Abstract
Conventional chemotherapy is limited by systemic toxicity, poor tumor penetration, and drug resistance. While mechano-chemo therapy combining mechanical stimulation with chemicals shows promise, existing techniques are in the early stages. A bubble-free acoustofluidic platform using high-frequency surface acoustic waves to apply localized mechanical forces, named ChemoTAP, was developed. This tunable method uses high-frequency surface acoustic wave pulses (~9.67 MHz) to specifically activate mechanosensitive ion channels. This activation triggers calcium flow and cellular stress reactions, which enhance cisplatin-induced apoptosis in cancer cells by 1.78 times. Compared with traditional ultrasound-driven cavitation or sonoporation approaches, ChemoTAP provides accurate, non-invasive mechanical stimulation without the addition of microbubbles. It serves as a precise, non-invasive platform for mechano-chemotherapy, offering new possibilities for overcoming chemotherapy resistance in precision oncology. We believe that with further development, this platform will strengthen its technological foundation, paving the way for its application as a tunable and effective tool in mechano-chemotherapy.
Keywords
Mechano-chemotherapy, ChemoTAP, Cancer, Drug resistance
Introduction
For decades, traditional chemotherapy has served as a cornerstone in cancer treatment, which relies on cytotoxic drugs to eliminate cancer cells for therapeutic purposes. These chemotherapy drugs are designed to eliminate cancer cells through various mechanisms, including DNA intercalation [1,2], microtubule disruption [3], and inhibition of essential metabolic pathways [4,5]. Despite considerable advances in drug development, systemic toxicity, poor tumor penetration, and the inevitable emergence of drug resistance continue to challenge the efficacy and feasibility of conventional chemotherapies [6–11]. In response to these issues, researchers have proposed a novel strategy that utilizes mechanical stimuli to temporarily enhance cellular activity and membrane permeability in target cancer cells, thereby improving drug delivery and potency [12–14]. The convergence of mechanical force and chemical therapeutics—mechano-chemotherapy-represents a frontier in oncology with significant potential to overcome the limitations of conventional chemotherapy.
To date, various mechanical stimulation modalities have been explored, including magnetic stress, hydrodynamic shear, and ultrasonic cavitation [15–19]. While each has demonstrated potential, they are burdened by significant limitations. Magnetic approaches often contend with issues of off-target accumulation [20]. Microfluidic-based shear forces, while precise, are difficult to scale for clinical use [21–23]. Among existing techniques, conventional low-frequency ultrasound techniques, sonoporation, which rely on inertial cavitation of microbubbles [19,24,25]. While this technique has demonstrated considerable efficacy, ongoing debates persist regarding the biosafety of micro/nanobubbles and the risk of off-target activation in healthy tissues due to bubble diffusion [26,27]. The clinical field, therefore, is in pressing need of a mechanical stimulation platform that offers non-invasiveness, high spatial precision, and dynamic tunability to safely and effectively modulate cell and tissue responses.
To circumvent these issues, the authors of this study developed a bubble-free acoustofluidic stimulation method, the Chemotherapy-enhanced tunable acoustofluidic permeabilization (ChemoTAP) platform. Moving beyond the paradigm of cavitation, this innovative approach utilizes high-frequency (9.63 MHz) standing surface acoustic waves (SSAWs) generated by focused interdigital transducers (fIDTs), which leverages strong, highly localized acoustic radiation forces to directly exert mechanical stress on cell membranes, eliminating the requirement for microbubbles and their associated variability. Previous studies have indicated that cavitation effects diminish significantly when ultrasound frequencies exceed 3 MHz [19], effectively eliminating the possibility of bubble-induced sonoporation. We further confirmed the absence of microbubble formation during experiments through high-magnification microscopic observation. The fundamental operating principle involves the generation of resonant acoustic fields that create precisely controlled mechanical stresses on target cells, resulting in transient and reversible modulation of membrane properties without the collateral damage associated with inertial cavitation.
Core Working Principle of the Proposed Platform
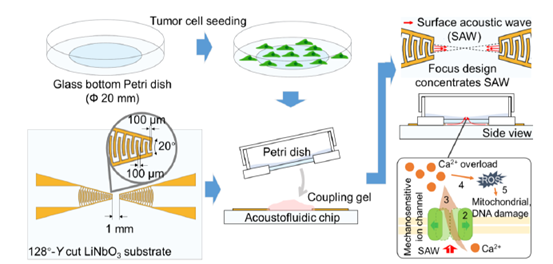
The ChemoTAP utilizes high-frequency SSAWs to operate a precise mechanotransductive stimulation to target tumor cells (Figure 1). To initiate localized and intense acoustic radiation forces capable of exerting direct mechanical stress on cellular membranes, we employed a pair of focused interdigital transducers with a 20° arc configuration (Figure 1). This specialized design concentrates acoustic energy into a highly focused region, enabling precise mechanical stimulation [28]. This stimulus reversibly activates mechanosensitive ion channels, triggering rapid ion influx that disrupts intracellular homeostasis. This microenvironment imbalance will cause reactive oxygen species (ROS), which subsequently induce functional impairment of organelles, including mitochondria, endoplasmic reticulum, nucleus, and so on [29–31], ultimately initiating the programmed cell apoptosis pathway (Figure 1). Conventional ultrasound strategies rely on high-power bulk acoustic wave pulses to physically disrupt cells, which may lead to damage to healthy tissue cells as well [17,27]. In contrast, the proposed ChemoTAP operates tumor cell killing through a fundamentally different mechanism. By selectively targeting mechanosensitive ion channels, the proposed method employs a more controllable method to either directly trigger apoptosis or synergistically enhance chemotherapeutic efficacy.
Figure 1. ChemoTAP operation process and mechanical stimulation pathway. Step 1: Surface acoustic wave (SAW) pulse stimulation; Step 2: The mechanosensitive ion channel is activated by SAW pulses; Step 3: Ca2+ overload; Step 4: intracellular ROS level is elevated; Step 5: High level ROS leads to mitochondrial damage, DNA disruption, and so on, which causes cell death.
Key Technological Advantage of the Proposed Platform
The core advantage of ChemoTAP lies in its exceptional tunability. By simply adjusting the input voltage and pulse duration of the SAW, the level of mechanical force applied can be precisely calibrated. In the work with HeLa cells, an optimal parameter set (90 Vpp, 250 ms) that maximized the permeabilization response was explored, inducing a 2.73-fold increase in intracellular calcium flux. This tunability is crucial, as it suggests the platform can be adapted to the unique "mechanophenotype" of different cancer types, which may express varying suites and sensitivities of mechanosensitive ion channels. To achieve this, different types of cancer cells can be separately seeded and cultured in glass-bottom Petri dishes. Leveraging the platform’s tunability, each cell type will receive SAW pulse stimulation with sweeping pulse voltages and duration. For mechanophenotypic characterization, we will continue to use the Fluo-4 AM fluorescence dye to measure calcium flux dynamics, supported by qRT-PCR to quantify the expression of mechanosensitive ion channels (MICs). This allows us to plot MIC activity changes vs SAW pulse duration/voltage curves across different cell types. Distinct cancer cell types may exhibit unique optimal SAW parameters for maximal responsiveness. We can generate "MIC activity-voltage" or “MIC activity-pulse duration” curves for each cell type—analogous to I-V curves—with distinct profiles expected across different cell lines. These curves will serve as the indicators for mechanophenotype properties for each cancer cell type.
The therapeutic efficacy of the ChemoTAP platform is rooted in its ability to engage specific molecular pathways through precisely controlled biomechanical stimulation. Through experiment, we have demonstrated that acoustic radiation forces directly activate mechanosensitive ion channels rather than causing non-specific membrane disruption. To support this conclusion, we utilized the gadolinium (Gd3+), a broad-spectrum inhibitor of mechanosensitive channels, to treat tumor cells. Being blocked by Gd3+, the viability of tumor cells being treated by the ChemoTAP platform was significantly increased. The essential role of specific ion channel activation distinguishes ChemoTAP from cavitation-based approaches that often cause non-selective membrane poration, highlighting the selectivity and biocompatibility of this acoustofluidic approach. This calcium-mediated signaling subsequently induces a measurable increase in intracellular ROS, creating oxidative stress that further propagates cell apoptosis. Concurrently, ChemoTAP stimulation induces mitochondrial membrane depolarization and endoplasmic reticulum stress. These coordinated effects disrupt cellular homeostasis and promote apoptosis.
Translational Potential of the Proposed Platform
The ChemoTAP platform holds particular promise for addressing some of the most challenging scenarios in clinical oncology. In this commentary, we extend our recent work for further discussion. Although the study successfully demonstrated that pulsed acoustic stimulation significantly activates mechanosensitive channel activities and enhances the intracellular delivery of chemotherapeutic agents, we identify two key areas for further investigation. First, beyond conventional solid tumors, ChemoTAP technology should be further developed for managing non-adherent tumor cells, such as circulating tumor cells (CTCs) [32]. To achieve this, the current ChemoTAP platform requires specific optimization for non-adherent cells. The first improvement will focus on the cell confinement system. The current experimental setup uses commercial glass-bottom Petri dishes to hold cells, but non-adherent cells will not stay in position under acoustic stimulation because of acoustic streaming. In addition, non-adherent cells distribute uniformly in the culture medium, particularly in the vertical direction. This means the SAW stimulation intensity will be attenuated when reaching cells located in the upper regions of the culture medium. A potential experimental setup should therefore focus on the design and integration of microfluidic channels within the Petri dish. By precisely tuning the dimensions of the microchannels, non-adherent cells can be restricted by the channel walls when passing through the acoustic field, ensuring they remain within the region of maximum acoustic intensity. Another promising experimental approach involves leveraging the siphon effect to construct a unidirectional fluidic pumping within the microchannel. This setup enables continuous, one-way flow of cells, preventing uneven stimulation. Our ongoing work to extend this research to circulating tumor cells represents a logical and potentially transformative application of the technology. The ability to target non-adherent cancer cells in circulation requires therapeutic approaches that can selectively sensitize tumor cells from the surrounding healthy tissues. Recent advances in acoustic technologies for biological manipulation provide encouraging precedent for this application, with researchers developing acoustic virtual 3D scaffolds that enable precise spatial organization of cells without physical substrates [33,34]. Such acoustic patterning techniques could potentially be integrated with ChemoTAP's permeabilization capabilities to create comprehensive platforms for flowing cells detection, trapping, and stimulation.
Another potential improvement lies in the in vivo therapy efficacy evaluation. While the in vitro efficacy of ChemoTAP is robust and compelling, the translation of this technology toward clinical impact requires thoughtful consideration of the necessary developmental pathway. The current acoustofluidic stimulation setup is designed for in vitro Petri dish cultures. Future work should transition from simple cell culture to various tumor models that accurately mimic the tissue architecture and microenvironmental conditions. Even though promising, transiting ChemoTAP to in vivo experiments is particularly challenging. First, current SAW stimuli are electrodes patterned on a rigid piezoelectric ceramic substrate. This rigid substrate restricts in vivo applications because it can neither be inserted into the body nor conform well to the surface of tumor tissues. Therefore, soft piezoelectric materials are crucially needed for in vivo translation. Secondly, the in vivo tumor microenvironment is much more complex than the in vitro Petri dish cultures. In vivo, tumor cells grow in a spheroid shape, which prevents acoustic wave penetration, therefore limiting the mechanical stimulation efficiency. Studies on quantifying acoustic attenuation in various tissue types will be essential for the in vivo translation. Refining dosage parameters for different anatomical locations and confirming the biocompatibility of the proposed acoustofluidic platform are potential research focal points when interacting with biological systems.
Conclusion
In conclusion, the ChemoTAP system represents a significant advance in the field of mechanobiology and oncology, providing a non-invasive, tunable, and highly effective approach for synergizing mechanical stimulation with chemotherapy. By enabling precise modulation of cell permeability through targeted activation of mechanosensitive ion channels, ChemoTAP significantly enhances cisplatin-induced apoptosis in cancer cells while maintaining exceptional control over mechanotransduction responses. Beyond its immediate applications, this technology offers key insights into the broader biological implications of acoustic stimulation, highlighting its potential for advancing targeted drug delivery, overcoming chemotherapy resistance, and enabling next-generation mechano-chemotherapy strategies. As research in this field progresses, the integration of acoustofluidic technologies with other therapeutic modalities promises to open new frontiers in precision oncology, potentially transforming our approach to some of the most challenging clinical scenarios in cancer management.
References
2. Agudelo D, Bourassa P, Bérubé G, Tajmir-Riahi HA. Intercalation of antitumor drug doxorubicin and its analogue by DNA duplex: structural features and biological implications. International journal of biological macromolecules. 2014 May 1;66:144–50.
3. Parker AL, Kavallaris M, McCarroll JA. Microtubules and their role in cellular stress in cancer. Frontiers in oncology. 2014 Jun 18;4:153.
4. McBride A, Garcia AJ, Sanders LJ, Yiu K, Cranmer LD, Kuo PH, et al. Sustained response to pembrolizumab in recurrent perivascular epithelioid cell tumor with elevated expression of programmed death ligand: a case report. Journal of Medical Case Reports. 2021 Jul 24;15(1):400.
5. Behranvand N, Nasri F, Zolfaghari Emameh R, Khani P, Hosseini A, Garssen J, et al. Chemotherapy: a double-edged sword in cancer treatment. Cancer immunology, immunotherapy. 2022 Mar;71(3):507–26.
6. Marin JJ, Romero MR, Blazquez AG, Herraez E, Keck E, Briz O. Importance and limitations of chemotherapy among the available treatments for gastrointestinal tumours. Anti-Cancer Agents in Medicinal Chemistry-Anti-Cancer Agents). 2009 Feb 1;9(2):162–84.
7. Tunggal JK, Cowan DS, Shaikh H, Tannock IF. Penetration of anticancer drugs through solid tissue: a factor that limits the effectiveness of chemotherapy for solid tumors. Clinical cancer research. 1999 Jun 1;5(6):1583–6.
8. Konstantinidis I, Tsokkou S, Gavriilaki E, Delis G, Papamitsou T. Protective Role of Key Micronutrients in Chemotherapy-Induced Organ Toxicity: A Comprehensive Review of Mechanistic Insights and Clinical Implications. Nutrients. 2025 Aug 31;17(17):2838.
9. Remesh A. Toxicities of anticancer drugs and its management. International Journal of Basic & Clinical Pharmacology. 2017;1:2–12.
10. Ramirez LY, Huestis SE, Yap TY, Zyzanski S, Drotar D, Kodish E. Potential chemotherapy side effects: what do oncologists tell parents?. Pediatric blood & cancer. 2009 Apr;52(4):497–502.
11. Mustapha A, Ismail A, Abdullahi SU, Hassan ON, Ugwunnaji PI, Berinyuy EB. Cancer chemotherapy: a review update of the mechanisms of actions, prospects and associated problems. BIOMED Nat Appl Sci. 2021;1(1):1–19.
12. Nagelkerke A, Bussink J, Rowan AE, Span PN. The mechanical microenvironment in cancer: How physics affects tumours. Seminars in Cancer Biology. 2015 Dec 1;35:62–70.
13. Zhang Y, Yu J, Bomba HN, Zhu Y, Gu Z. Mechanical force-triggered drug delivery. Chemical reviews. 2016 Oct 12;116(19):12536–63.
14. Mura S, Nicolas J, Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nature materials. 2013 Nov;12(11):991–1003.
15. Sim T, Choi B, Kwon SW, Kim KS, Choi H, Ross A, et al. Magneto-activation and magnetic resonance imaging of natural killer cells labeled with magnetic nanocomplexes for the treatment of solid tumors. ACS nano. 2021 Jun 24;15(8):12780–93.
16. Mitchell MJ, King MR. Fluid shear stress sensitizes cancer cells to receptor-mediated apoptosis via trimeric death receptors. New journal of physics. 2013 Jan 18;15(1):015008.
17. Shen Z, Shao J, Zhang J, Qu W. Ultrasound cavitation enhanced chemotherapy: In vivo research and clinical application. Experimental Biology and Medicine. 2020 Aug;245(14):1200–12.
18. Yin H, Hu X, Xie C, Li Y, Gao Y, Zeng H, et al. AT‐Cell Inspired Sonoporation System Enhances Low‐Dose X‐Ray‐Mediated Pyroptosis and Radioimmunotherapy Efficacy by Restoring Gasdermin‐E Expression. Advanced Materials. 2024 Jun;36(26):2401384.
19. Rich J, Tian Z, Huang TJ. Sonoporation: Past, present, and future. Advanced materials technologies. 2022 Jan;7(1):2100885.
20. Chenyang Y, Fang Y, Li S, Yuanyuan M, Stanciu SG, Zihou L, et al. Magnetically switchable mechano-chemotherapy for enhancing the death of tumour cells by overcoming drug-resistance. Nano Today. 2020 Dec 1;35:100967.
21. Chen NX, Ryder KD, Pavalko FM, Turner CH, Burr DB, Qiu J, et al. Ca2+ regulates fluid shear-induced cytoskeletal reorganization and gene expression in osteoblasts. American Journal of Physiology-Cell Physiology. 2000 May 1;278(5):C989–97.
22. Sharei A, Zoldan J, Adamo A, Sim WY, Cho N, Jackson E, et al. A vector-free microfluidic platform for intracellular delivery. Proceedings of the National Academy of Sciences. 2013 Feb 5;110(6):2082–7.
23. Yang SH, Kun SH, Min ZH, Hongjing LI, Jianhua QI. Effects of combined the fluid shear stress and tumor necrosis factor-α on cartilage phenotype in a dynamic microfluidic chip. Chinese Journal of Chromatography. 2017 Apr 8;35(4):458–65.
24. Shen Z, Shao J, Zhang J, Qu W. Ultrasound cavitation enhanced chemotherapy: In vivo research and clinical application. Experimental Biology and Medicine. 2020 Aug;245(14):1200–12.
25. Li M, Sankin G, Vu T, Jing Y, Zhong P, Yao J. Three-dimensional cavitation mapping in shock wave and laser lithotripsy. The Journal of the Acoustical Society of America. 2021 Oct 1;150(4_Supplement):A330-.
26. Yoo S, Mittelstein DR, Hurt RC, Lacroix J, Shapiro MG. Focused ultrasound excites cortical neurons via mechanosensitive calcium accumulation and ion channel amplification. Nature communications. 2022 Jan 25;13(1):493.
27. O’Reilly MA. Exploiting the mechanical effects of ultrasound for noninvasive therapy. Science. 2024 Sep 13;385(6714):eadp7206.
28. Zhong R, Yang S, Ugolini GS, Naquin T, Zhang J, Yang K, et al. Acoustofluidic droplet sorter based on single phase focused transducers. Small. 2021 Nov;17(46):2103848.
29. Lin H, Peng Y, Li J, Wang Z, Chen S, Qing X, et al. Reactive Oxygen Species Regulate Endoplasmic Reticulum Stress and ER‐Mitochondrial Ca2+ Crosstalk to Promote Programmed Necrosis of Rat Nucleus Pulposus Cells under Compression. Oxidative medicine and cellular longevity. 2021;2021(1):8810698.
30. Vietri M, Miranda MR, Amodio G, Ciaglia T, Bertamino A, Campiglia P, et al. The link between endoplasmic reticulum stress and lysosomal dysfunction under oxidative stress in cancer cells. Biomolecules. 2025 Jun 25;15(7):930.
31. Malla S, Neupane R, Sood S, Hussein N, Abou‐Dahech M, Terrero D, et al. Mitochondria as regulators of nonapoptotic cell death in cancer. MedComm. 2025 Aug;6(8):e70244.
32. Soffe R. Discontinuous dielectrophoresis enabled investigation of high shear stress flows on intracellular calcium signalling of non-adherent cells. Thesis, RMIT University; 2016.
33. Yuan Z, Lu C, Liu C, Bai X, Zhao L, Feng S, et al. Ultrasonic tweezer for multifunctional droplet manipulation. Science Advances. 2023 Apr 19;9(16):eadg2352.
34. Melde K, Kremer H, Shi M, Seneca S, Frey C, Platzman I, et al. Compact holographic sound fields enable rapid one-step assembly of matter in 3D. Science advances. 2023 Feb 8;9(6):eadf6182.