Abstract
Background: Contrast-induced acute kidney injury (CI-AKI) is a potential complication following percutaneous coronary intervention (PCI), particularly in patients with pre-existing kidney conditions. Previous research has identified hyperuricemia as a predictor for CI-AKI. Allopurinol, a medication commonly used to manage hyperuricemia, also possesses anti-inflammatory properties. This study aims to assess the impact of adding allopurinol to hydration therapy on CI-AKI incidence in patients undergoing PCI.
Results: We enrolled 107 patients undergoing PCI with moderate to high Mehran risk scores; Participants were randomly assigned to either an allopurinol group (n=52) or a control group (n=55). The allopurinol group received 300 mg of allopurinol 24 hours before and one hour before PCI, along with intravenous normal saline at 1 ml/kg/hour. The control group received conventional hydration only. Serum creatinine and urea levels were measured before and 48 hours after the procedure. An increase in serum creatinine by ≥25% or ≥0.5 mg/dL 48 hours after PCI was used to detect contrast-induced acute kidney injury (CI-AKI).
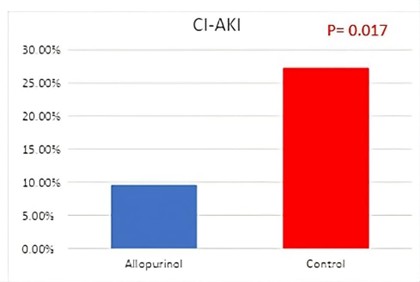
The mean age of the patients was 59.8±8.9 years, with 63% being male. The baseline Mehran risk score was 9.5±3.0. Baseline characteristics, including age, gender, risk factors, kidney function, and uric acid levels, were similar between the two groups. CI-AKI occurred in 5 patients (9.6%) in the allopurinol group and 15 patients (27.3%) in the control group (P=0.017). No significant difference was found in serum uric acid levels between patients with and without CI-AKI (7.2±2.8 mg/dL vs. 6.8±2.0 mg/dL, p-value: 0.49).
Conclusion: Our results suggest that allopurinol may reduce CI-AKI incidence beyond its hypouricemic effect following elective PCI in patients with moderate to high Mehran risk score. However, further large-scale, multi-center studies are needed to confirm these findings.
Keywords
Allopurinol, Contrast-induced acute kidney injury, Coronary artery disease, Percutaneous coronary intervention
Background
Contrast-induced acute kidney injury (CI-AKI) is a significant complication following elective percutaneous coronary intervention (PCI), accounting for approximately 12% of hospital-acquired acute kidney injury cases [1]. Assessing the true incidence of CI-AKI is challenging due to variations in definitions, patient populations, clinical outcomes, types of contrast media used, and preventive protocols [2]. The mechanisms underlying CI-AKI include direct nephrotoxic effects of contrast agents, hemodynamic changes, oxidative stress, apoptosis, immune/inflammation responses, and epigenetic regulation [3].
Patients with CI-AKI showed a higher adjusted risk of adverse cardiac events at 5 years in patients undergoing a first-ever PCI, and this risk was severity-dependent [4]. Intravascular volume expansion is pivotal in preventing CA-AKI along with other well-studied measures [5,6]. Other preventive measures include high-intensity statin, minimal low-osmolar and iso-osmolar contrast [3,7,8], and stopping nephrotoxic medications 24 hours before and 48 hours after the procedure [9].
In many studies, serum uric acid level was an independent predictor of CI-AKI [10–12]. On the other hand, many studies contradict this and have found that serum uric acid levels are an insignificant contributor. Inflammation and subsequent formation of reactive oxygen species are among the postulated mechanisms of CI-AKI development. Allopurinol is a drug that inhibits the xanthine oxidase enzyme, reducing the blood level of uric acid. It also exhibits anti-inflammatory effects by reducing oxygen-free radicals and other inflammatory mediators [13]. The drug has emerged as a preventive measure of CI-AKI and is still under evaluation with different contradictory studies that support or refute its suggested role [14–16].
This study aims to evaluate the impact of adding allopurinol to an intravenous hydration protocol in preventing CI-AKI in high-risk PCI patients. Allopurinol is available, inexpensive, and has a relatively good safety profile.
Methods
This interventional study enrolled 107 patients scheduled for elective PCI between December 2021 and May 2022. After screening for inclusion and exclusion criteria, participants were randomly assigned to either the intervention group (allopurinol plus hydration) or the control group (hydration only) in a 1:1 ratio. The simple random allocation sequence was generated using Microsoft Excel (Version 2308; Microsoft Corporation, 2022). All patients were informed about the study's purpose and provided written consent. The local ethics committee approved the study.
Inclusion criteria
Adult patients indicated for elective PCI with moderate to high Mehran risk score (risk score above 5) of (CI-AKI), as laid out by Mehran et al. [17], which includes (Table 1):
|
Risk factor |
Points |
|
Hypotension (SBP <80 mmHg for one hour or more requiring inotropic support) |
5 |
|
IABP within 24 hours periprocedural |
5 |
|
Congestive heart failure NYHA III/IV and/or pulmonary oedema |
5 |
|
Age >75 years |
4 |
|
Anemia (HCT <39% for males and <36% for females) |
3 |
|
Diabetes Mellitus |
3 |
|
Contrast media volume |
1 for each 100 ml |
|
Serum creatinine greater than 1.5 mg/dl |
4 |
|
Creatinine clearance |
2 for 40–60 4 for 20–40 6 for <20 |
Exclusion criteria
- Patients refused to participate in the study
- End-stage renal insufficiency (CrCl less than 15 ml/minute/1.73m2)
- Acute renal insufficiency
- Pregnancy and lactation
- Acute pulmonary edema
- Cardiogenic shock
- Multiple myeloma
- History of an allergic reaction to contrast agents or allopurinol
- Contrast media exposure within seven days before the procedure or history of CI-AKI
- The administration of NAC, metformin, dopamine, theophylline, sodium bicarbonate, mannitol, fenoldopam, and nephrotoxic medicines within 48 hours before a procedure
Methodology
Clinical Assessment
All patients were subjected to a detailed history and examination with particular emphasis on age, gender, and body mass index in addition to conventional risk factors, including diabetes mellitus [18], hypertension [19], dyslipidemia [20], and smoking [21].
Laboratory workup
In this study, all patients underwent laboratory tests, including complete blood count, uric acid, and lipid profile. Kidney function was assessed by measuring urea and creatinine levels before and 48 hours after the procedure. Creatinine clearance (CrCl) was estimated using the Modification of Diet in Renal Disease (MDRD) formula [22,23].
Intervention
The intervention group received 300 mg of allopurinol (Zyloric) administered 24 hours and 1 hour before the procedure, in addition to intravenous hydration with normal saline at a rate of 1 mL/kg/hour, up to a maximum of 100 mL/hour, for 12 hours before and after PCI. The control group received the same intravenous hydration regimen without allopurinol. Both groups were scheduled for elective PCI and received the same type of contrast media: low-osmolar contrast medium, iopromide (Ultravist® 300). The volume of contrast media used during the procedure was recorded for all patients.
Outcome
Development of CI-AKI was defined as an absolute rise in serum creatinine of ≥0.5 mg/dL or a relative rise in serum creatinine of ≥25% from baseline within 48 to 72 hours after exposure to contrast media during percutaneous coronary intervention [24].
Statistical analysis
The sample size in this study was based on the anticipated CI-AKI event rate in patients with Mehran risk scores greater than 5, which is approximately 15% [17]. For a 95% confidence level, an anticipated event rate of 0.15, and a margin of error of 0.07, the required sample size is 100 participants.
Statistical Package for the Social Sciences 25 (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp.) was used for statistical analysis. Continuous data were represented using mean, median, and standard deviation, while categorical data were represented using count and percentage. The non-parametric Kruskal-Wallis and Mann- Whitney tests were used to compare the continuous variables. Serial measurements within each patient were compared using a non-parametric Wilcoxon signed-rank test. The tests used for categorical data were the Chi-square test or the Fisher’s exact test. Correlations were done using the Spearman correlation coefficient. Regression analysis for the predictors of CI-AKI was performed using logistic regression analysis. Results with a p-value less than 0.05 were considered statistically significant.
Results
The mean age of the 107 patients enrolled in our study was 59.8±8.9 years, with 63% of the patients being male. The majority had diabetes mellitus (DM) and hypertension. Baseline creatinine levels were mildly elevated at 1.35±0.60 mg/dL. The average Mehran score was 9.5±3.0, indicating a moderate to high risk for contrast-induced acute kidney injury (CI-AKI). Most patients (94%) were on statin therapy. There were no significant differences in baseline characteristics between the allopurinol and control groups (Table 2).
|
|
Total |
Allopurinol |
Control |
p-value |
|
(n: 107) |
(n: 52) |
(n: 55) |
|
|
|
Age |
60.0±9.0 |
60.0 ± 10.0 |
59.0± 8.0 |
0.67 |
|
Male Gender |
63 (58.9) |
32 (61.5) |
31 (56.4) |
0.59 |
|
Diabetes Mellitus |
97 (90.7) |
49 (94.2) |
48 (87.3) |
0.22 |
|
Hypertension |
92 (86.0) |
46 (88.5) |
46 (83.6) |
0.47 |
|
Smoking |
58 (54.2) |
29 (55.8) |
29 (52.7) |
0.75 |
|
Heart Failure |
21 (19.6) |
12 (23.1) |
9 (16.4) |
0.38 |
|
BMI |
26.9±4.5 |
26.3±4.7 |
27.4±4.2 |
0.06 |
|
SBP (mmHg) |
129.0±14.0 |
131.0±14.0 |
129.0±14.0 |
0.49 |
|
DBP (mmHg) |
80.0±8.0 |
81.0± 8.0 |
80.0±8.0 |
0.65 |
|
Heart rate (bpm) |
80.0±9.0 |
81.0± 9.0 |
79.0±8.0 |
0.14 |
|
Hemoglobin (g/dl) |
11.9±1.4 |
11.8± 1.2 |
11.8±1.4 |
0.75 |
|
TLC (*1000/cm) |
7.6 ±2.6 |
7.7±2.5 |
7.5±2.6 |
0.47 |
|
Platelets(*1000/cm) |
251.3±84.6 |
261.8±96.6 |
243.6±71.6 |
0.53 |
|
Total cholesterol (mg/dl) |
157.1±39.9 |
153.0±36.0 |
163.0±45.0 |
0.21 |
|
Triglycerides (mg/dl) |
154.9±82.3 |
161.0±87.0 |
157.0±94.0 |
0.87 |
|
HDL (mg/dl) |
35.5±8.4 |
36.0±9.0 |
35.0±7.0 |
0.78 |
|
LDL (mg/dl) |
94.3±32.2 |
90.0±31.0 |
100.0±36.0 |
0.13 |
|
Uric acid (mg/dl) |
6.8±2.5 |
6.8±1.8 |
7.0±2.6 |
0.66 |
|
Baseline creatinine (mg/dl) |
1.35±0.60 |
1.39±0.45 |
1.30±0.72 |
0.44 |
|
Post-PCI creatinine (mg/dl) |
1.48±0.77 |
1.37 ±0.51 |
1.58 ±0.96 |
0.15 |
|
Baseline urea (mg/dl) |
47.7±26.5 |
46.0±21.2 |
49.4±31.0 |
0.51 |
|
Post PCI urea (mg/dl) |
50.8±31.4 |
46.3±20.3 |
55.3±39.3 |
0.13 |
|
Baseline CrCl (ml/min) |
64.5±26.4 |
60.1±25.3 |
68.8±26.9 |
0.09 |
|
Post PCI CrCl (ml/min) |
61.1±27.3 |
62.5±27.7 |
59.7±27.2 |
0.60 |
|
EF (%) |
52.3±13.1 |
52.8±13.8 |
52.9±12.6 |
0.67 |
|
Insulin |
54 (50.5) |
29 (55.8) |
25 (45.5) |
0.29 |
|
RASi |
55 (51.4) |
25 (48.1) |
30 (54.5) |
0.51 |
|
Statin |
101 (94.4) |
50 (96.2) |
51 (92.7) |
0.70 |
|
Beta Blocker |
96 (89.7) |
44 (84.6) |
52 (94.5) |
0.09 |
|
Diuretic |
36 (33.6) |
18 (34.6) |
18 (32.7) |
0.84 |
|
Mehran score |
9.5±3.0 |
9.3±3.2 |
8.7±2.4 |
0.45 |
|
Contrast volume (ml) |
167.5 ±71.2 |
179.8 ± 68.1 |
153.8 ± 57.9 |
0.052 |
|
BMI: Body Mass Index; CrCl: Creatinine Clearance; DBP: Diastolic Blood Pressure; EF: Ejection Fraction; HDL: High-Density Lipoprotein; LDL: Low-Density Lipoprotein; RASi: Renin- Angiotensin System Inhibitor; SBP: Systolic Blood Pressure; TLC: Total Leucocytic Count |
||||
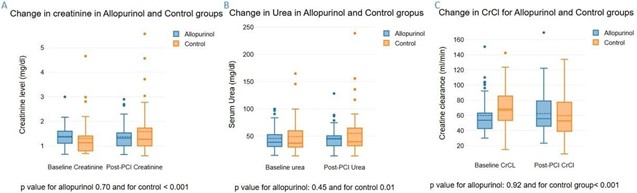
Twenty patients (18.9%) developed CI-AKI. The allopurinol group had a lower incidence of CI-AKI compared to the control group (9.6% vs. 27%, p=0.017) (Figure 1). In the allopurinol group, there were no significant changes in creatinine, urea, or creatinine clearance levels before and after PCI. Conversely, the control group exhibited significant increases in creatinine and urea levels as well as a significant decrease in creatinine clearance post-PCI (Figure 2). No clinically relevant adverse side effects were observed in the allopurinol group.
Figure 1. The incidence of contrast-induced nephropathy (CIN) in Allopurinol and Control groups. CI-AKI: Contrast-Induced Acute Kidney Injury.
Figure 2. Changes in kidney function 24 hours after PCI in Allopurinol and Control groups, A. Change in creatinine level. B. Change in urea level. C. Change in Creatinine clearance (CrCl).
In this study, baseline serum uric acid levels were similar between the allopurinol and control groups, with no significant difference observed (Table 2). Additionally, uric acid levels did not significantly differ between patients who developed contrast-induced acute kidney injury (CI-AKI) and those who did not (7.2±2.8 mg/dL vs. 6.8±2.0 mg/dL, p=0.49) (Table 3).
Logistic regression analysis revealed that the odds of developing CI-AKI were significantly lower with allopurinol use (OR 0.04, 95% CI 0.01–0.33, p=0.002) and higher with increased total leukocyte count (TLC) (OR 1.48, 95% CI 1.14–1.92, p=0.004). Hypertension and heart failure were significantly more prevalent in patients who developed CI-AKI. Additionally, these patients had higher TLC values, increased use of diuretics, and higher Mehran risk scores.
|
|
Total |
Allopurinol |
Control |
||||||
|
|
CI-AKI (n: 20) |
No CI-AKI (n: 87) |
p- value |
CI-AKI (n: 5) |
No CI-AKI (n: 47) |
p- value |
CI-AKI (n: 15) |
No CI-AKI (n: 40) |
p- value |
|
Male Gender |
9 (45.0) |
54 (62.1) |
0.16 |
1 (20.0) |
29 (61.7) |
0.14 |
8 (53.3) |
25 (45.5) |
0.41 |
|
Diabetes Mellitus |
19 (95.0) |
78 (89.7) |
0.46 |
5 (100.0) |
42 (89.4) |
0.55 |
14 (93.3) |
36 (90.0) |
0.33 |
|
Hypertension |
20 (100) |
72 (82.8) |
0.046 |
5 (100.0) |
39 (83.0) |
0.38 |
15 (100.0) |
33 (82.5) |
0.043 |
|
Smoking |
9(45.0) |
49(56.3) |
0.36 |
1(20.0) |
26(55.3) |
0.17 |
8(53.3) |
23(57.5) |
0.8 |
|
Heart Failure |
8 (40.0) |
13 (14.9) |
0.01 |
5 (100) |
7 (15.9) |
<0.001 |
3 (20.0) |
6 (15.0) |
0.99 |
|
Age |
59.9±6.2 |
59.9±9.4 |
0.99 |
62.2±7.5 |
59.0±10.7 |
0.34 |
59.2±5.3 |
60.1±8.5 |
0.69 |
|
BMI |
27.0±4.8 |
26.9±4.5 |
0.95 |
25.2±2.9 |
26.8±5.2 |
0.47 |
27.9±5.1 |
27.2±4.0 |
0.65 |
|
SBP (mmHg) |
126.3±15.6 |
130.2±13.8 |
0.27 |
125.4±19.7 |
132.2±12.1 |
0.38 |
127.7±14.1 |
128.1±14.3 |
0.91 |
|
DBP (mmHg) |
80.3±8.8 |
80.0±8.5 |
0.91 |
79.2±9.0 |
80.8±8.0 |
0.2 |
81.7±7.7 |
79.1±9.1 |
0.35 |
|
Heart rate (bpm) |
84.2±11.3 |
79.0±8.0 |
0.02 |
85.0±9.4 |
79.6±9.2 |
0.59 |
84.0±11.9 |
77.1±5.7 |
0.67 |
|
Hemoglobin (g/dl) |
11.8± 1.4 |
11.9±1.4 |
0.87 |
11.7±0.8 |
11.8±1.4 |
0.3 |
11.8±1.5 |
12.0±1.4 |
0.61 |
|
TLC (*1000/cm) |
9.4±3.2 |
7.2±2.2 |
<0.001 |
8.4±3.2 |
7.4±2.3 |
0.38 |
9.4±3.1 |
6.9±1.9 |
0.009 |
|
Platelets (*1000/cm) |
247.2±74.6 |
251.9±87.3 |
0.83 |
262.2±74.7 |
261.1±105.7 |
0.1 |
229.9±66.5 |
246.6±68.1 |
0.4 |
|
Total cholesterol (mg/dl) |
153.9±34.3 |
157.9±41.3 |
0.69 |
167.7±34.7 |
152.4±37.9 |
0.41 |
149.3±34.5 |
164.4±45.0 |
0.24 |
|
Triglycerides (mg/dl) |
187.7±89.4 |
148.5±77.4 |
0.14 |
246.2±104.7 |
159.2±89.9 |
0.95 |
156.2±88.6 |
135.7±55.7 |
0.33 |
|
HDL (mg/dl) |
35.3±7.3 |
35.5±8.6 |
0.94 |
39.6±12.1 |
35.7±9.4 |
0.4 |
33.9±4.5 |
35.2±7.7 |
0.54 |
|
LDL (mg/dl) |
89.5±30.0 |
95.7±32.6 |
0.44 |
84.1±29.7 |
90.4±31.0 |
0.2 |
91.2±31.0 |
102.1±34.2 |
0.3 |
|
Uric acid (mg/dl) |
7.2±2.8 |
6.7±1.8 |
0.33 |
6.4±1.8 |
6.9±1.8 |
0.24 |
7.5±3.1 |
6.8±2.3 |
0.38 |
|
Insulin |
11 (55.0) |
41 (51.3) |
0.76 |
3 (60.0) |
25 (53.2) |
0.99 |
8 (53.3) |
16 (40.0) |
0.5 |
|
RASi |
12 (60.0) |
44 (53.8) |
0.62 |
5 (100.0) |
20 (42.6) |
0.02 |
7 (46.7) |
24 (60.0) |
0.21 |
|
Statins |
19 (95.0) |
76 (95.0) |
0.99 |
5 (100.0) |
43 (91.5) |
0.63 |
14 (93.3) |
33 (82.5) |
0.9 |
|
Beta Blockers |
20 (100.0) |
68 (85.0) |
0.07 |
5 (100.0) |
37 (87.7) |
0.3 |
15 (100.0) |
31 (77.5) |
0.24 |
|
Diuretics |
12 (60.0) |
22 (27.5) |
0.006 |
5 (100.0) |
13 (27.7) |
0.002 |
7 (46.7) |
9 (22.5) |
0.08 |
|
EF |
53.5±14.7 |
52.3±12.6 |
0.73 |
50.6±12.2 |
52.1±12.9 |
0.44 |
55.1±10.8 |
52.7±12.8 |
0.36 |
|
Contrast volume (ml) |
187.5±90.1 |
163.1±66.4 |
0.18 |
210.0±89.4 |
175.6±73.6 |
0.34 |
180.0±92.2 |
145.7±52.0 |
0.19 |
|
Baseline creatinine (mg/dl) |
1.34±0.73 |
1.31±0.42 |
0.8 |
1.10±0.57 |
1.42±0.43 |
0.13 |
1.42±0.78 |
1.25±0.70 |
0.47 |
|
Post-PCI creatinine (mg/dl) |
1.87±1.04 |
1.32±0.47 |
0.001 |
1.53±0.73 |
1.35±0.49 |
0.48 |
1.98±1.12 |
1.41±0.85 |
0.06 |
|
Baseline urea (mg/dl) |
54.2±37.3 |
44.9±20.1 |
0.13 |
42.5±30.7 |
46.4±20.4 |
0.7 |
58.2±39.4 |
45.6±26.3 |
0.19 |
|
Post-cath urea (mg/dl) |
69.3±50.4 |
44.6±19.2 |
0.001 |
54.4±20.7 |
45.4±20.2 |
0.35 |
74.3±56.7 |
47.2±26.0 |
0.095 |
|
Baseline CrCl (ml/min) |
65.9±34.8 |
64.5±23.6 |
0.83 |
67.2±34.2 |
59.3±24.5 |
0.51 |
65.5±36.2 |
70.3±22.3 |
0.64 |
|
Post-cath CrCl (ml/min) |
48.1±27.7 |
65.1±26.0 |
0.01 |
52.2±32.7 |
63.7±27.3 |
0.39 |
46.8±26.9 |
65.3±25.7 |
0.03 |
|
Mehran score |
10.8±3.4 |
9.2±2.7 |
0.02 |
14.2±2.2 |
9.9±3.0 |
0.003 |
9.7±2.9 |
8.3±2.1 |
0.07 |
|
BMI: Body Mass Index; CI-AKI: Contrast-Induced Acute Kidney Injury; CrCl: Creatinine Clearance; DBP: Diastolic Blood Pressure; EF: Ejection Fraction; HDL: High-Density Lipoprotein; LDL: Low-Density Lipoprotein; PCI: Percutaneous Coronary Intervention; RASi: Renin-Angiotensin System Inhibitor; SBP: Systolic Blood Pressure; TLC: Total Leucocytic Count |
|||||||||
Discussion
Contrast-associated acute kidney injury (CA-AKI) is a significant complication following PCI, associated with increased short- and long-term mortality and major adverse cardiac events (MACE) [9]. Preventive strategies are crucial to mitigate the risk of CA-AKI. Key measures include minimizing the volume of contrast media used, ensuring adequate hydration to prevent volume depletion, and avoiding nephrotoxic agents such as non-steroidal anti-inflammatory drugs (NSAIDs) [3,7,8].
In addition to these standard approaches, recent studies have explored the role of pharmacological interventions in preventing CA-AKI. For instance, the administration of allopurinol, a xanthine oxidase inhibitor, has been investigated for its potential protective effects against CI-AKI. Allopurinol may exert its benefits through mechanisms beyond uric acid reduction, including anti-inflammatory and antioxidant effects [25,26].
In our study, the incidence of contrast-induced acute kidney injury (CI-AKI) was notably higher among patients with hypertension and heart failure. Specifically, all patients who developed CI-AKI were hypertensive, and 40% of those with heart failure experienced this complication. These findings align with existing literature, which identifies hypertension and heart failure as significant risk factors for CI-AKI. The use of diuretics was significantly higher among patients with CI-AKI. This result can be explained by the higher prevalence of heart failure and hypertension among CI-AKI. On the other hand, there was no difference between the allopurinol and control groups regarding the use of diuretics. There were no differences between the two groups regarding DM, anemia, gender, and smoking.
The Mehran risk score, a widely used tool for assessing the risk of contrast-induced nephropathy, incorporates factors such as age, diabetes mellitus, renal function, heart failure, and the volume of contrast media used. Our study's observation that CI-AKI occurred significantly more frequently in patients with higher Mehran scores underscores the utility of this scoring system in identifying individuals at elevated risk for CI-AKI.
In our study, the administration of allopurinol, in conjunction with intravenous hydration, was associated with a lower incidence of contrast-induced acute kidney injury (CI-AKI) compared to the control group. Specifically, the control group exhibited significant increases in urea and creatinine levels, accompanied by a decrease in creatinine clearance, indicating renal impairment. Conversely, the allopurinol group maintained stable levels of urea, creatinine, and creatinine clearance, suggesting renal protection.
Our study's findings align with existing research supporting the use of allopurinol as a preventive measure against contrast-induced acute kidney injury (CI-AKI). For instance, Erol et al. conducted a randomized controlled trial involving 79 patients with serum creatinine levels exceeding 1.1 mg/dL. They administered 300 mg of oral allopurinol 24 hours prior to cardiac catheterization, alongside intravenous hydration. The results demonstrated a significant reduction in the incidence of contrast-induced nephropathy (CIN) within 48 and 96 hours post-procedure in the allopurinol group compared to the control group [2].
Similarly, a study by Sadineni et al. compared the efficacy of N-acetylcysteine (NAC) and allopurinol in preventing contrast nephropathy in patients with chronic kidney disease. The study found that while there was no significant difference between NAC and allopurinol in preventing contrast-induced nephropathy, allopurinol, when combined with hydration, was superior to placebo [16].
Our observation regarding the study by Iranirad et al. highlights the variability in research findings concerning the efficacy of allopurinol in preventing contrast-induced acute kidney injury (CI-AKI). In their randomized controlled trial, Iranirad et al. administered a single 300 mg dose of allopurinol 24 hours prior to coronary angiography. They found no significant difference in the incidence of CI-AKI between the allopurinol and control groups [15]. This discrepancy may be attributed to differences in study design, particularly the timing and dosing regimen of allopurinol administration. In contrast, our study employed a two-dose regimen of 300-mg of allopurinol, administered 24 hours before and just before the procedure, which may have enhanced its protective effect against CI-AKI.
Collectively, these studies highlight the potential of allopurinol, particularly when combined with intravenous hydration, as an effective strategy for preventing CI-AKI in high-risk patients undergoing procedures involving contrast media. However, further large-scale, well-designed randomized controlled trials are necessary to establish the efficacy of allopurinol in this context conclusively.
Our study's findings regarding the lack of association between baseline uric acid levels and contrast-induced acute kidney injury (CI-AKI) contrast with some existing literature. For instance, a study by Chung et al. concluded in their retrospective study that hyperuricemia did not affect CI- AKI incidence in patients with CKD and non-CKD patients undergoing PCI [27]. Conversely, Toprak et al. found that patients with elevated uric acid levels and creatinine levels ≥1.2 mg/dL had an increased risk of CI-AKI following percutaneous coronary intervention (PCI) [10]. Similarly, a study by Liu et al. concluded that hyperuricemia could increase the incidence of CI-AKI even in patients with normal serum creatinine levels after PCI [11]. These studies suggest that elevated uric acid levels may be a risk factor for CI-AKI. However, our study did not find a significant association between uric acid levels and CI-AKI, indicating that the relationship between uric acid and CI-AKI may be complex and influenced by other factors.
Regarding the role of inflammation, our regression analysis revealed that total leukocyte count is an independent predictor of CI-AKI, aligning with recent research findings. A study published in PeerJ found that patients in the CI-AKI group had significantly higher white blood cell (WBC) counts than those in the non-CI-AKI group, suggesting that elevated WBC counts may be associated with an increased risk of CI-AKI [28]. Additionally, a study published in European Heart Journal identified elevated WBC count as a predictor of CI-AKI in patients undergoing primary percutaneous coronary intervention [29]. These findings support the hypothesis that inflammation plays a significant role in the pathogenesis of CI-AKI. Elevated WBC counts may reflect an inflammatory response that contributes to kidney injury following contrast exposure.
Miotto et al. demonstrated that allopurinol can target the ryanodine receptor 2, thereby modulating calcium handling and enhancing cardiac contractility. Improved cardiac output can directly enhance renal blood flow and glomerular filtration rate, which may be associated with a reduction in the incidence of CI-AKI. This hemodynamic effect could explain the protective effect of allopurinol against CI-AKI, especially in patients with heart failure and hypertension [30].
In summary, while some studies suggest a link between hyperuricemia and CI-AKI, our study did not find this association, indicating that other factors may influence the relationship. However, the consistent finding across studies that elevated WBC counts are associated with an increased risk of CI-AKI underscores the importance of inflammation in the development of this condition. Whether the use of non-purine xanthine inhibitors (e.g., febuxostat) or other anti-inflammatory agents could have similar effects, this can be tested in the future.
Limitations
Sample Size and Statistical Power
While our study demonstrated a significant reduction in CI-AKI incidence with allopurinol despite a small sample size, larger studies are essential to confirm these findings and ensure statistical power.
Anti-Inflammatory properties
The proposed anti-inflammatory properties of allopurinol could not be proven. Inflammatory biomarkers, such as high-sensitivity C-reactive protein, can serve as a surrogate biomarker for inflammation.
Dosing regimen
Whether different doses of allopurinol could have distinct clinical outcomes can be studied in future trials.
Conclusion
In this study, allopurinol administration, in conjunction with intravenous hydration, was associated with a reduced incidence of contrast-induced acute kidney injury (CI-AKI) following elective percutaneous coronary intervention (PCI) in patients identified as high-risk based on the Mehran risk score. Our findings highlight the potential of allopurinol as a preventive strategy in at-risk populations. Notably, this protective effect was observed regardless of baseline serum uric acid levels. Given the promising results from this study, larger, multicenter randomized controlled trials are warranted to confirm these findings and establish optimal dosing regimens.
List of Abbreviations
BMI: Body Mass Index; CI-AKI: Contrast-Induced Acute Kidney Injury; CrCl: Creatinine Clearance; DBP: Diastolic Blood Pressure; EF: Ejection Fraction; HDL: High-Density Lipoprotein; LDL: Low-Density Lipoprotein; PCI: Percutaneous Coronary Intervention; RASi: Renin-Angiotensin System Inhibitor; SBP: Systolic Blood Pressure; TLC: Total Leucocytic Count
Funding
No funding has been received from any donor, and this funding clause is not applicable.
Declarations Ethics Approval and Consent to Participate
The Institutional Review Board (IRB) of the Faculty of Medicine, Cairo University, gave ethical approval for patient data. Written informed consent was obtained from all patients before the procedure for their participation in the study.
Consent for Publication
We give full consent for the publication of our study.
Clinical Trial Number
ms-354-2020.
References
2. Kumar A, Bhawani G, Kumari N, Murthy KS, Lalwani V, Raju ChN. Comparative study of renal protective effects of allopurinol and N-acetyl-cysteine on contrast induced nephropathy in patients undergoing cardiac catheterization. J Clin Diagn Res. 2014 Dec;8(12):HC03–7.
3. Zhang F, Lu Z, Wang F. Advances in the pathogenesis and prevention of contrast-induced nephropathy. Life Sci. 2020 Oct 15;259:118379.
4. Ng AK, Ng PY, Ip A, Lam LT, Ling IW, Wong AS, et al. Impact of contrast-induced acute kidney injury on long-term major adverse cardiovascular events and kidney function after percutaneous coronary intervention: insights from a territory-wide cohort study in Hong Kong. Clin Kidney J. 2021 Oct 22;15(2):338–46.
5. Walther CP, Podoll AS, Finkel KW. Summary of clinical practice guidelines for acute kidney injury. Hosp Pract (1995). 2014 Feb;42(1):7–14.
6. Ellis JH, Cohan RH. Prevention of contrast-induced nephropathy: an overview. Radiol Clin North Am. 2009 Sep;47(5):801–11, v.
7. Yuan N, Latif K, Botting PG, Elad Y, Bradley SM, Nuckols TK, et al. Refining Safe Contrast Limits for Preventing Acute Kidney Injury After Percutaneous Coronary Intervention. J Am Heart Assoc. 2021 Jan 5;10(1):e018890.
8. Mamoulakis C, Tsarouhas K, Fragkiadoulaki I, Heretis I, Wilks MF, Spandidos DA, et al. Contrast-induced nephropathy: Basic concepts, pathophysiological implications and prevention strategies. Pharmacol Ther. 2017 Dec;180:99–12.
9. Mandurino-Mirizzi A, Munafò A, Crimi G. Contrast-Associated Acute Kidney Injury. J Clin Med. 2022 Apr 13;11(8):2167.
10. Toprak O, Cirit M, Esi E, Postaci N, Yesil M, Bayata S. Hyperuricemia as a risk factor for contrast-induced nephropathy in patients with chronic kidney disease. Catheter Cardiovasc Interv. 2006 Feb;67(2):227–35.
11. Liu Y, Tan N, Chen J, Zhou Y, Chen L, Chen S, et al. The relationship between hyperuricemia and the risk of contrast-induced acute kidney injury after percutaneous coronary intervention in patients with relatively normal serum creatinine. Clinics (Sao Paulo). 2013 Jan;68(1):19–25.
12. Erol T, Tekin A, Katırcıbaşı MT, Sezgin N, Bilgi M, Tekin G, et al. Efficacy of allopurinol pretreatment for prevention of contrast-induced nephropathy: a randomized controlled trial. Int J Cardiol. 2013 Aug 20;167(4):1396–9.
13. Schlesinger N, Brunetti L. Beyond urate lowering: Analgesic and anti-inflammatory properties of allopurinol. Semin Arthritis Rheum. 2020 Jun;50(3):444–50.
14. Bodagh H, Esfahani Z, Aslanabadi N, Amiri B, Sarvestani AH. Effect of allopurinol in the prevention of contrast-induced nephropathy in patients undergoing angioplasty: randomized clinical trial. J Anal Res Clin Med. 2019 Dec 20;7(4):118–21.
15. Iranirad L, Sadeghi MS, Bagheri A, Doostali K, Norouzi S, Hejazi SF, et al. Allopurinol prophylactic therapy and the prevention of contrast-induced nephropathy in high-risk patients undergoing coronary angiography: A prospective randomized controlled trial. ARYA Atheroscler. 2017 Sep;13(5):230–5.
16. Sadineni R, Karthik KR, Swarnalatha G, Das U, Taduri G. N-acetyl cysteine versus allopurinol in the prevention of contrast nephropathy in patients with chronic kidney disease: A randomized controlled trial. Indian J Nephrol. 2017 Mar-Apr;27(2):93–8.
17. Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004 Oct 6;44(7):1393–9.
18. American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020 Jan;43(Suppl 1):S14–31.
19. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018 Sep 1;39(33):3021–104.
20. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020 Jan 1;41(1):111–88.
21. McClave AK, McKnight-Eily LR, Davis SP, Dube SR. Smoking characteristics of adults with selected lifetime mental illnesses: results from the 2007 National Health Interview Survey. Am J Public Health. 2010 Dec;100(12):2464–72.
22. Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006 Aug 15;145(4):247–54.
23. Goldwasser P. Prediction equation for glomerular filtration rate. Ann Intern Med. 1999 Oct 19;131(8):629; author reply 630.
24. McCullough PA. Contrast-induced acute kidney injury. J Am Coll Cardiol. 2008 Apr 15;51(15):1419–28.
25. Landmesser U, Spiekermann S, Dikalov S, Tatge H, Wilke R, Kohler C, et al. Vascular oxidative stress and endothelial dysfunction in patients with chronic heart failure: role of xanthine-oxidase and extracellular superoxide dismutase. Circulation. 2002 Dec 10;106(24):3073–8.
26. George J, Struthers AD. The role of urate and xanthine oxidase inhibitors in cardiovascular disease. Cardiovasc Ther. 2008 Spring;26(1):59–64.
27. Chung W, Kim AJ, Ro H, Chang JH, Lee HH, Jung JY. Hyperuricemia is an independent risk factor for mortality only if chronic kidney disease is present. Am J Nephrol. 2013;37(5):452–61.
28. Fu C, Ouyang C, Yang G, Li J, Chen G, Cao Y, et al. Impact of white blood cell count on the development of contrast-induced acute kidney injury in patients receiving percutaneous coronary intervention. PeerJ. 2024 Jun 28;12:e17493.
29. Narula A, Mehran R, Weisz G, Dangas GD, Yu J, Généreux P, et al. Contrast-induced acute kidney injury after primary percutaneous coronary intervention: results from the HORIZONS-AMI substudy. Eur Heart J. 2014 Jun 14;35(23):1533–40.
30. Miotto MC, Luna-Figueroa E, Tchagou C, Bahlouli L, Reiken S, Dridi H, et al. Targeting ryanodine receptors with allopurinol and xanthine derivatives for the treatment of cardiac and musculoskeletal weakness disorders. Proc Natl Acad Sci U S A. 2025 Jun 17;122(24):e2422082122.