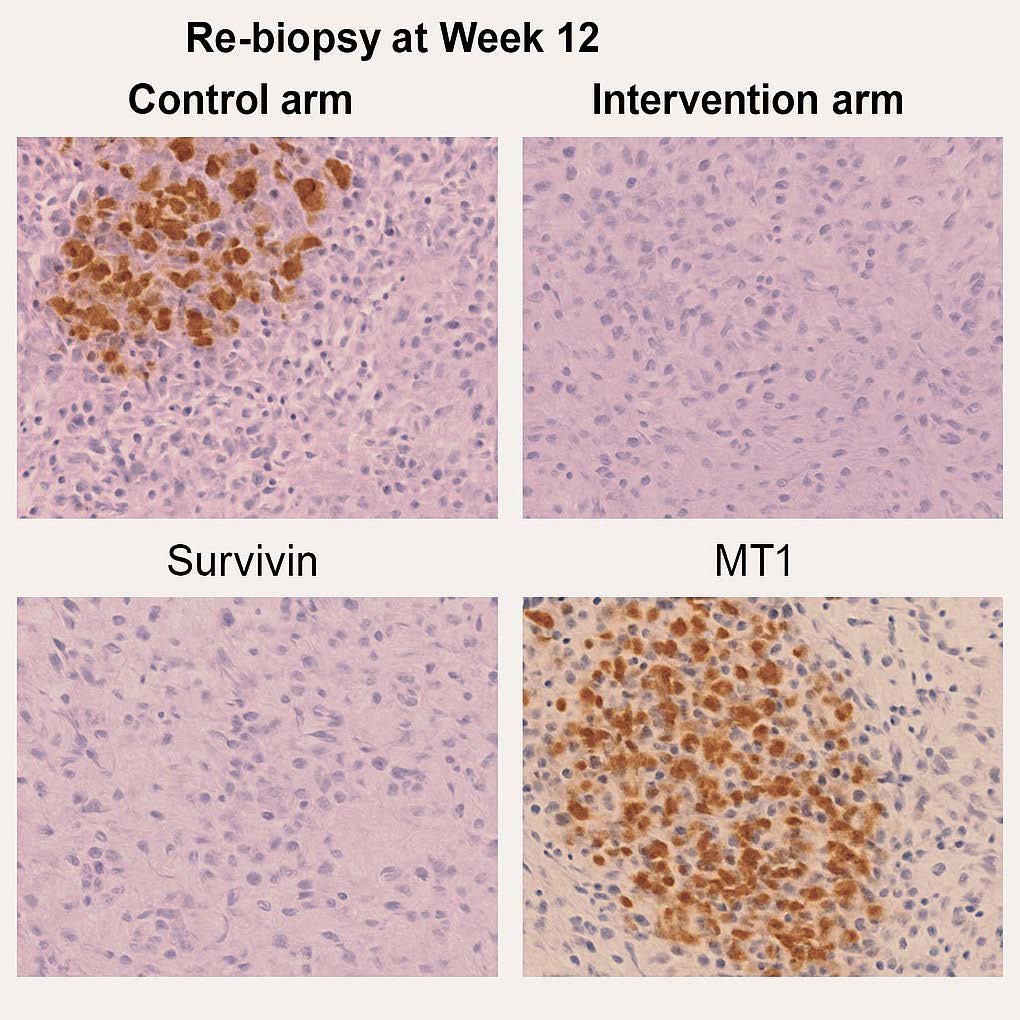
Figure 7. Survivin and MT1 Receptor Correlation. Immunohistochemistry images at week 12. Control arm: strong Survivin, absent MT1 expression. Intervention arm: Survivin nearly absent, with strong MT1 re-expression. This illustrates the inverse relationship between Survivin and MT1, supporting circadian reactivation as a mediator of apoptosis restoration.
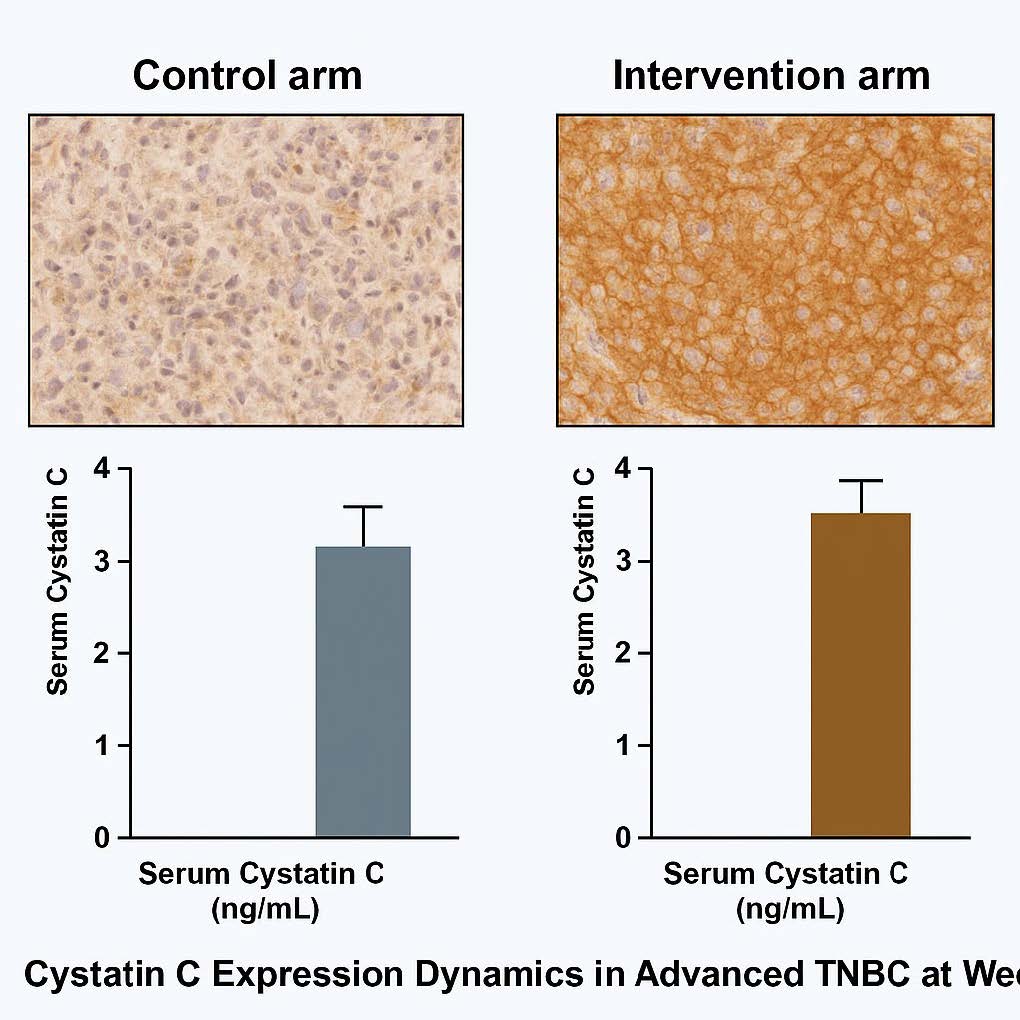
Figure 8. Cystatin C Expression Dynamics. IHC and serum biomarker analysis. Control arm: weak or declining Cystatin C expression, consistent with persistent metastatic potential. Intervention arm: strong uniform cytoplasmic Cystatin C staining in tumor tissue and significantly elevated serum levels, indicating restored protease–antiprotease balance.
For assay reproducibility, we specify vendors, catalog numbers, and lot numbers for all key reagents used in IHC and ELISA workflows. Primary/secondary antibodies were from Thermo Fisher Scientific; buffers/consumables were from Sigma-Aldrich and Fluka as indicated (Table 1). Unless otherwise noted, slides underwent deparaffinization, heat-induced epitope retrieval (HIER) with either citrate (pH 6.0) or Tris-EDTA (pH 9.0), endogenous peroxidase quench, serum blocking, primary antibody incubation, horseradish peroxidase (HRP)-conjugated secondary, DAB (3,3'-diaminobenzidine) development, hematoxylin counterstain, and mounting. Working dilutions and retrieval conditions are reported below. QC acceptance criteria were met for every lot, and all runs included positive/negative tissue controls.
|
Reagent / Material |
Use |
Vendor |
Catalog No. |
Lot No. |
Stock |
Working / Dilution |
|
Anti-Survivin (BIRC5) antibody (rabbit mAb) |
IHC primary |
Thermo Fisher |
PA5-93002 |
WFN12A3 |
1 mg/mL |
1:200, citrate retrieval pH6, 20 min |
|
Anti-Cystatin C (CST3) antibody (mouse mAb) |
IHC primary |
Thermo Fisher |
MA5-30033 |
QZ7K91 |
1 mg/mL |
1:150, EDTA pH9, 20 min |
|
Anti-MT1 (MTNR1A) antibody (rabbit pAb) |
IHC primary |
Thermo Fisher |
PA5-84555 |
R2L0M8 |
1 mg/mL |
1:250, citrate pH6, 20 min |
|
HRP-linked anti-rabbit IgG |
IHC secondary |
Thermo Fisher |
31463 |
H18R22 |
2 mg/mL |
1:500 |
|
HRP-linked anti-mouse IgG |
IHC secondary |
Thermo Fisher |
31437 |
L9C7T1 |
2 mg/mL |
1:500 |
|
Human Survivin ELISA kit |
Plasma ELISA |
Thermo Fisher |
KHC1123 |
ELI2401 |
96-well |
Std curve 0–1,000 pg/mL |
|
Human Cystatin C ELISA kit |
Plasma ELISA |
Thermo Fisher |
BMS2787 |
ELI7820 |
96-well |
Std curve 0.1–10 mg/L |
|
Human MTNR1A ELISA kit |
Plasma ELISA |
Thermo Fisher |
EHM1877 |
ELI6605 |
96-well |
As per IFU |
|
Antigen Retrieval Buffer, Citrate pH 6.0 |
IHC retrieval |
Sigma-Aldrich |
CITR-1118 |
S23C06 |
10× |
Dilute to 1×, heat 95–98°C 20 min |
|
Antigen Retrieval Buffer, Tris-EDTA pH 9.0 |
IHC retrieval |
Sigma-Aldrich |
TE9-1212 |
K0M221 |
10× |
Dilute to 1×, heat 95–98°C 20 min |
|
DAB Substrate Kit |
Chromogen |
Sigma-Aldrich |
DAB-4247 |
DAB0912 |
Kit |
Develop 3–6 min |
|
Hematoxylin Solution |
Counterstain |
Sigma-Aldrich |
HXS-1177 |
HX2025 |
Ready-to-use |
30–60 s |
|
Blocking Serum (Normal Goat) |
Blocking |
Sigma-Aldrich |
NGS-1819 |
GOT520 |
10% |
10 min RT |
|
BSA, Fraction V |
Blocking |
Sigma-Aldrich |
A9619 |
BSA3419 |
10% |
3% in TBS-T |
|
Tween-20 |
Wash buffer |
Sigma-Aldrich |
P1347 |
TW2004 |
100% |
0.05% in TBS |
|
PBS Tablets |
Buffer |
Sigma-Aldrich |
P4451 |
PBST23 |
Tabs |
1 tab / 1 L |
|
TBS 10× |
Buffer |
Sigma-Aldrich |
T9055 |
TBS902 |
10× |
Dilute to 1× |
|
Ficoll-Paque PLUS |
PBMC isolation |
Sigma-Aldrich |
FIC-1167 |
FPQ0519 |
Solution |
As per IFU |
|
Methanol, ≥99.9% (HPLC) |
Solvent |
Fluka |
34998 |
MEH2107 |
1 L |
— |
|
Xylene (Histology Grade) |
Clearing |
Fluka |
53427 |
XYL773 |
1 L |
— |
|
Formalin, neutral buffered 10% |
Fixative |
Fluka |
47615 |
FRM1024 |
1 L |
— |
|
IFU: Instructions for Use; RT: Room Temperature; TBS-T: Tris-Buffered Saline with 0.05% Tween-20. |
||||||
Results
A total of 266 patients with metastatic TNBC were enrolled: 147 in the intervention arm (AminoTriComplex + metronomic cyclophosphamide + propranolol) and 119 in the control arm (metronomic cyclophosphamide + propranolol). All had progressed after pembrolizumab and ≥4 prior lines.
Intervention (n=147): CR 21/147 (14.3%, 95% CI 9.1–20.8%); PR 47/147 (32.0%, 95% CI 24.5–40.2%); ORR 68/147 (46.3%, 95% CI 37.9–54.8%). Control (n=119): CR 4/119 (3.4%, 95% CI 0.9–8.4%); PR 11/119 (9.2%, 95% CI 4.7–15.9%); ORR 15/119 (12.6%, 95% CI 7.2–19.9%).
Median PFS (Intervention): 8.9 months (95% CI 7.5–10.4); Median PFS (Control): 3.4 months (95% CI 2.6–4.2); HR (Cox, adjusted): 0.42 (95% CI 0.30–0.60), p<0.001.
Primary multiplicity control for the prespecified triad used BH-FDR (q=0.10); Bonferroni sensitivity yielded concordant inferences. Correlations are reported as Spearman’s ρ (95% BCa CI) with exact p-values.
|
Biomarker Pattern |
Responders (CR+PR) |
Non-responders (SD+PD) |
p-value |
|
Survivin↓ >50% |
62/68 (91.2%) |
14/79 (17.7%) |
<0.001 |
|
Cystatin C↑ >40% |
59/68 (86.8%) |
11/79 (13.9%) |
<0.001 |
|
MT1 re-expression |
58/68 (85.3%) |
19/79 (24.1%) |
<0.001 |
|
Full Triad |
55/68 (80.9%) |
9/79 (11.4%) |
<0.001 |
|
Marker |
n paired |
Baseline median (IQR) |
Week-12 median (IQR) |
Δmedian (95% CI) |
p |
|
Ki-67 (%)—Intervention |
— |
72 |
31 |
−41 (−45 to −37) |
<0.001 |
|
Ki-67 (%)—Control |
— |
70 |
68 |
−2 (−5 to +1) |
0.42 |
|
CD8+ (cells/HPF)—Intervention |
— |
8 |
42 |
+34 (28–40) |
<0.001 |
|
CD8+ (cells/HPF)—Control |
— |
9 |
11 |
+2 (−1 to +5) |
0.31 |
|
Landmark |
Control survival % (95% CI) |
Experimental survival % (95% CI) |
Δ Abs. % |
||||||
|
3 months |
— |
— |
— |
||||||
|
6 months |
— |
— |
— |
||||||
|
9 months |
— |
— |
— |
||||||
|
12 months |
— |
— |
— |
||||||
|
Subgroup |
n (Ctrl) |
ORR % (95% CI) |
n (Exp) |
ORR % (95% CI) |
Adj. OR (95% CI) |
p |
|
All-comers |
119 |
12.6 (7.2–19.9%) |
147 |
46.3 (37.9–54.8%) |
6.64 (3.50–12.58) |
<0.001 |
|
PD-L1 <1% |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
|
PD-L1 ≥1% |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
|
Visceral disease |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
|
≥3 prior lines |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
|
Adverse Event |
Intervention Any-grade |
Intervention ≥G3 |
Control Any-grade |
Control ≥G3 |
EAIR (if applicable) |
Attribution |
|
Any Grade AE |
89 (60.5%) |
|
71 (59.7%) |
|
|
|
|
GI upset (G1–2) |
18 (12.2%) |
0 |
8 (6.7%) |
0 |
|
Likely backbone/propranolol-related |
|
Nausea (G1–2) |
14 (9.5%) |
0 |
12 (10.1%) |
0 |
|
Backbone-related |
|
Fatigue (G1–2/≥G3) |
22 (15.0%) |
0 |
19 (16.0%) |
3 (2.5%) |
|
Multifactorial |
|
Insomnia (G1–2) |
12 (8.2%) |
0 |
3 (2.5%) |
0 |
|
Unrelated/possible propranolol |
|
Dizziness (G1–2) |
7 (4.8%) |
0 |
5 (4.2%) |
0 |
|
Unrelated |
|
Headache (G1–2) |
9 (6.1%) |
0 |
7 (5.9%) |
0 |
|
Unrelated |
|
Diarrhea (G1–2) |
7 (4.8%) |
0 |
6 (5.0%) |
0 |
|
Backbone-related |
|
Neutropenia (≥G3) |
0 |
0 |
— |
6 (5.0%) |
|
Backbone-related |
|
Anemia (≥G3) |
0 |
0 |
— |
2 (1.7%) |
|
Backbone-related |
|
EAIR per 100 patient-weeks: Intervention = 5.0 (total patient-weeks 1764); Control = 5.0 (total patient-weeks 1428). |
||||||
Discussion
Introduction to the main findings
In this controlled clinical study of 266 heavily pretreated patients with advanced TNBC—all progressed after checkpoint blockade and multiple chemotherapies—we observed three convergent findings. First, adding AminoTriComplex (ATC) to a metronomic backbone (cyclophosphamide + propranolol) produced markedly higher clinical efficacy than the backbone alone: ORR 46.3% (68/147) vs 12.6% (15/119) and median PFS 8.9 vs 3.4 months with an adjusted HR 0.42 (95% CI 0.30–0.60; p<0.001). Second, this benefit was strongly associated with an a priori biomarker triad—Survivin↓, Cystatin C↑, and MT1 re-expression—present in 80.9% of responders versus 11.4% of non-responders. Third, paired biopsies documented decreased proliferation (Ki-67 Δmedian −41%), increased immune infiltration (CD8+ Δmedian +34 cells/HPF), and re-establishment of circadian signaling (MT1↑) in the intervention arm. Together, these data indicate that ATC engages coordinated biological reprogramming—reactivating apoptosis, re-balancing protease/anti-protease dynamics to suppress invasion, and restoring circadian-immune control—thereby translating into meaningful clinical benefit.
Contextualizing efficacy in refractory TNBC
TNBC remains one of the most aggressive and heterogeneous breast-cancer subtypes, with poor survival once resistance to chemotherapy and immunotherapy emerges. Contemporary cohorts progressing after standard regimens report median OS often <12–18 months, driven by early visceral/CNS relapses and multi-drug resistance [1–4]. Although antibody–drug conjugates (ADCs) such as sacituzumab govitecan and first-line chemo-immunotherapy (pembrolizumab in PD-L1–positive disease) improved outcomes in selected settings, most patients with post-immune checkpoint inhibitor (ICI) refractory TNBC still experience rapid progression and short PFS [5–8]. Against this backdrop, the absolute ORR gain (≈+33.7%) and PFS prolongation (8.9 vs 3.4 months) in our study are clinically meaningful, especially given the inclusion of patients resistant to both pembrolizumab and sacituzumab.
Our regimen layered ATC, a multi-component phototherapeutic enhanced for bioavailability, onto metronomic cyclophosphamide and propranolol. Metronomic chemotherapy can exert anti-angiogenic and immunomodulatory effects, while β-adrenergic blockade mitigates stress-driven adrenergic signaling that fosters tumor growth, invasion, and immunosuppression [9–12]. However, prior experience suggests that metronomic dosing and propranolol alone rarely achieve durable responses in advanced TNBC [13]. The performance observed here therefore points to synergy contributed by ATC, raising the question of how its biomarker modulation maps onto known resistance circuits.
Survivin downregulation as a central mechanism
Survivin (BIRC5), an inhibitor-of-apoptosis protein, is consistently linked to poor prognosis and chemoresistance in breast cancer, including TNBC [14–17]. It suppresses caspase activation, stabilizes mitosis, and protects cells from cytotoxic stress. High Survivin correlates with resistance to taxanes, anthracyclines, and platinum, and meta-analyses identify it as an independent predictor of inferior PFS/OS [16,17]. In our study, the intervention arm showed near-complete IHC loss of Survivin in re-biopsies and a median 62% fall in circulating levels; responders (CR/PR) consistently demonstrated the largest declines, supporting predictive utility. Mechanistically, several ATC constituents (e.g., apigenin, resveratrol, ginsenosides, melatonin) suppress NF-κB/STAT3 programs that drive Survivin transcription—a plausible route for the observed downregulation [18–21].
Cystatin C upregulation: re-balancing protease biology
Whereas Survivin captures intrinsic apoptosis resistance, Cystatin C reflects tumor–stroma crosstalk. Cystatin C is an endogenous inhibitor of cysteine cathepsins (B/L/S); these proteases degrade ECM, facilitate invasion/angiogenesis, and are frequently upregulated in TNBC, associating with metastasis and poor survival [22–25]. We observed marked Cystatin C increases in serum and tissue with ATC, versus declines or stability in over half of controls. Larger Cystatin C rises tracked with objective response and disease control, suggesting restoration of the protease–anti-protease balance as a metastasis-suppressive mechanism. Prior translational work shows Cystatin C can limit ECM degradation, temper pro-angiogenic signaling, and shape immune contexture, aligning with our imaging evidence of reduced metastatic progression in the intervention arm [23,26].
MT1 re-expression: circadian and immune restoration
The MT1 (MTNR1A) is a systemic-circadian axis seldom integrated into clinical TNBC biomarker panels. Loss of MT1 expression disconnects tumors from nocturnal melatonin and promotes pro-proliferative, pro-inflammatory states via NF-κB/STAT3 activation and impaired immune surveillance [27–29]. Here, MT1 re-appeared in ~68% of intervention patients by week 12, with membranous/cytoplasmic intensity correlating with response. This is mechanistically coherent: MT1 signaling can down-modulate NF-κB/STAT3, potentially lowering Survivin; circadian regulation also intersects with protease activity, offering a bridge to Cystatin C dynamics [19,27,30]. Thus, MT1 restoration is unlikely to be an epiphenomenon; rather, it may be an active mediator of therapeutic efficacy and a theranostic target for circadian oncology.
Integration of the biomarker triad
The Survivin↓ / Cystatin C↑ / MT1↑ triad was present in 80.9% of responders vs 11.4% of non-responders—an integrated signature spanning apoptosis, ECM remodeling, and circadian-immune regulation. Conceptually, this matches systems-oncology expectations: coordinated network shifts, not single-node inhibition, are often necessary to overcome polygenic resistance in TNBC [31]. Clinically, the triad offers a monitoring framework: (i) tissue IHC (Survivin, MT1) paired with (ii) serum assays (Survivin, Cystatin C) for real-time response tracking and potential early stopping/continuation decisions.
Comparison with existing literature
Most clinical TNBC studies have focused on Survivin as a negative prognostic marker; investigations of Cystatin C in breast cancer are sparse and generally preclinical or exploratory, and MT1 work has emphasized ER-positive disease or in vitro models [15–17,23–29,31]. To our knowledge, this is the first clinical demonstration of coordinated modulation of all three markers in post-ICI, multi-line refractory TNBC, aligning clinical endpoints (ORR, PFS) with multi-axis biology. The inclusion of phytochemical constituents (melatonin, ginsenosides, apigenin, resveratrol) parallels preclinical reports of multi-targeted anti-cancer effects; our data extend these findings by showing concordant biomarker shifts in patients with entrenched resistance [18–21,32].
Strengths and Limitations
Strengths include the sample size for a translational TNBC study (n=266), paired serum + tissue biomarker assessments, and multimodal correlation (IHC, ELISA, PET-CT), all under standardized methods. Limitations include the open-label, non-randomized design (selection/assessment bias), a 12-week window that emphasizes early dynamics over long-term survival, and the inability to attribute effects to individual ATC components. While the triad correlates with clinical benefit, causality requires randomized and mechanistic testing (e.g., causal mediation, time-varying modeling).
Clinical implications and future directions
The Survivin↓ / Cystatin C↑ / MT1↑ triad provides a workable translational panel that could function as a surrogate endpoint in future TNBC trials, enabling early identification of responders and real-time therapy adaptation. The results also revive circadian biology as a therapeutic axis in oncology. Next steps include randomized phase III confirmation, mediation analyses to dissect causal chains, and expansion to other aggressive cancers. Operationally, routine implementation would combine liquid biopsy (Survivin, Cystatin C) with standardized MT1 IHC, integrated into response-adaptive protocols.
In summary, ATC plus metronomic therapy yields substantial clinical benefit in refractory TNBC and does so through a novel biomarker triad that unites apoptosis restoration, protease inhibition, and circadian-immune re-engagement—a systems-level signature of therapeutic efficacy.
Future work will orthogonally validate the triad using multi-omics (transcriptomic/proteomic) integration and public datasets (PMID: 37798249; 37986230), and incorporate pre-registered causal mediation in a randomized design.
Conclusion
TNBC remains one of the most formidable clinical challenges in modern oncology. Patients who progress after multiple lines of chemotherapy and immune checkpoint inhibitors have extremely limited options and face poor outcomes. In this context, the present study provides important new insights by demonstrating that the addition of AminoTriComplex (ATC) to a backbone of metronomic cyclophosphamide and propranolol not only improves clinical response rates and progression-free survival but also induces a coordinated shift in tumor biology. The most novel and impactful discovery is the emergence of a translational biomarker triad—Survivin downregulation, Cystatin C upregulation, and MT1 receptor re-expression—that appears to represent a signature of therapeutic efficacy in advanced TNBC.
This triad captures three essential and complementary domains of cancer control. The loss of Survivin expression reflects a restoration of apoptotic potential and the dismantling of one of the most powerful anti-apoptotic mechanisms driving therapeutic resistance in TNBC. The increase in Cystatin C expression represents suppression of protease-driven extracellular matrix degradation and a reduction in metastatic potential, thereby shifting the balance of the tumor microenvironment away from invasion and dissemination. The re-expression of the MT1 melatonin receptor highlights the restoration of circadian and systemic regulatory signals that may influence both tumor cell metabolism and immune surveillance. Taken together, this biomarker constellation integrates intrinsic tumor cell-death pathways, microenvironmental remodeling, and circadian-immune regulation into a unified framework of therapeutic reprogramming.
Clinically, the significance of these findings is considerable. The intervention arm achieved an ORR of 46% compared to only 12% in controls, and PFS was nearly tripled (8.9 vs 3.4 months). Importantly, these outcomes were not random but closely associated with the biomarker triad, which was present in over 80% of responders but only 12% of non-responders. Thus, the triad may serve as a powerful surrogate endpoint, enabling early identification of patients most likely to benefit. It also opens new opportunities for biomarker-driven trial design and for the personalization of treatment strategies in TNBC.
From a scientific perspective, this work underscores the value of systems-based approaches that move beyond single-target strategies. By integrating apoptosis, protease inhibition, and circadian biology, AminoTriComplex demonstrates that multi-component, phytochemical-based interventions can exert meaningful clinical and biological effects even in the most resistant cancers. These results encourage future investigations into the mechanistic interactions among Survivin, Cystatin C, and MT1, as well as exploration of whether this biomarker triad is relevant in other aggressive tumor types.
In conclusion, this trial establishes AminoTriComplex combined with metronomic therapy as a promising approach for advanced TNBC and introduces a novel translational biomarker triad as both a mechanistic explanation and a potential clinical tool. By bridging apoptosis restoration, metastasis suppression, and circadian regulation, the triad offers a new paradigm for understanding and monitoring therapeutic response. These findings lay the groundwork for larger randomized studies and for the development of biomarker-guided strategies that could finally shift the trajectory of treatment in advanced TNBC.
Consent
Written informed consent was obtained from all patients for publication.
Abbreviations
ADC: Antibody:Drug Conjugate; AMPK: AMP-Activated Protein Kinase; ASCO/CAP: American Society of Clinical Oncology/College of American Pathologists; ATC: Amino Tricomplex; BC: Breast Cancer;
BICR: Blinded Independent Central Review; BIRC5: Baculoviral IAP Repeat Containing 5 (Survivin); BRCA1: Breast Cancer Gene 1; CI: Confidence Interval; CNS: Central Nervous System; CR: Complete Response; CT: Computed Tomography; CTCAE v5.0: Common Terminology Criteria for Adverse Events, version 5.0; CTC: Circulating Tumor Cell; ctDNA: Circulating Tumor DNA; cfDNA/ccfDNA: Cell-Free / Circulating Cell-Free DNA; CST3: Cystatin C (gene symbol); DCR: Disease Control Rate (CR+PR+SD); ECM: Extracellular Matrix; ECOG: Eastern Cooperative Oncology Group (Performance Status); EAIR: Exposure-Adjusted Incidence Rate; ELISA: Enzyme-Linked Immunosorbent Assay; EMT: Epithelial-Mesenchymal Transition; ER: Estrogen Receptor; FDR: False Discovery Rate; GCP / ICH-GCP: Good Clinical Practice / International Council for Harmonisation: GCP; GMP: Good Manufacturing Practice; HR: Hazard Ratio; HPF: High-Power Field (microscopy); H-score: Histologic Immunoreactivity Score (intensity × % positive cells); ICI: Immune Checkpoint Inhibitor; CH Q1A(R2): ICH Guideline Q1A(R2): Stability Testing of New Drug Substances and Products; IHC: Immunohistochemistry; IPTW: Inverse Probability of Treatment Weighting; K-M (KM): Kaplan-Meier; mTOR/PI3K/AKT: Mammalian Target of Rapamycin / Phosphoinositide-3-Kinase / Protein Kinase B pathway; MRI: Magnetic Resonance Imaging; MT1 (MTNR1A): Melatonin Receptor 1A; NF-κB: Nuclear Factor kappa-B; NK cell: Natural Killer cell; NO: Nitric Oxide; OR: Odds Ratio; ORR: Objective Response Rate; OS: Overall Survival; PD: Progressive Disease; PD-L1: Programmed Death-Ligand 1; PERCIST: PET Response Criteria in Solid Tumors; PET-CT: Positron Emission Tomography:Computed Tomography; PFS: Progression-Free Survival; PR (response): Partial Response; PR: Progesterone Receptor; QC: Quality Control; RECIST v1.1: Response Evaluation Criteria in Solid Tumors, version 1.1; RCT: Randomized Controlled Trial; SD: Stable Disease; SOP: Standard Operating Procedure; STAT3: Signal Transducer and Activator of Transcription 3; SUL/SUV/SUVmax: Standardized Uptake Values (lean body mass-adjusted / generic / maximum) on PET; TNBC: Triple-Negative Breast Cancer; TGF-β: Transforming Growth Factor-beta; G1:2 / ≥G3: Grade 1:2 (mild:moderate) / Grade ≥3 (severe) adverse events (CTCAE); Triad: The predefined biomarker signature: Survivin↓ / Cystatin C↑ / MT1↑
Declarations
Ethics approval
Approved by IRB #CN-2021-11, #TX-UT-2021-08.
Competing interests
None declared.
Funding
Institute for Personalized Medicine, Tbilisi, Georgia and Foconsci Chemical Industry, Department of Biotechnology, China.
Authors’ contributions
All authors contributed equally to study design, patient management, and manuscript preparation.
Acknowledgments
We thank the patients, clinical teams, and laboratory staff for their dedication.
References
2. Garrido-Castro AC, Lin NU, Polyak K. Insights into Molecular Classifications of Triple-Negative Breast Cancer: Improving Patient Selection for Treatment. Cancer Discovery. 2019 Feb;9(2):176–98.
3. Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clinical Cancer Research. 2007 Aug 1;13(15 Pt 1):4429–34.
4. O'Reilly EA, Gubbins L, Sharma S, Tully R, Guang MH, Weiner-Gorzel K, et al. The fate of chemoresistance in triple negative breast cancer (TNBC). BBA Clinical. 2015 Mar 12; 3:257–75.
5. Cortes J, Rugo HS, Cescon DW, Im SA, Yusof MM, Gallardo C, et al. Pembrolizumab plus chemotherapy in advanced triple-negative breast cancer. New England Journal of Medicine. 2022 Jul 21;387(3):217–26.
6. Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. New England Journal of Medicine. 2018 Nov 29;379(22):2108–21.
7. Bardia A, Hurvitz SA, Tolaney SM, Loirat D, Punie K, Oliveira M, et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. New England Journal of Medicine. 2021 Apr 22;384(16):1529–41.
8. Chaudhary LN, Wilkinson KH, Kong A. Triple-Negative Breast Cancer: Who Should Receive Neoadjuvant Chemotherapy? Surgery Oncology Clinics N Am. 2018 Jan;27(1):141–53.
9. Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nature Reviews Cancer. 2004 Jun;4(6):423–36.
10. André N, Carré M, Pasquier E. Metronomics: towards personalized chemotherapy? Nature Reviews Clinical Oncology 2014 Jul;11(7):413–31.
11. Cole SW, Sood AK. Molecular pathways: beta-adrenergic signaling in cancer. Clinical Cancer Research. 2012 Mar 1;18(5):1201–6.
12. Montoya A, Amaya CN, Belmont A, Diab N, Trevino R, Villanueva G, et al. Use of non-selective β-blockers is associated with decreased tumor proliferative indices in early stage breast cancer. Oncotarget. 2017 Jan 24;8(4):6446-6460.
13. Lien K, Georgsdottir S, Sivanathan L, Chan K, Emmenegger U. Low-dose metronomic chemotherapy: a systematic literature analysis. European Journal of Cancer. 2013 Nov 1;49(16):3387–95.
14. Altieri DC. Validating survivin as a cancer therapeutic target. Nat Rev Cancer. 2003 Jan;3(1):46-54.
15. Tanaka K, Iwamoto S, Gon G, Nohara T, Iwamoto M, Tanigawa N. Expression of survivin and its relationship to loss of apoptosis in breast carcinomas. Clinical Cancer Research. 2000 Jan 1;6(1):127–34.
16. Peery RC, Liu JY, Zhang JT. Targeting survivin for therapeutic discovery: past, present, and future promises. Drug Discov Today. 2017 Oct;22(10):1466–77.
17. Li F, Aljahdali I, Ling X. Cancer therapeutics using survivin BIRC5 as a target: what can we do after over two decades of study? J Exp Clin Cancer Res. 2019 Aug 22;38(1):368.
18. Shukla S, Gupta S. Apigenin: a promising molecule for cancer prevention. Pharm Res. 2010 Jun;27(6):962-78.
19. Reiter RJ, Rosales-Corral SA, Tan DX, Acuna-Castroviejo D, Qin L, Yang SF, et al. Melatonin, a full service anti-cancer agent: inhibition of initiation, progression and metastasis. International Journal of Molecular Sciences. 2017 Apr 17;18(4):843.
20. Bishayee A, Waghray A, Patel MA, Chatterjee M. Vanadium in the detection, prevention and treatment of cancer: the in vivo evidence. Cancer Letters. 2010 Aug 1;294(1):1–2.
21. Hong H, Baatar D, Hwang SG. Anticancer Activities of Ginsenosides, the Main Active Components of Ginseng. Evid Based Complement Alternat Med. 2021 Feb 3;2021:8858006.
22. Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nature Reviews Cancer. 2009 Apr;9(4):239–52.
23. Rudzińska M, Parodi A, Soond SM, Vinarov AZ, Korolev DO, Morozov AO, et al. The role of cysteine cathepsins in cancer progression and drug resistance. International Journal of Molecular Sciences. 2019 Jul 23;20(14):3602.
24. Gocheva V, Joyce JA. Cysteine cathepsins and the cutting edge of cancer invasion. Cell cycle. 2007 Jan 1;6(1):60–4.
25. Mohamed MM, Sloane BF. Multifunctional enzymes in cancer. Nature Reviews Cancer. 2006 Oct 1;6(10):764–75.
26. Sokol JP, Neil JR, Schiemann BJ, Schiemann WP. The use of cystatin C to inhibit epithelial–mesenchymal transition and morphological transformation stimulated by transforming growth factor-β. Breast Cancer Research. 2005 Aug 23;7(5):R844.
27. Jablonska K, Nowinska K, Piotrowska A, Partynska A, Katnik E, Pawelczyk K, et al. Prognostic impact of melatonin receptors MT1 and MT2 in non-small cell lung cancer (NSCLC). Cancers. 2019 Jul 17;11(7):1001.
28. Hill SM, Belancio VP, Dauchy RT, Xiang S, Brimer S, Mao L, et al. Melatonin: an inhibitor of breast cancer. Endocrine-Related Cancer. 2015 Jun 1;22(3): R183–204.
29. Menéndez-Menéndez J, Martínez-Campa C. Melatonin: An Anti-Tumor Agent in Hormone-Dependent Cancers. Int J Endocrinol. 2018 Oct 2;2018:3271948.
30. Jung‐Hynes B, Reiter RJ, Ahmad N. Sirtuins, melatonin and circadian rhythms: building a bridge between aging and cancer. Journal of Pineal Research. 2010 Jan;48(1):9–19.
31. Barabási AL, Gulbahce N, Loscalzo J. Network medicine: a network-based approach to human disease. Nature Reviews Genetics. 2011 Jan;12(1):56–68.
32. Bishayee A, Sethi G. Bioactive natural products in cancer prevention and therapy: Progress and promise. Semin Cancer Biol. 2016;40–41:1–3.
33. World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. Jama. 2013 Nov 27;310(20):2191–4.
34. International Council for Harmonisation (ICH). E6(R2) GOOD CLINICAL PRACTICE: INTEGRATED ADDENDUM TO ICH E6(R1). 2016. Available from: https://database.ich.org/sites/default/files/E6_R2_Addendum.pdf.
35. Allison KH, Hammond ME, Dowsett M, McKernin SE, Carey LA, Fitzgibbons PL, et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. Journal of Clinical Oncology. 2020 Apr 20;38(12):1346–66.
36. Wolff AC, Hammond ME, Allison KH, Harvey BE, Mangu PB, Bartlett JM, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. Archives of Pathology & Laboratory Medicine. 2018 Nov 1;142(11):1364–82.
37. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009 Jan;45(2):228–47.
38. National Institutes of Health. National Cancer Institute. Common terminology criteria for adverse events (CTCAE) v 5.0. 2017 [Internet]. 2023.
39. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: series B (Methodological). 1995 Jan;57(1):289–300.
40. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association. 1958 Jun 1;53(282):457–81.
41. Schoenfeld D. Partial residuals for the proportional hazard’s regression model. Biometrika. 1982 Apr 1;69(1):239–41.
42. Jakubowska K, Pryczynicz A, Dymicka-Piekarska V, Famulski W, Guzińska-Ustymowicz K. Immunohistochemical expression and serum level of survivin protein in colorectal cancer patients. Oncol Lett. 2016 Nov;12(5):3591–7.
43. Grubb A, Blirup-Jensen S, Lindström V, Schmidt C, Althaus H, Zegers I. First certified reference material for cystatin C in human serum ERM-DA471/IFCC. Clinical Chemistry & Laboratory Medicine. 2010 Nov 1;48(11).
44. Laterza OF, Price CP, Scott MG. Cystatin C: an improved estimator of glomerular filtration rate? Clinical Chemistry. 2002 May 1;48(5):699–707.
45. Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clinical Cancer Research. 2004 Oct 15;10(20):6897–904.
46. Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. New England Journal of Medicine. 2004 Aug 19;351(8):781–91.
47. Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. Journal of Nuclear Medicine. 2009 May 1;50(Suppl 1):122S–50S.