Abstract
Objective: Data on umbilical vein varix (UVV) and associated perinatal outcomes are sparse.
Study Design: Retrospective cohort study from a single institution using a standard care plan for pregnancies affected by UVV from 2012-2021. Univariate and multivariate analyses were utilized as appropriate with intent to stratify UVV by isolated cases without anatomic defects or abnormal aneuploidy screening/testing vs. non-isolated cases.
Results: 132 pregnancies with fetal UVV were included. Among these,131 livebirths occurred. Mean gestational age at delivery of the livebirths was 36 weeks and 6 days (SD 1.7 weeks). 51 (38.9%) livebirths required NICU admission, which occurred more commonly those preterm (58.1% preterm vs. 25.0% term, respectively; OR 4.17 95 CI 1.98, 8.80). NICU admission was not affected by size of UVV or presence of filling defect (NS). There were no perinatal deaths among pregnancies affected by isolated UVV; however, among the 33 pregnancies affected by non-isolated UVV, there were 2 (6.1%) perinatal deaths.
Conclusion: Non-isolation of UVV was predictive of poor perinatal outcome, whereas size of UVV and presence of a filling defect was not. Evidence to justify antenatal testing and 37-week delivery remains insufficient in isolated UVV.
Keywords
Umbilical vein varix, Perinatal mortality, Antenatal testing, Prenatal diagnosis, Adverse pregnancy outcome, Prenatal diagnosis
At a Glance
A. Why was this study conducted? Current recommendations for delivery of all pregnancies affected by umbilical vein varix (UVV), isolated or not, at 37 weeks are not well-supported by published data and needs to be evaluated further.
B. What are the key findings? Stratification by isolated UVV versus non-isolated UVV is helpful in determining fetuses at risk for poor outcomes. UVV size or presence of filling defect does not affect perinatal outcomes.
C. What does this study add to what is already known? Data from a single center and with care delivered in a uniform approach builds on recommendations from prior publications to identify risk factors for poor perinatal outcomes among pregnancies affected by UVV.
Introduction
Umbilical vein varix (UVV) is a dilation of the intra-abdominal portion of the umbilical vein that affects 0.1% of livebirths having prenatal ultrasounds [1-7]. The intra-abdominal portion of the umbilical vein typically measures about 2 mm at 15 weeks gestational age and increases proportionately to achieve an upper limit of normal diameter of 8 mm at term [7]. When exceeding 9 mm, or >50% the diameter of intrahepatic portion of the vein, an intra-abdominal dilation of umbilical vein meets criteria for UVV [8-10]. UVV is differentiated from other intra-abdominal masses, such as fluid-filled intra-abdominal cysts or enteric duplication cysts) using color Doppler interrogation [11].
UVV has been associated with structural anomalies, fetal growth restriction (FGR), aneuploidy, hydrops fetalis, and intrauterine fetal demise (IUFD) [9-15]. To date, published collections of data sets have consisted of small retrospective cohorts and heterogeneous approaches to management. Consequently, no clear guideline exists for management of pregnancies affected by UVV [1-17].
The aim of this study was to characterize perinatal outcomes among pregnancies affected by UVV at a single institution with a uniform clinical management approach. Additionally, perinatal outcomes among pregnancies affected by isolated and non-isolated UVV were compared.
Methods
This retrospective cohort study examined pregnancies affected by UVV between January 1, 2012 and December 31, 2021 evaluated by Wake Forest University Physicians (WFUP). This study was approved by the institutional review board of Wake Forest University (IRB00045630). During the study period, WFUP served as a tertiary referral group in northwest North carolina and performed over 3200 deliveries annually. WFUP provided prenatal care, ultrasound evaluation, and delivery for all maternal-fetal pairs included in the study.
Diagnosis and measurements
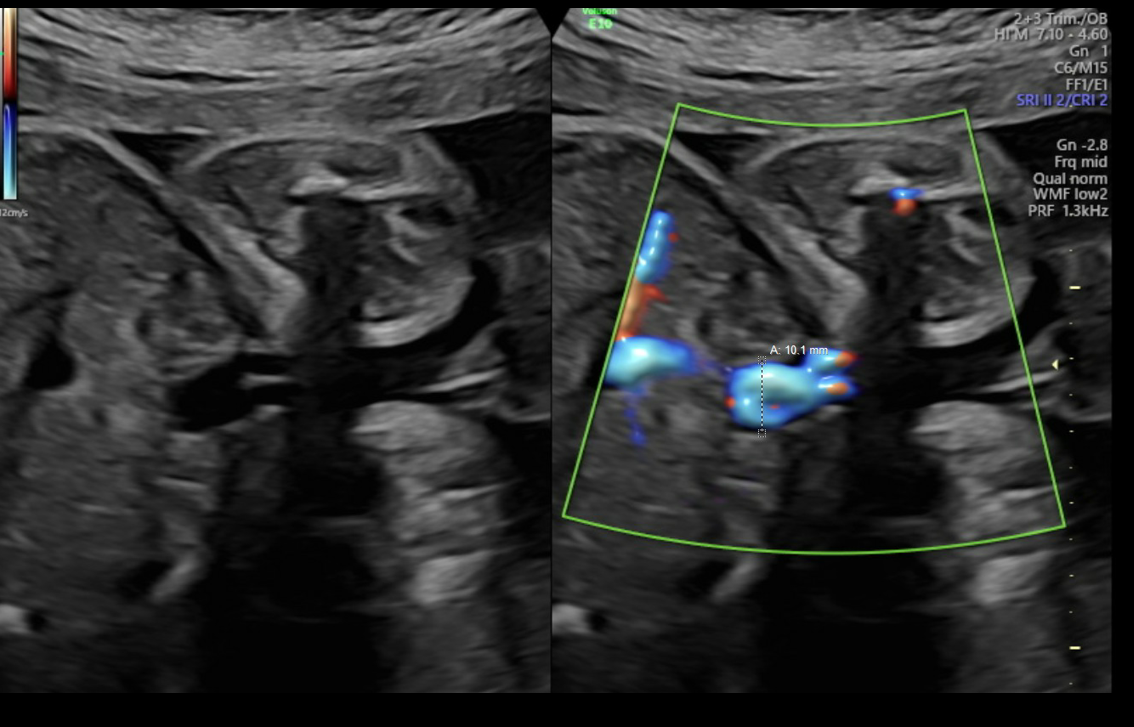
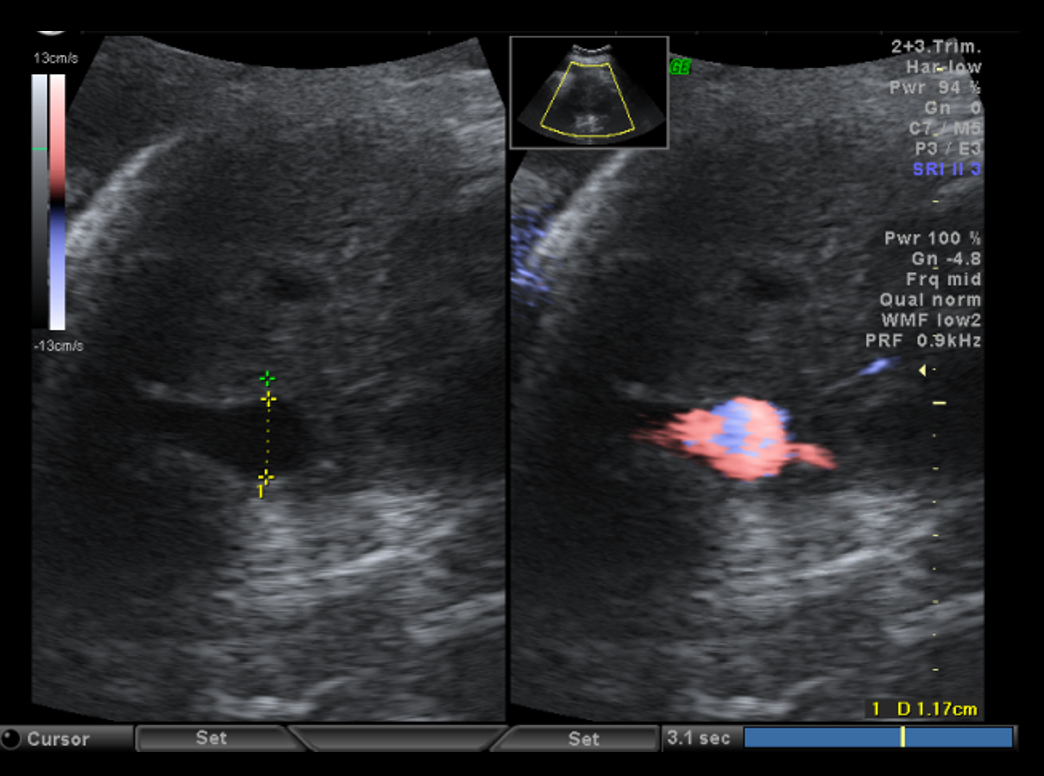
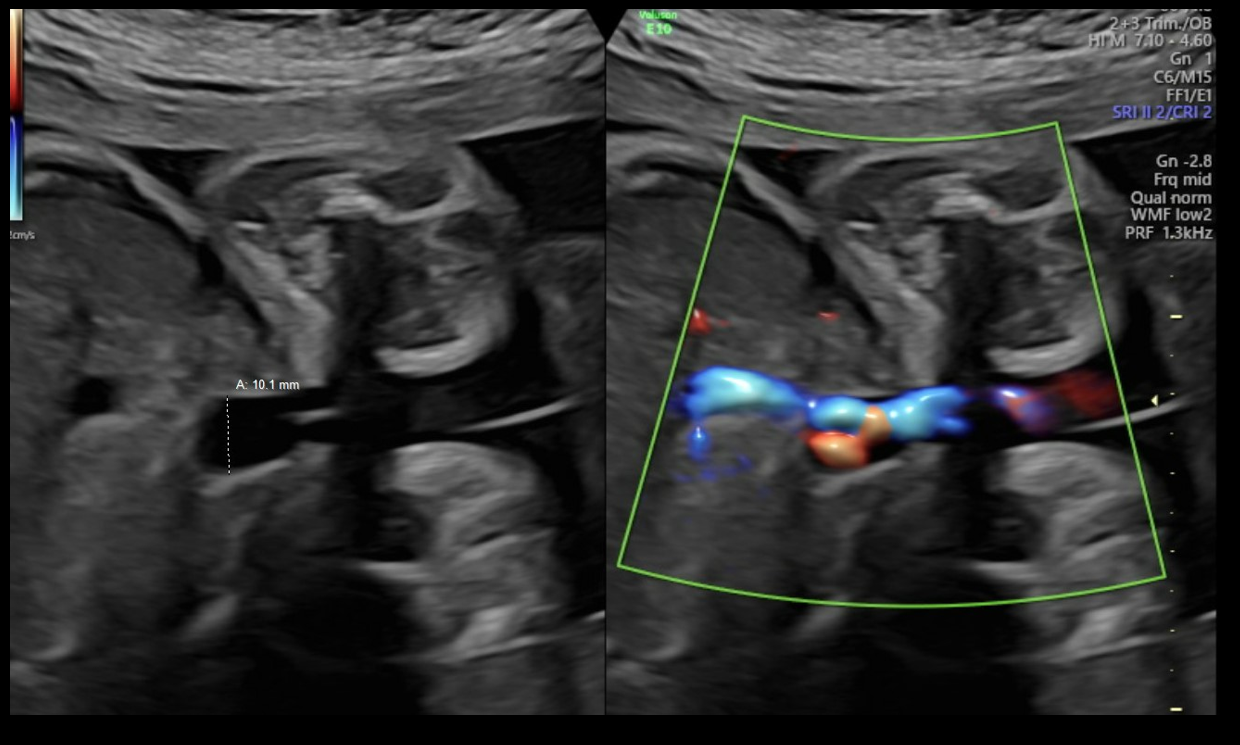
Diagnostic criteria for UVV and the WFUP protocol for subsequent evaluation and surveillance is described in (Table 1) and was followed for all maternal-fetal pairs. UVV characteristics documented during each ultrasound included: size, directionality of blood flow (unidirectional or bidirectional), presence of turbulence, and presence of a filling defect; see (Figures 1a-1c).
|
DIAGNOSTIC CRITERIA |
|
|
UPON DIAGNOSIS |
|
|
GROWTH ULTRASOUNDS |
|
|
ANTENATAL TESTING |
|
|
DELIVERY TIMING |
|
Figure 1a. Unidirectional or nonturbulent flow without filling defect.
Figure 1b. Bidirectional or Turbulent flow without filling defect.
Figure 1c. Bidirectional flow with suspected filling defect.
Filling defects were recorded when sonolucent absence of color flow was demonstrated with color Doppler within the UVV. Filling defects were interrogated in transverse and sagittal abdominal views along with color Doppler sweeps to confirm the filling defect and overcome the artifact loss of signal with flow perpendicular to insonation. The presence of UVV and its characteristics was confirmed on repeat ultrasound within 7 days while having monitoring in the interim for fetal well-being. All recorded fetal sonographic measures were uniformly collected by WFUP’s American Institute of Ultrasound in Medicine (AIUM) accredited ultrasound unit by a Registered Diagnostic Medical Sonographer. Voluson E10 General Electric ultrasound machines, with abdominal probe 4-8 and 4 mHz frequency, were used. All WFUP patients are offered genetic counseling and genetic screening as appropriate, including this study’s participants.
Data collection
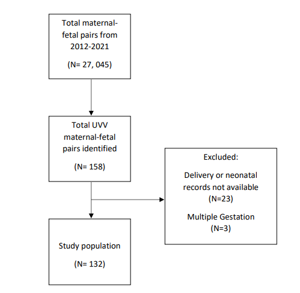
Data were abstracted from the ultrasound database (AS-OBGYN, AS Software, Englewood Cliffs, NJ) [18] and Epic Systems Corporation (Verona, WI), and managed using REDCap software (Vanderbilt University, Nashville, TN). Maternal-fetal pairs affected by UVV with a singleton gestations and complete delivery and neonatal records were included. A total of 27,045 maternal fetal pairs were identified in the study time period. Among these, 158 were affected by UVV and 132 were included in the final analysis; exclusion criteria included multiple gestation (n=3) and incomplete delivery or neonatal records (n=23).
Pertinent sonographic markers were tracked as follows: varix size in millimeters (mm), directionality of flow, presence of turbulence, presence of filling defect of the UVV, hydrops, any structural defect(s), marker(s) of aneuploidy, and fetal weight. Fetal weight changes were monitored by the Hadlock curve [19]. The primary exposure was isolated versus non-isolated UVV. Maternal-fetal pairs were determined to have isolated UVV in the absence of other structural defects or markers of aneuploidy. Non-isolated UVV was defined as the presence of UVV and other structural defects or markers of aneuploidy.
Primary outcomes were neonatal intensive care unit (NICU) admission and perinatal mortality defined as either intrauterine fetal demise (IUFD) or neonatal death within 30 days of delivery. Secondary outcomes included admission for fetal monitoring due to filling defect, mode of delivery, and delivery for fetal distress.
Statistics and data
All analyses were conducted using STATA statistical software (Version 15.1, College Station, TX [20]). Baseline characteristics were summarized with frequencies and proportions for categorical data and mean with standard deviation (SD) for continuous data. Continuous outcome data were evaluated by Student T tests, as appropriate. Differences between dichotomized groups were evaluated with Chi-square or Fisher’s exact tests, as appropriate. Multivariable logistic regression was used to estimate adjusted odds ratios (aOR) and corresponding 95% confidence intervals (95% CI), controlling for gestational age, maternal age, body mass index (BMI), and parity.
Results
A total of 27,045 maternal-fetal pairs were delivered by WFUP during the study time period. Among these, 158 were affected by UVV and 132 were included in the final analysis; maternal-fetal pairs with multiple gestation (n=3) and incomplete delivery or neonatal records (n=23) were excluded. Flow of enrollment is detailed in Figure 2. In total, 99 (75.0%) maternal-fetal pairs had isolated UVV and 33 (25.0%) had non-isolated UVV (see Tables 2 and 3, respectively). All maternal-fetal pairs in the non-isolated UVV group had other structural defects or markers of aneuploidy identified on ultrasound, and two also had abnormal non-invasive prenatal screening (NIPS), including trisomy 21 (n=1) and triploidy (n=1). Of note, 8 of the 132 (6.1%) maternal-fetal pairs had an abnormal initial aneuploidy screen, followed by negative NIPS, and no other ultrasound findings of structural defects, and these were included in the isolated UVV group. No patients underwent amniocentesis for confirmatory karyotyping. A livebirth occurred in 131 of 132 (99.2%) maternal-fetal pairs; the suspected triploid fetus demised prior to delivery. This fetus had several abnormal ultrasound findings (see Table 3). Following joint counseling emphasizing that this was a life-limiting or lethal condition by Neonatology, Genetics, and WFUP, the patient elected for expectant management until spontaneous delivery or maternal indication for delivery occurred. At 36 weeks 3 days on interval growth evaluation, the fetus was identified to have met criteria for hydrops and was demised. Table 4 lists aneuploidy testing and birth outcome data.
Figure 2. Enrollment Flow Chart.
|
CHARACTERISTIC (N=132) |
VALUE (STD/STE) |
|
Maternal age (years) |
28.4 (7.2) |
|
Gravidity |
3.0 (2.0) |
|
Parity |
1.8 (1.3) |
|
Gestational age at delivery (weeks) |
36.6 (1.7) |
|
Caucasian (%) |
88 (66.7) |
|
African-American Race (%) |
17 (13.3) |
|
Hispanic/Latino (%) |
27 (21.1) |
|
CHARACTERISTIC (N=132) |
VALUE (%) |
|
Varix size in mm (STD) |
11.7 (2.1) |
|
Area suspicious for filling defect requiring monitoring/reevaluationa |
32 (25) |
|
Non-isolatedb |
33 (25) |
|
Abnormal genetic screeningc |
10 (7.9) |
|
Abnormal NIPS |
2 (1.5) |
|
FGRd |
10 (7.6) |
|
IUFD (% total, %isolated, %non-isolated) |
1 (0.75, 0, 3) |
|
afilling defects were suspected when color flow Doppler on the varix demonstrated absence of color flow in a region of the varix that could be demonstrated by Doppler flow interrogation in more than 1 plane (eg, transverse, saggital) bnon-isolated: varix was seen in association with addition finding(s) on ultrasound (eg, structural defect, marker of aneuploidy) cfirst trimester screen or second trimester quad screen dfetal growth restriction (FGR): defined as <10th%’le for estimated fetal weight on Hadlock curve [19]. |
|
|
CHARACTERISTIC (N=132) |
VALUE (%) |
|
Admitted for monitoring |
34 (26.7) |
|
Delivery for non-reassuring testing + filling defect preterm |
8 (6.3) |
|
Spontaneous labor |
34 (25.8) |
|
UVV-indicated deliverya |
62 (48.8) |
|
Non-spontaneous birthb |
98 (74.2) |
|
Indicated preterm birth for preeclampsia |
2 (1.5) |
|
NICU admission |
23 (17.4) |
|
Neonatal Demise |
1 (0.7) |
|
aUVV-indicated: if spontaneous delivery or other obstetrical delivery indication did not arise, expectant management was not pursued past 37 weeks 0 days and all patients with UVV varix (both isolated UVV and non-isolated UVV) were scheduled for delivery at 37 weeks 0 days. bnon-spontaneous birth: all births induced or delivered by cesarean section for any reason leading to iatrogenic delivery (eg, preeclampsia, fetal growth restriction, UVV, non-reassuring testing) |
|
Mean gestational age at which betamethasone (BMZ) was administered was 31 weeks 5 days (SE 0.7 wks) when indicated for UVV filling defect or other obstetrical indication. 48 of 132 patients (36.4%) received BMZ given concern for potential preterm delivery resulting from fetal growth restriction (FGR) with abnormal Doppler studies, evidence of hydrops, suspicion of filling defect, or equivocal antenatal testing prompting physician to recommend heightened surveillance; monitoring in this setting, consisted of either extended monitoring or interval ultrasound in less than 7 days.
The majority (65.9%) of participants had a scheduled delivery at or shortly after 37 weeks with median delivery of 258 days (36 weeks 6 days) gestational age (GA), (IQR 256d, 259d). Spontaneous labor occurred in 25 of 132 (18.9%) prior to reaching scheduled delivery date for UVV at or shortly after 37 weeks. No patients were expectantly managed beyond the protocol recommendation, and there were zero iatrogenic protocol violations. 39 of 132 (29.5%) were delivered by cesarean section, not for UVV, but for usual obstetrical indications (eg, non-reassuring fetal heart tracing, labor dystocia). 3 of the 93 (3.2%) vaginal births were operative vaginal deliveries (2 vacuum-assisted and 1 forceps-assisted).
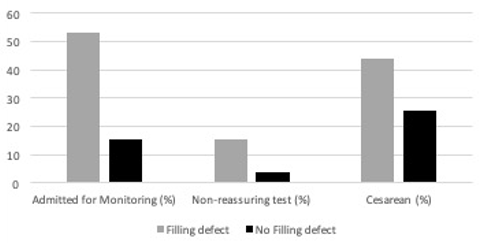
Among maternal-fetal pairs with liveborn fetuses, those with a filling defect of the UVV were more likely to be admitted inpatient for prolonged fetal monitoring (53.1% vs. 15.2%; OR 6.3; p<0.001; 95% CI 2.6, 15.4), delivered for non-reassuring fetal heart tracing or non-reassuring biophysical profile (BPP) (15.6% vs 4.0%; OR 4.4; 95% CI 1.1, 17.5), and delivered by cesarean section (43.8% vs. 24.3%, OR 2.3, p=0.049, 95% CI 1.001, 5.3); see Figure 3. Of note, among the 32 maternal-fetal pairs admitted for prolonged fetal monitoring, 24 demonstrated resolution of the filling defect and were discharged home after reassuring inpatient fetal monitoring.
Figure 3. Impact of filling defect.
Isolated UVV in absence of a filling defect were less likely than non-isolated UVV to have a stillbirth (0% vs 3.1%; OR 0.3, CI 0.23, 0.52, p=0.01). There was additionally one neonatal death among participants with non-isolated UVV. This fetus was identified as having the following findings: cerebellar hypoplasia, ventriculomegaly, dilated 4th ventricle, absent cavum septum pellucidum, cleft lip, cleft palate, gallstones, and bilateral absent radii. Delivery of this non-isolated UVV fetus occurred at 35 weeks 2 days weeks for spontaneous preterm labor (PTL). This neonate was admitted to NICU for comfort care, as selected by the parents, and demised on the first day of life.
NICU admission was required in 51 of 131 (38.9%) liveborn neonates as determined by the neonatology team at delivery. This occurred more commonly among preterm infants [32 of 55 (58.2%) preterm births admitted to NICU vs. 19 of 76 (25%) term births admitted to NICU; OR 4.17 95 CI 1.98, 8.80]. Outcomes of NICU admissions were as follows: Required initial respiratory support (n=29), surfactant administered (n=9), respiratory distress syndrome (RDS, n=0), bronchopulmonary dysplasia (BPD, n=0), necrotizing enterocolitis (NEC, n=1), intraventricular hemorrhage (IVH, n=2), and neonatal sepsis (n=5). No isolated UVV cases were noted to have any of the more severe morbidities associated with preterm delivery with respect to RDS, BPD, IVH, or NEC. None of the fetuses were admitted to NICU solely for UVV. Conversely, delivery at gestational age <34 weeks was a significant factor contributing to composite neonatal morbidity in the NICU as defined by having one or more of RDS, BPD, IVH, NEC or sepsis (4/7 vs. 01/44; OR 57.3, 95 CI 4.8, 687.6; p<0.01). When stratifying by NICU admission, filling defect, isolated UVV, and mean size of varix did not vary significantly; see Table 5.
|
|
|
Mean size of varix, mm (SD) |
p-value (T-test) |
|
NICU admission |
Admitted |
11.6 (2.2) |
0.56 |
|
Not Admitted |
11.9 (1.8) |
||
|
Filling defect |
Present |
11.8 (2.6) |
0.74 |
|
Absent |
11.6 (1.7) |
||
|
Isolated UVV |
Isolated |
11.6 (1.9) |
0.61 |
|
Non-isolated |
11.8 (2.7) |
Discussion
Principal findings
This study describes one of the largest single institution cohorts of maternal-fetal pairs receiving uniform care for management of pregnancies complicated by UVV.
Results in the context of what is known
Rate of IUFD, FGR, and aneuploidy were all lower than previous publications on UVV; such rates are even lower in the non-isolated UVV subset. In general, most previous publications featured smaller numbers of cases and none had a cohort managed via a uniform protocol. No prior studies or this study demonstrate adequately powered data to identify a significantly increased risk for IUFD with isolated UVV.
Clinical implications
Data from this retrospective cohort show that size of UVV and presence of filling defect did not affect perinatal outcomes. Prior case series have indicated the importance of identifying whether UVV is in isolation or not by ensuring detailed anatomic survey and aneuploidy testing as clinically appropriate [21].
Outcomes were overall reassuring in all cases of isolated UVV. Conversely, maternal-fetal pairs with non-isolated UVV will benefit from adherence to delivery at 37 weeks, as has previously been recommended; non-isolated UVV is associated with adverse outcomes for the fetus and neonate [21].
Research implications
Iatrogenic preterm or early term delivery may pose unnecessary risks due to fetal immaturity among maternal-fetal pairs with isolated UVV [21,22]. Accordingly, this study indicates the need for evaluation of management to term of maternal-fetal pairs with isolated UVV.
Strengths and limitations
There is no clinical control group available to determine the clinical effect of the approach used in this cohort study. However, use of a standard protocol for diagnosis and management is a strength of this study; effectively, this study yields descriptive data generated from a standardized care pathway employed at a single institution. Despite being one of the largest cohorts described, the small sample size is small overall due to low incidence of UVV in pregnancy. Additionally, these data are limited by potential for sampling error due to missing the diagnosis of UVV given WFUP is not the only practice performing anatomic surveys in the study region. Many umbilical vein varices may not be diagnosable until later in pregnancy when there may be no medical or obstetrical indication for ultrasound.
Retrospective cohort studies are habitually susceptible to selection bias. However, this study was designed to minimize such bias in two discrete ways. First, all cases of UVV diagnosed under the care of Wake Forest University Physicians were collected. During the study period, Wake Forest University was the only Maternal-Fetal Medicine practice providing care to a large catchment population in northwest North Carolina. Therefore, it is unlikely that recognized cases of UVV were diagnosed and managed elsewhere. Second, this study identified cases over a nine-year timeframe, which served to balance any unknown seasonal or point-exposure linked cases of UVV not yet described. Given these two strengths, these data serve to represent the targeted population during the 9-year time frame.
Despite minimizing selection bias, UVV diagnosis was also particularly susceptible to information bias given the condition was diagnosed via ultrasound. A retrospective chart abstraction precludes inadvertent probing in perceived high-risk populations; as such, abstracting relevant information should provide unbiased information. However, cases in certain populations, such as in women with a high BMI, may go under-diagnosed. Notwithstanding, such under-diagnosis was not specific to the study site and can be reasonable assumed to be representative of the current clinical practice throughout the United States. Lastly, all patients were given the same management, thus, limiting comparison and cause-and-effect type analysis.
Conclusions
Adverse outcomes appear to be limited to non-isolated UVV fetuses. These study data suggest recommendations regarding timing of delivery at 37 weeks due to isolated UVV without filling defect and reassuring fetal growth, amniotic fluid and antenatal testing may need to be reevaluated. Doppler poses risk for false positive “filling defect” leading to admissions for monitoring. While there are no definitive data or clear established guidelines on how to manage a suspected filling defect, this cohort highlights potential risks for harm and unnecessary costs and maternal anxiety in acting on suspected filling defects whether admission or delivery.
Acknowledgements
The authors gratefully acknowledge Christina Tulbert as our study coordinator in conjunction with use of services and facilities of Wake Forest School of Medicine Clinical and Translational Science Institute (CTSA) as funded by National Center for Advancing Translational Sciences (NCATS) and National Institutes of Health, through Grant Award Number 21. Likewise, this study was sponsored by Wake Forest University School of Medicine, Department of Obstetrics & Gynecology. Study data were collected and managed using REDCap electronic data capture tools.
Conflict of Interest Disclosure Statement
The authors have no conflicts of interest to report. Data accumulated from 2012-2019 were previously accepted for oral presentation at the American Institute for Ultrasound in Medicine (AIUM) Conference in New York, New York, March 2020 (Abstract/Report ID 770622).
Compliance with Ethical Standards
During preparation, authors complied with HIPAA guidelines to protect anonymity and confidentiality. This study was conducted in accordance with guidelines set forth by Wake Forest University School of Medicine Institutional Review Board (IRB). Informed consent for aneuploidy testing was obtained from individuals undergoing testing. Our Wake Forest University School of Medicine IRB policy was adhered to for de-identified radiographic images and data stored in REDCap.
Author Contributions
All authors participated in the conception, data collection and analysis, preparation, write-up, review and submission process of the manuscript with authorship assigned by level of contribution.
References
2. Sciaky-Tamir Y, Cohen SM, Hochner-Celnikier D, Valsky DV, Messing B, Yagel S. Three-dimensional power Doppler (3DPD) ultrasound in the diagnosis and follow-up of fetal vascular anomalies. Am J Obstet Gynecol. 2006 Jan;194(1):274-81.
3. Beraud E, Rozel C, Milon J, Darnault P. Umbilical vein varix: Importance of ante- and post-natal monitoring by ultrasound. Diagn Interv Imaging. 2015 Jan;96(1):21-6.
4. Fung TY, Leung TN, Leung TY, Lau TK. Fetal intra-abdominal umbilical vein varix: what is the clinical significance? Ultrasound Obstet Gynecol. 2005 Feb;25(2):149-54.
5. Valsky DV, Rosenak D, Hochner-Celnikier D, Porat S, Yagel S. Adverse outcome of isolated fetal intra-abdominal umbilical vein varix despite close monitoring. Prenat Diagn. 2004 Jun;24(6):451-4.
6. Fuster JS, Benasco C, Saad I. Giant dilatation of the umbilical vein. J Clin Ultrasound. 1985 Jun;13(5):363-5.
7. Mahony BS, McGahan JP, Nyberg DA, Reisner DP. Varix of the fetal intra-abdominal umbilical vein: comparison with normal. J Ultrasound Med. 1992 Feb;11(2):73-6.
8. Mankuta D, Nadjari M, Pomp G. Isolated fetal intra-abdominal umbilical vein varix: clinical importance and recommendations. J Ultrasound Med. 2011 Feb;30(2):273-6.
9. Byers BD, Goharkhay N, Mateus J, Ward KK, Munn MB, Wen TS. Pregnancy outcome after ultrasound diagnosis of fetal intra-abdominal umbilical vein varix. Ultrasound Obstet Gynecol. 2009 Mar;33(3):282-6.
10. Weissmann-Brenner A, Simchen MJ, Moran O, Kassif E, Achiron R, Zalel Y. Isolated fetal umbilical vein varix--prenatal sonographic diagnosis and suggested management. Prenat Diagn. 2009 Mar;29(3):229-33.
11. di Pasquo E, Kuleva M, O'Gorman N, Ville Y, Salomon LJ. Fetal intra-abdominal umbilical vein varix: retrospective cohort study and systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2018 May;51(5):580-85.
12. Novoa V, Shazly S, Ibirogba ER, Sutton L, Tonni G, Prefumo F, et al. Perinatal outcomes of fetal intra-abdominal umbilical vein varix: a multicenter cohort study. J Matern Fetal Neonatal Med. 2021 Oct;34(20):3393-96.
13. Rahemtullah A, Lieberman E, Benson C, Norton ME. Outcome of pregnancy after prenatal diagnosis of umbilical vein varix. J Ultrasound Med. 2001 Feb;20(2):135-9.
14. Ozek MA, Calis P, Bayram M, Karcaaltincaba D. Fetal intraabdominal umbilical vein varix: antenatal diagnosis and management. J Matern Fetal Neonatal Med. 2018 Jan;31(2):245-50.
15. Lee SW, Kim MY, Kim JE, Chung JH, Lee HJ, Yoon JY. Clinical characteristics and outcomes of antenatal fetal intra-abdominal umbilical vein varix detection. Obstet Gynecol Sci. 2014 May;57(3):181-6.
16. Novoa V, Shazly S, Ibirogba ER, Sutton L, Tonni G, Prefumo F, et al. Perinatal outcomes of fetal intra-abdominal umbilical vein varix: a multicenter cohort study. J Matern Fetal Neonatal Med. 2021 Oct;34(20):3393-96.
17. PJ KW, Soahey R, Byrne JL, Oh KY, Puchalski MD. Diagnostic imaging obstetrics. Salt Lake City: Amirsys Inc; 2005.
18. AS Medical Information Systems, www.as-software.com Ver 6.9425, copyright 1991-2010.
19. Hadlock FP, Harrist RB, Martinez-Poyer J. In utero analysis of fetal growth: a sonographic weight standard. Radiology. 1991 Oct;181(1):129-33.
20. StataCorp LP. College Station, TX: StataCorp LP; 2015. Stata Surv data Ref Man release. 2020;14.
21. Lallar M, Phadke SR. Fetal intra abdominal umbilical vein varix: Case series and review of literature. Indian J Radiol Imaging. 2017 Jan-Mar;27(1):59-61.
22. Wetta L, Tita AT. Early term births: considerations in management. Obstet Gynecol Clin North Am. 2012 Mar;39(1):89-97.