Abstract
A 42 years old male, with a recent COVID-19 infection, presented with multiple progressive neurologic symptoms over one month; starting as numbness around the mouth, reduced facial sensation, and a feeling of band-like sensation below the costal margins. On further examination, he had left abduction restriction, diplopia on left gaze and upbeat nystagmus, reduced facial sensation, and hyperesthesia. The reflexes were 1+ in the upper limbs, 3+ in the lower limbs, upgoing planters, tingling from the feet up to T6 level, and postural tremor bilaterally. His cerebrospinal fluid (CSF) showed a high protein level. Magnetic resonance imaging (MRI) brain/spine revealed left frontal juxtacortical white matter and bilateral middle cerebral peduncles lesions with post-contrast enhancement and long-segment spinal cord demyelinating plaques. He was initially treated as a case of acute disseminated encephalomyelitis (ADEM) post-viral infection in a background of CLL. The delayed diagnosis was due to the temporal relation of neurological manifestation to viral infection, similar MRI lesions to ADEM, and multiple negative CSF results of cytology and flow cytometry. He was managed for ADEM based on responsiveness to the recommended therapy step by step. Firstly, he received a high dose of corticosteroids, secondly IV immunoglobulin but he was still progressing. Lastly, plasma exchange was done and he exhibited progressive symptoms with fair improvement. Interestingly, the patient showed significant improvement in the clinical and radiological parameters after starting him with anti-leukemia medication (Acalabrutinib) for his active CLL. He ran out of that chemotherapy, so he experienced a recurrence of the neurological manifestation and the previous lesions in the images. Repeated flow cytometry for the third time came positive for CLL cells and the final diagnosis of CNS involvement by CLL was established. The patient received Ibrutinib at a standard dose and as a monotherapy. The patient is back to his work and his daily activities have improved.
Abbreviations
CNSi: Central Nervous System Involvement; CLL: Chronic Lymphocytic Leukemia; ADEM: Acute Disseminated Encephalomyelitis; DDx: Differential Diagnosis; MS: Multiple Sclerosis; MOG: Myelin Oligodendrocyte Glycoprotein; NMOSD: Neuromyelitis Optica Spectrum Disorder; AQP4: Aquaporin-4; IVMP: High-Dose Intravenous Methylprednisolone; IVIG: Intravenous Immunoglobulin; FLAIR: Fluid Attenuated Inversion Recovery
Introduction
Chronic Lymphocytic Leukemia (CLL) is a mature B cell neoplasm characterized by a progressive accumulation of monoclonal B lymphocytes. It manifests primarily in the blood [1]. Infiltration of CLL lymphocytes outside of this site is relatively rare and is defined as extra medullary CLL [2]. These sites include the central nervous system [3]. CNS involvement by CLL is a rare condition. It may occur at any stage of CLL, either as the first manifestation or after several years of treatment, often in the phase of progression or transformation into a more aggressive clinical form [3] that is why prognosis seemed to be related to CLL characteristics rather than to CNS involvement itself [4]. Specific CNS involvement by CLL has been involved in only 20% of cases [4], symptoms could be due to other etiologies in 80% of cases [2].
Aim
This case is unique as neurological manifestations in an immunocompromised patient due to underlying malignancy (CLL). Firstly, the final diagnosis was tricky and extremely challenging. The patient could have had CNSi CLL or any other central/ peripheral nervous system demyelination conditions in view of symptoms of one post-COVID-19 infection. The patient was initially misdiagnosed and the treatments were not completely effective. Secondly, there is no standard protocol for CNSi CLL treatment due to the rarity of these conditions. It is important to report similar cases to understand this condition; which will contribute to early diagnosis and help in choosing the most effective drugs for administration at an early stage of the disease. Thirdly, the outcome in this setting is poorly reported in the literature [4]. The report aims to alert the doctors to consider CNSi CLL even in the presence of negative results; as it indicates underlying progressive CLL, particularly late stage that needs to be urgently treated to achieve a better outcome. This case is going to open our minds for the importance of repeating the diagnostic tests to detect the pathologic cells and choosing the most effective treatment for both hematological and neurological complications.
Case Presentation
A 42 years old male, who is known to have Chronic Lymphocytic Leukemia and recently successfully treated mild COVID-19 infection, presented with multiple neurological symptoms. Since infection, he had been having progressive neurological symptoms. It started as numbness around the mouth, which progressed to involve the buccal cavity. He described a reduced taste sensation and “feeling of sand” and sometimes sore in the mouth, fluid was dropping from his mouth. He also developed a reduction in the sensation of the left side of the face and later the right side was involved. He felt the uneasiness of hands, he noticed that things were dropping from hand. One month later, he developed a feeling of band-like sensation below the costal margins, followed by hyperesthesia; he cannot tolerate his clothes. A few weeks later, he suffered tingling in his both feet and cramps in the lower limbs, so his gait became unsteady. He experienced urinary urgency and used to strain for a long time to complete evacuation. There was no incontinence or sexual dysfunction. ROS was negative. His past medical history is significant for CLL in 2016 and received 6 cycles of Fludarabine, Cyclophosphamide, Rituximab (FCR), and bone marrow transplantation with complete remission. On examination; cranial nerves showed mild left abduction restriction, diplopia on left gaze, and upbeat nystagmus evoked with a left gaze. There was a reduction in facial sensation and hyperesthesia in V1, V2, and V3 distribution bilaterally, including the buccal cavity. Sensation exam was positive for impairment of joint position in toes and hyperpathia from the feet up to T6 level. Reflexes were 1+ in the upper limbs bilaterally, 3+ in the lower limbs bilaterally and planters were up going bilaterally. Coordination examinations showed mild postural tremor of bilateral upper limb but no intention tremor. He was able to stand and walk in a wide-based gait, with difficulties due to pain in the lower limbs, there was gross sensory ataxia and a tendency for buckling of knees. He could not perform tandem walking. Romberg’s test was positive. General examination revealed a palpable spleen 2 cm below the costal margin.
Method
His basic laboratory investigations were within the normal levels. MRI Brain with contrast on admission and before starting any treatment revealed abnormal T2 & FLAIR high signal intensity seen in the left frontal juxtacortical white matter and bilateral middle cerebral peduncles with post-contrast enhancement (Figure 1). MRI of the cervical and dorsal spine with contrast revealed multilevel and long-segment spinal cord demyelinating plaques extending for more than 3 contiguous segments; elongated white matter lesion seen at C7 level, T3-T4 disc and extending from T6 to T10 levels showing post-contrast enhancement (Figure 2). The patient underwent further thorough investigation to rule out causes of CNS demyelination conditions, infections, neoplastic and paraneoplastic causes explaining his clinical and radiological findings. CNSi CLL was on top of our differential diagnosis (DDx) but it was misdiagnosed due to the initial mislead of negative CSF cytology test and Cytospin for Blast for Leukemic Lymphoblasts until it came positive after 1 year of the presentation. A biopsy is needed to distinguish between CLL, primary brain tumors, or metastatic malignancy [2]. It was difficult to be performed in this patient because the lesions wherein non-accessible locations; in the brainstem, cerebellum, and the spinal cord. Neoplastic and paraneoplastic syndromes were considered in view of his underlying CCL. These were excluded by negative tumor markers, paraneoplastic panel and CT chest, abdomen and pelvis. He underwent MRI Spectroscopy to look for tumors or other DDx (Figure 5). In our case, the trickiest part of the DDx was to distinguish between CNSi CLL and cute disseminated encephalomyelitis (ADEM). This patient presented with multiple rapidly progressive polyfocal clinical CNS events in temporal relation to COVID 19 infection. The neuroimaging features represent typical ADEM features such as multiple hyperintense bilateral, asymmetric patchy and poorly marginated lesions in MRI T2-weighted and FLAIR images. The CSF studies revealed mild pleocytosis, increased CSF protein and elevated CSF IgG index. However, there was one important missing criterion in his clinical presentation that made the diagnosis of ADEM is less likely which is the absent of encephalopathy. The progressive nature and recurrent of the neurological complications were against ADEM; because it is classically considered a monophasic illness. Despite that, he received the treatment for it as the picture of CNSi CLL was not clear and it actually helped in relieving the severity of his symptom and he was at least able to live his daily life. These treatments were used in treating CNSi CLL as reported in a few cases [5,6]. The fact that the clinical presentation followed a prodromal viral illness, presenting with polyfocal neurological symptoms, the absence of previous demyelinating attacks, the absence of multiple sclerosis (MS) features in brain imaging excluded MS. Features of MS-like relapsing and remitting the course of the disease were absent. ADEM can be a manifestation of Myelin oligodendrocyte glycoprotein (MOG) antibody-associated disorder [7]. But, the negative serum MOG-IgG makes this diagnosis less likely. Neuromyelitis optica spectrum disorder (NMOSD) was one of the DDx investigated for this case as he presented with myelitis symptoms and MRI showed long segment spinal cord demyelinating plaques extending for more than 3 contiguous segments. NMOSD target the optic nerves and spinal cord as well as the brain and brainstem [7]. This patient’s lack of bilateral optic neuritis and the disease-specific aquaporin-4 (AQP4) antibody made this diagnosis down the list. Infectious meningoencephalitis was excluded clinically due to the absence of fever, headache, meningismus, and seizures at the presentation. CSF cultures, meningitis, and encephalitis panels were negative (including virology tests and CSF cultures). Progressive multifocal leukoencephalopathy (PML) was considered in this patient in view of the history of malignancy and exposure to chemotherapy put him in immune suppression status. He presented similarly to PML as his symptoms were progressive, multifocal, and radiologically (white matter involvement). He was tested by Polymerase chain reaction (PCR) for John Cunningham (JC) virus DNA in the CSF and it was negative. The vasculitis workup such as ANF, DSNA, Cardiolipin, etc. was negative making the vasculitis and other systemic inflammatory causes of CNS demyelination is less likely. The clinical progressive course of the condition and the absence of the sudden, acute neurological deficits as well as the MRI findings were not typical for acute embolic infarcts.
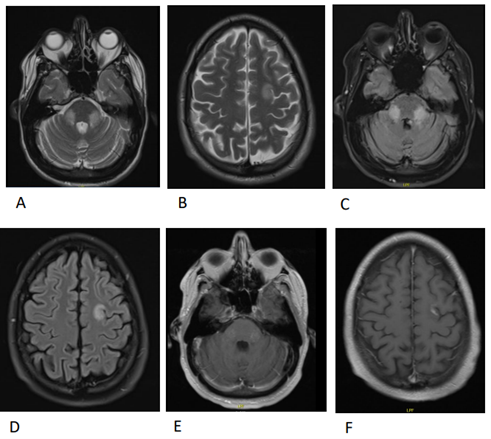
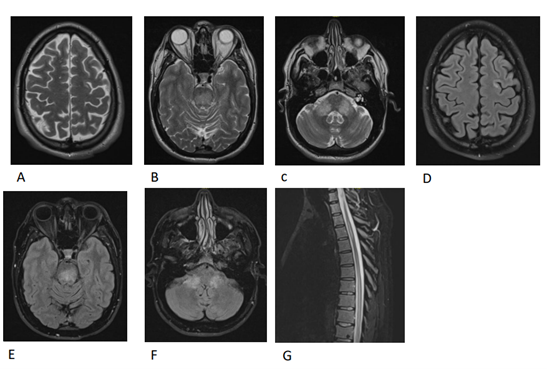
Figure 1. Brain MRI images with contrast on admission before treatment on June 24th, 2020. (A) T2 axial image revealing high signal intensity in the bilateral middle cerebral peduncles. (B) T2 axial image revealing high signal intensity in the left frontal juxtacortical white matter. (C) FLAIR axial image revealing high signal intensity in the bilateral middle cerebral peduncles. (D) FLAIR axial image revealing high signal intensity in the left frontal juxtacortical white matter. (E) Axial T1 post contrast showing enhancement of the lesions in the bilateral middle cerebral peduncles. (F) Axial T1 post contrast showing enhancement of the lesions in the left frontal juxtacortical white matter.
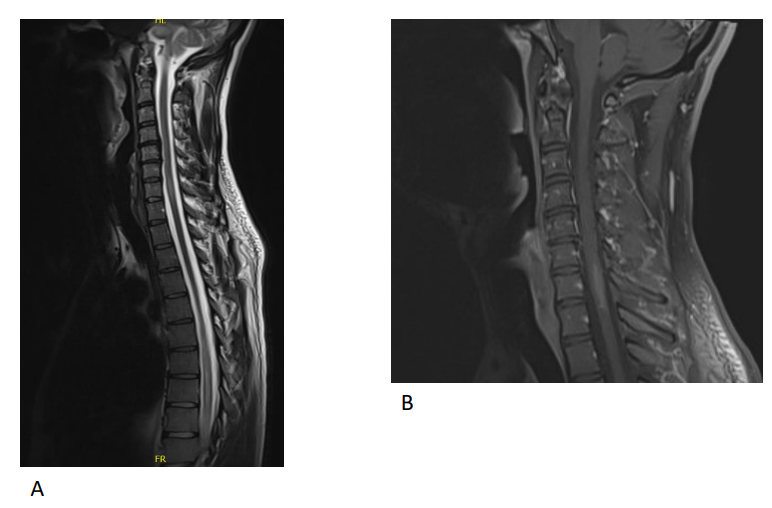
Figure 2. MRI of the cervical and dorsal spine with contrast on June 24th, 2020 showed multilevel and long segment spinal cord demyelinating plaques extending for more than 3 contiguous segments. (A) T2 sagittal view of the spinal cord revealing demyelinating plaques from T3-T4 disc (blue arrow) and long segment demyelinating plaques extending from T6 to T10 levels (red arrow). (B) T1 sagittal view post contrast showing enhancement of the spinal cord lesion.
Treatment
Based on the clinical, laboratory, CSF tests and imaging findings, he was initially treated as post-COVID ADEM. He has been given high-dose intravenous methylprednisolone (IVMP) over 5 days and it was followed with a course of oral prednisolone. This course produced partial improvement in his neurological symptoms. Unfortunately, the symptoms got worsened a few weeks later and the MRI findings showed disease progression (Figure 3), so he was started on intravenous immunoglobulin (IVIG) for 5 days. The disabling symptoms were getting worse and repeated MRI brain revealed persistent enhancing lesions with mild interval disease progression and the treatment was accelerated to plasmapheresis (Figure 4). He received 5 cycles with only modest improvement of his symptoms. There were residual sensory deficits in lower limbs, sensory ataxia, band-like hyperesthesia from T6-T12, and diplopia. He underwent bone marrow examination after discharge as a follow-up for CLL. The result was consistent with the persistent disease of CLL. As a result, he was started on Acalabrutinib (tyrosine kinase inhibitor) for it. Interestingly the patient reported significant neurological improvement after starting Acalabrutinib, almost completely resolved. He was able to go back to his work after 6 months of sick leave. There was also a radiological response to chemotherapy, the MRI brain, thoracic and cervical spine without contrast three months after starting Acalabrutinib showed near-complete regression of the disease (Figure 6). That is what promotes the consideration of the other DDx of CNSi CLL despite 2 negative CSF flow cytometry in the past for the malignant cells. A few months later, he presented with resurfacing of his previous neurological symptoms after stopping the acalabrutinib for 1 week because of the non-availability of the medication. MRI brain and spinal cord showed increasing in the size and the extension of the previous lesions (Figure 7). He underwent lumbar puncture again to look for CNS leukemia. The flow cytometry on CSF findings was consistent with CSF involvement of CCL. The hematology team was counseled immediately in regards to the confirmed diagnosis. The decision was made to switch his treatment to Ibrutinib (Antineoplastic Agent, Bruton Tyrosine Kinase Inhibitor) as it crosses the blood-brain barrier [4,8]. Using Ibrutinib showed rapid shrinkage of bulky lymphadenopathy and splenomegaly, resolution of constitutional symptoms, and radiological improvement [6].
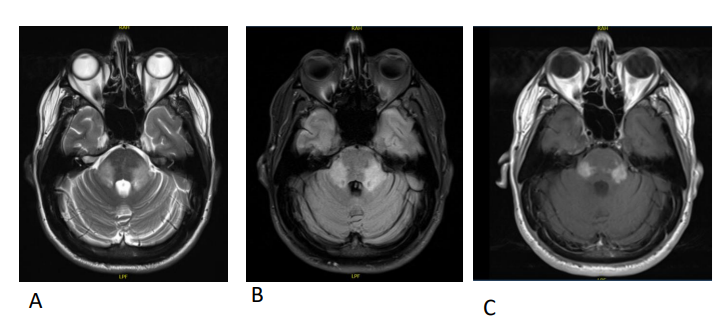
Figure 3. MRI Brain with contrast after 1 month from receiving IV Methylprednisolone course on July 14th, 2020 (A) T2 axial view revealed increasing in the signal intensity in the bilateral middle cerebral peduncles. (B) FLAIR axial view revealed increasing in the signal intensity in the bilateral middle cerebral peduncles. (C) Axial T1 post contrast showing increased enhancement of the lesions in the bilateral middle cerebral peduncles.
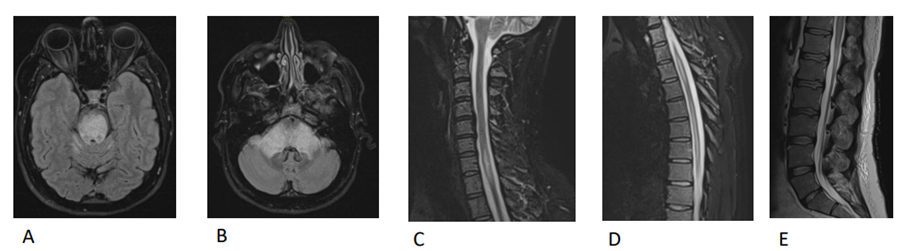
Figure 4. MRI Brain with contrast after 1 month from receiving IVIG course on October 19th, 2020. (A) T2 axial view revealed further increasing in the signal intensity in the bilateral middle cerebral Peduncles. (B) T2 axial view revealed further increasing in the signal intensity in the left frontal juxtacortical white matter. (C) FLAIR axial view revealed further increasing in the signal intensity in the bilateral middle cerebral peduncles. (D) FLAIR axial view revealed further increasing in the signal intensity in the left frontal juxtacortical white matter. (E) FLAIR sagittal view revealed further increasing in the signal intensity in the central aspect of pons. (F) Axial T1 post contrast showing further increasing in the enhancement of the lesions in the bilateral middle cerebral peduncles. (G) Axial T1 post contrast showing increasing in the enhancement of the left frontal juxtacortical white matter.
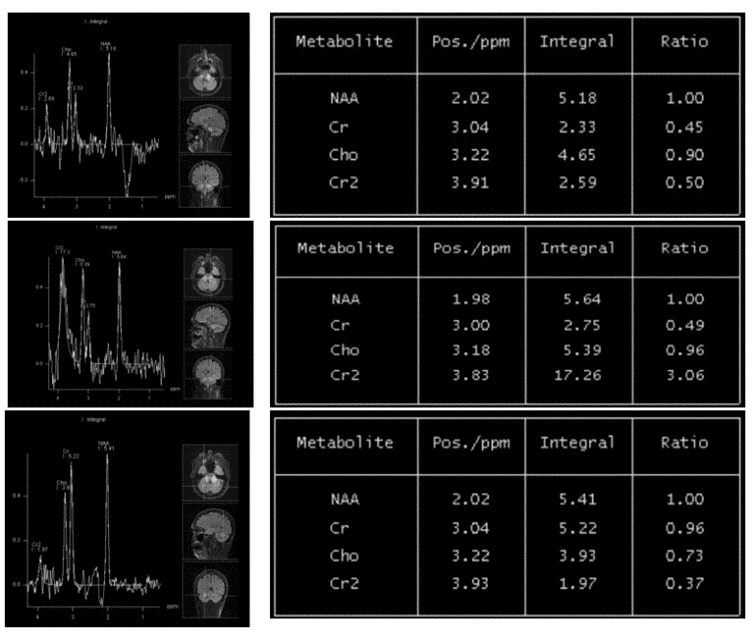
Figure 5. MRI Spectroscopy on October 26th, 2020 showed multi voxel spectroscopy with voxel placement within the bilateral symmetrical FLAIR abnormal signal in superior cerebellar peduncles shows, Mild elevation of Choline peak, with increased cho/cr ratio (>1.5). Mildly elevated lipid lactate peak also seen. No significant reduction in the NAAÂ peak. Impression: Features are possibly suggestive of inflammatory etiology like drug induced acute toxic demyelination/ leukoencephalopathy.
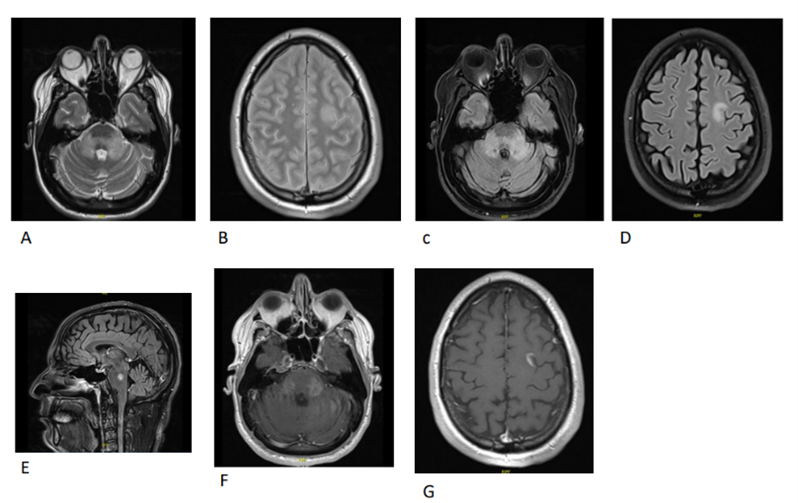
Figure 6. MRI brain, thoracic and cervical spine without contrast on March 8th, 2021 three months after starting Acalabrutinib. (A) T2 axial view of the brain revealed near complete regression of the previous hyperintensity in the left frontal juxtacortical white-matter. (B) T2 axial view of the brain revealed faint and inactive lesion in the central pons. (C) T2 axial view of the brain revealed faint and inactive lesion in the bilateral middle cerebral peduncles. (D) FLAIR axial view of the brain revealed near complete regression of the previous hyperintensity in the left frontal juxtacortical white-matter. (E) FLAIR axial view of the brain revealed faint and inactive lesion in central pons. (F) FLAIR axial view of the brain revealed faint and inactive lesion in bilateral middle cerebral peduncles (G) T2 sagittal view of thoracic spine revealed regression of the prior diffuse swelling of the spinal cord at D6 through D10 level.
Figure 7. MRI brain, cervical, thoracic, Lumbo-Sacral spine with contrast on May 11th, 2021 around 2 weeks of stopping Acalabrutinib. (A) FLAIR axial view of the brain revealed increase in the size and the extension of the hyper intensity in the entire pons (B) FLAIR axial view of the brain revealed increase in the size and the extension of the hyper intensity in the bilateral cerebellar peduncles (C) T2 sagittal view of cervical spine revealed increase in hyper intensity signal at C6-C7 level and cord swelling (D) T2 sagittal view of thoracic spine revealed increase in hyper intensity signal at D3, D6-D10 levels and cord swelling (E) T2 sagittal view of lumbo-sacral spine revealed diffuse increase in hyperintensity signal in conus medullaris with increase in cord swelling.
Results
He was doing very well with this chemotherapy he took. He is doing all his activities of daily living independently including driving and he was able to join his work with mild residual symptoms only.
Discussion
There are multiple similar reported cases. The next section is going to compare our case with the other reported cases. Neurological symptoms of CNSi CLL were variable. They were either central (related to supra-tentorial cortical infiltration) or peripheral (related to infra-tentorial meningitis, cranial or spinal nerves, nerve roots or plexus infiltration) [6]. These symptoms could manifest as peripheral neuropathy, progressive multifocal leukoencephalopathy, encephalitis, neuromuscular junction dysfunction, and paraneoplastic conditions. Median age at CLL diagnosis and at CNSi CLL onset was 62 years (range 41–85) and 69 years (range 42-87), respectively as described in a retrospective cohort of 30 CLL patients with CNSi CLL [6]. Our patient showed a younger and an aggressive disease course of age 42 years, like also described in one case report [4]. When a mass lesion is identified on imaging and a tissue biopsy can be performed, the evaluation and diagnosis for CNSi CLL is relatively straightforward. Biopsy is needed in these cases to distinguish between CLL, primary brain tumors, or metastatic malignancy, as illustrated in our series [2]. In view of the various presentation of the condition, there is no standard protocol to diagnose the CNSi CLL, therefore it is the patient-based approach. For example, the diagnosis should be made with CSF study whenever applicable, CSF study was not done due to risk of herniation from a brain mass in one case report [4]. However, a biopsy was obtained which is considered a gold standard for the diagnosis in that patient with right temporal lobe [4] and in other different locations (brain mass, vertex subcutaneous tumor, orbital mass, vitrectomy, and peripheral nerve) as described in a retrospective cohort of 30 CLL patients with CNSi CLL [6]. Another interesting presentation with peripheral nervous system manifestations and the MRI brain and spine were unremarkable and the diagnosis was made by CSF study rather than biopsy [5]. There are some cases that the neurological symptoms improved significantly with corticosteroids [4], however in our case and another case report it was associated with partial and no clinical response respectively [5]. Moreover, MRI showed worsening previous lesions similar to our case. The radiological findings by MRI vary in this condition, it might reveal brain mass, hydrocephalus, leptomeningeal enhancement, and brainstem enhancement, less specific diffuse white matter fluid-attenuated inversion recovery (FLAIR) hyperintensities, or spinal cord infiltration\myelitis [6]. A frequent negative CSF test should always be considered. In a retrospective cohort of 30 CLL patients with CNS involvement, 47% of the patients experienced a delay between their first neurological manifestations and a positive CNS involvement diagnosis. The median delay was 6 months (range 1-90) and some patients [5] were diagnosed more than 1 year after their first symptoms (19, 22, 50, 86, and 90 months) [6]. A patient was diagnosed with Sjogren syndrome based on the diagnostic tests’ findings and the fact that autoimmune disorders are common in CLL cases. The neurological symptoms improved temporarily with corticosteroid and that warranted reinvestigation. Similar to our patient, the third CSF flow cytology test revealed CLL cells, and the patient was started on chemotherapy and responded very well to it without any relapse [5]. To date, there is no proven treatment recommended for CLL patients with CNS involvement. There are multiple methods of treating the conditions with traditional chemotherapies and radiation with a significant clinical improvement that was mentioned in case reports and case series [4,5]. In our case, the patient received Ibrutinib at a standard as a monotherapy. Recent studies have shown a clear benefit of ibrutinib treatment in CNSi CLL. Ibrutinib is a Bruton tyrosine kinase inhibitor that acts by blocking B-cell antigen receptor signaling and changing the tumor microenvironment, thereby reducing the malignant proliferation of B cells and inducing apoptosis [7]. The clinical results following using this medication and the drug level that was found in high concentration in CSF samples of patients who are on it support the fact that ibrutinib penetrates the blood-brain barrier [5,6]. The CSF penetration was 21-100% for ibrutinib [6]. Using it showed rapid shrinkage of bulky lymphadenopathy and splenomegaly, and resolution of constitutional symptoms as reported in one case. It was associated with either complete remission or partial response defined as the disappearance of neurological symptoms, CSF clearance, normalization to the radiological findings after a few months of using it (after 4 and 6 months) [6], and no relapse that improves the patient’s quality of life [3,5-7].
Conclusion
CNSi CLL is rare and there is a usual delay in diagnosis due to its variable manifestations, challenging diagnosis process, and possible misdiagnosis with a mimicker condition which delays the treatment. The presence of neurological symptoms should prompt consideration of an active CLL disease. The diagnosis of clinically significant CNSi CLL is based on positive CSF analysis [2] with or without image evidence of brain tumor and tissue biopsy (when CSF analysis is negative or not performed) [2,4,9]. In case of inconclusive work up, CSF analysis should be repeated testing for cytology and flow cytometry\immunophenotypes as the false-negative results are common [4,5]. Our patient had an active CLL proved in his investigations, and the fact that the patient responded very well to the new chemotherapy should alert the diagnosis of CNS involvement by CLL and directs towards repeating investigations and introducing aggressive treatment strategies to target both hematological and neurological complications of the condition by a systemic immuno-chemotherapy. Treatment options with complicated backgrounds are more challenging. Ibrutinib monotherapy is efficient alternative chemotherapy as it crosses the blood-brain barrier and has shown a favorable clinical, biological and radiological outcome. The prognosis of underlying CLL is poor as CNSi appears mostly in late-stage disease and could be the only sign of progression of CLL [4]. That is why CNS involvement is always an indication to start specific therapy [4,8].
Acknowledgment
We are thankful to the patient for consent to publish his case. The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing Interests
None.
Author’s Contribution
Dr. Sara Alshamali is the main author and Dr. Jihad Inshasi is the treating physician of the patient and he revised the manuscript.
References
2. Strati P, Uhm JH, Kaufmann TJ, Nabhan C, Parikh SA, Hanson CA, et al. Prevalence and characteristics of central nervous system involvement by chronic lymphocytic leukemia. Haematologica. 2016 Apr;101(4):458.
3. Szczepanek D, Wąsik-Szczepanek E, Szymczyk A, Wach M, Cioch M, Podhorecka M, et al. Central nervous involvement by chronic lymphocytic leukaemia. Neurologia i Neurochirurgia Polska. 2018;52(2):228-34.
4. Wanquet A, Birsen R, Bonnet C, Boubaya M, Choquet S, Dupuis J, et al. Management of central nervous system involvement in chronic lymphocytic leukaemia: a retrospective cohort of 30 patients. British Journal of Haematology. 2017 Jan;176(1):37-49.
5. Nakanishi T, Ito T, Fujita S, Satake A, Konishi A, Hotta M, et al. Refractory chronic lymphocytic leukemia with central nervous system involvement: a case report with literature review. Journal of Blood Medicine. 2020;11:487.
6. Tam CS, Kimber T, Seymour JF. Ibrutinib monotherapy as effective treatment of central nervous system involvement by chronic lymphocytic leukaemia. British Journal of Haematology. 2017 Mar;176(5):829-831.
7. Lotze TE, Chadwick DJ. Acute disseminated encephalomyelitis (ADEM) in children: Pathogenesis, clinical features, and diagnosis.
8. Peter E, Roux M, Lachenal F. Chronic lymphocytic leukemia with central nervous system infiltration versus CLL-associated auto-immune disease with neurological involvement: A tricky differential diagnosis. Rev Neurol (Paris). 2020;176:120-3.
9. Wanquet A, Birsen R, Lemal R, Hunault M, Leblond V, Aurran-Schleinitz T. Ibrutinib responsive central nervous system involvement in chronic lymphocytic leukemia. Blood, The Journal of the American Society of Hematology. 2016 May 12;127(19):2356-8.