Abstract
Introduction: Treatment burden significantly impacts patient adherence and quality of life when it comes to chronic conditions requiring frequent medical interventions. Intravitreal injections are administered to over 20 million patients globally annually, yet the associated treatment burden remains poorly quantified in Irish healthcare settings. This study aimed to assess treatment burden and identify key predictors among patients receiving intravitreal injections for retinal conditions at a tertiary eye center in Ireland.
Materials & Methods A cross-sectional questionnaire-based study was conducted using a modified Treatment Burden Questionnaire (TBQ) alongside demographic and qualitative assessments. Eligible participants included adults undergoing intravitreal injection treatment, excluding first-time attendees and those with insufficient English proficiency. The TBQ assessed treatment demands, medical follow-up, and financial burdens on a 0-10 Likert scale (total range 0-130). Supplementary questions addressed travel logistics, costs, and service perceptions. Correlation analysis and multiple linear regression identified predictors of treatment burden.
Results We enrolled 73 participants (52.1% male, mean age 74.2 years). Most participants (74%) required transport assistance, with mean travel times of 54.9 minutes and average costs of €22.08 per appointment. The mean TBQ score was 34.1 (SD 21.71), with 13.7% experiencing high treatment burden (≥59). Parking difficulties affected 89.8% of respondents. Correlation analysis identified commute duration (r=0.400, p<0.001) and difficulty arranging transport (r=0.465, p<0.001) as the strongest predictors of treatment burden. Age, gender, and costs showed no significant associations.
Conclusions We demonstrate that transportation logistics are the primary drivers of treatment burden in Irish patients receiving intravitreal therapy, rather than demographic or financial factors. These findings highlight the urgent need for satellite treatment centers and enhanced transport support along with possible extended dosing intervals and bilateral same day injections to reduce patient burden and improve treatment sustainability.
Keywords
Intravitreal therapy, Burden of treatment, Age related macular degeneration, Diabetic macular oedema, Carbon footprint, Patient experience, Bevacizumab, Faricimab, Aflibercept, Treatment-related burnout
Introduction
Burden of treatment (BOT) is defined as the workload imposed by healthcare experienced by patients and its consequences on well-being [1]. Effective management of chronic disease often requires substantial effort and inflicts significant burden on patients. The Treatment Burden Questionnaire (TBQ) was developed to assess treatment burden across different conditions and treatment contexts. Higher TBQ scores have been shown to correlate with an increased risk of patients becoming overwhelmed and experiencing treatment-related burnout [2]. Treatment-related burnout refers to the physical and emotional exhaustion patients experience when the time, effort and energy needed to adhere to their treatment regimen become unsustainable, ultimately leading to disengagement from health services [2].
Diabetic retinopathy is the leading cause of blindness among the working-age population in industrialized countries [3], while age-related macular degeneration is the third most common cause of blindness worldwide [3]. Overproduction of VEGF (Vascular Endothelial Growth Factor) is implicated in the pathophysiology of both DMO (diabetic macular oedema) and nAMD (neovascular age related macular degeneration) [4]. Anti-VEGF intravitreal injections (IVIs) are a cornerstone in the treatment of both conditions, and it is estimated that over 20 million IVIs are administered globally on an annual basis [5].
VEGF production promotes neovascularization. In the eye, this leads to pathological vascular permeability and fragile new vessels prone to leakage and hemorrhage [6]. In AMD, these vessels invade the subretinal space causing fluid accumulation, oedema, and scarring that destroys macular photoreceptors, resulting in irreversible vision loss [6].
Inhibiting VEGF is therefore critical for preventing blindness globally. However, despite their efficacy, long-term intravitreal anti-VEGF regimens present significant challenges. Many patients, particularly those with nAMD, will undergo IVIs as frequently as every four weeks for many years. This frequency not only imposes logistical and emotional burden but also contributes substantially to environmental impact, primarily through travel-related emissions [7].
This study aimed to quantify the treatment burden associated with attending intravitreal injection appointments at the South Infirmary Victoria University Hospital (SIVUH) in Cork, Ireland. To our knowledge, this is the first study assessing treatment burden in an Irish cohort of IVI patients using a modified TBQ instrument.
Materials & Methods
A single-center, cross-sectional questionnaire-based observational study was conducted using the TBQ in conjunction with demographic and qualitative questions. The TBQ, validated for assessing treatment burden across various conditions, was modified to include items specific to intravitreal injections. The Treatment Burden Questionnaire (TBQ) was adapted to better reflect the experiences of patients receiving intravitreal injections. The following items were removed as they were not relevant to this treatment context: impact of self-monitoring (e.g. checking blood pressure or blood sugar), effort not to forget taking medication, burden of dietary changes, burden of physical activity, and impact of laboratory tests and other examinations. New items were added to capture injection-specific aspects of treatment burden, including the impact of anxiety prior to the injection, burden of finding parking nearby, and burden associated with commute time. Several items were modified for contextual relevance. The question regarding taste, shape, and size of tablets and/or annoyances caused by your injections was revised to discomfort caused by injections. Similarly, the item necessary precautions when taking medication was reworded as necessary precautions post-injection. The final adapted version comprised 13 items, compared to 15 items in the original TBQ.
Post-modification Cronbach’s alpha was tested which gave a result of 0.82 demonstrating good internal consistency. Responses were rated on a Likert scale from 0 (“not a problem”) to 10 (“a big problem”), yielding a total TBQ score ranging from 0 to 130.
Qualitative data was collected via supplementary questionnaire. Participants were asked about travel time, appointment frequency, need for assistance attending appointments, impressions of the injection service, potential improvements, parking facilities at SIVUH and stress associated with arranging transportation.
Eligible participants included adults undergoing intravitreal injection treatment at SIVUH who were capable of providing informed consent and completing the questionnaire. First-time attendees and individuals with insufficient English proficiency were excluded. First time attendees were excluded due to potential bias i.e. perceptions influenced by anticipation rather than actual experience. It was felt that first time attendees would not be able to accurately reflect on how the treatment affected their quality of life over time, therefore making their input less valuable for assessing cumulative burden.
Given the prevalence of visual impairment among participants, many required assistance from the research team to complete the questionnaire, while those with adequate vision did so independently. Ethical approval was obtained from the Clinical Research Ethics Committee of the Cork Teaching Hospitals (CREC) and all participants provided consent to participate in the study. This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments.
Following data collection, responses were anonymized and analyzed using correlation and multiple linear regression analyses. Records with missing data were excluded. Data transformations included:
- Age ranges were converted to midpoints.
- Commute times were standardized (e.g., "<10 mins" as 10 minutes, "30m-1h" as 45 minutes).
- Cost descriptions were converted to numeric Euro values, with non-specific terms like "unsure" treated as missing and "minimal" coded as 0 Euro.
A Python script (available on request) facilitated these transformations. Results were presented using histograms, correlation barplots, and variance explained plots as can be seen in Figures 1–3 respectively.
Pearson correlation coefficients were calculated to assess relationships between Burden of Treatment scores and continuous or binary variables. Statistical significance was established at p<0.05, with additional notation for highly significant correlations (p<0.001). For categorical variables (diagnosis, location, travel method, gender), mean burden scores were calculated for each group and compared descriptively.
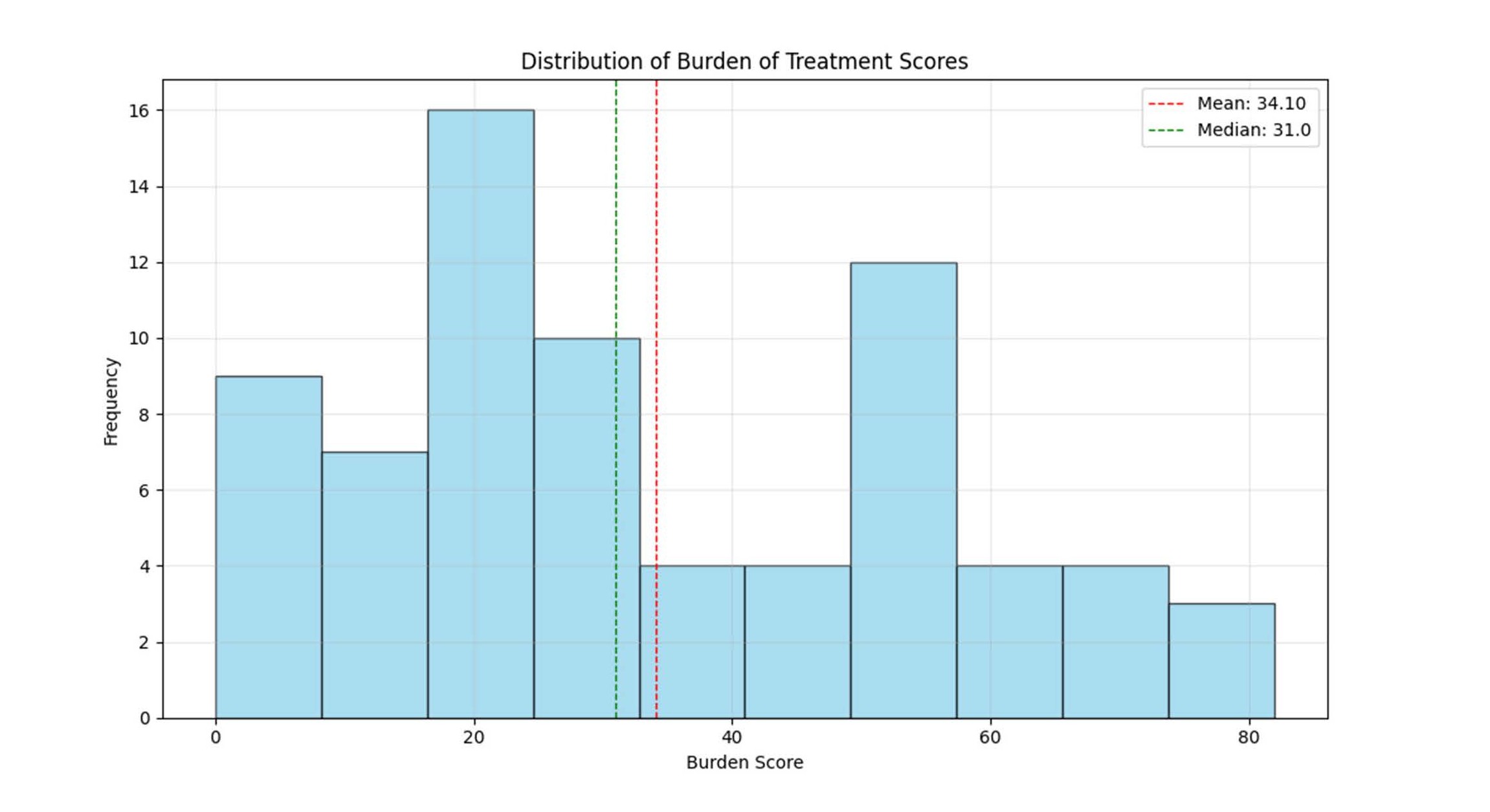
Figure 1. Distribution of burden of treatment Scores: Burden of treatment scores showed a right-skewed distribution, with most patients reporting low to moderate scores (mean 34.1, median 31.0).
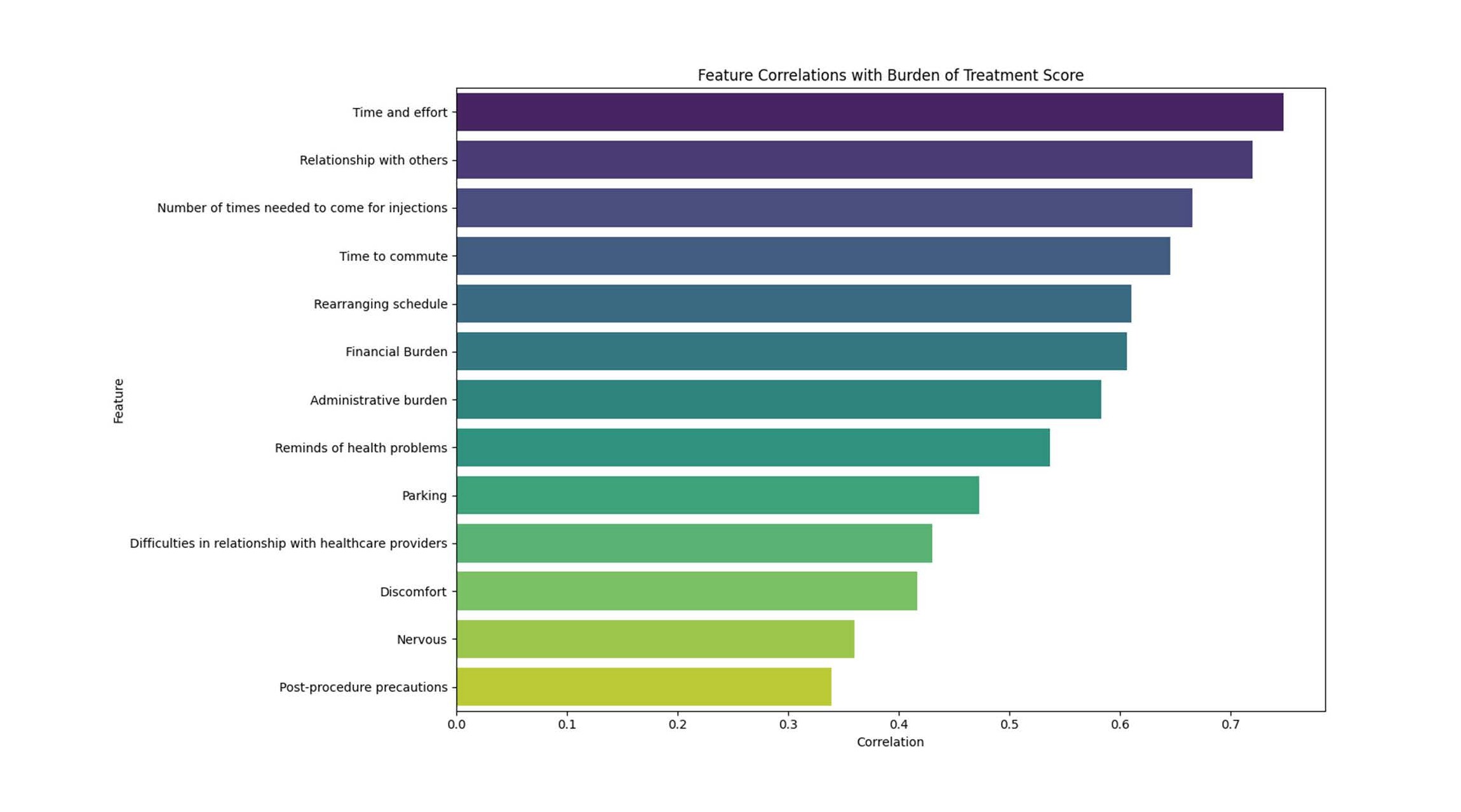
Figure 2. Feature correlations with burden of treatment score. Correlation with burden of treatment marked on x-axis. Each category is listed on the Y axis. Correlation analysis demonstrated that overall burden of treatment was most strongly associated with time and effort, frequency of injections, commuting, scheduling disruptions, financial burden, and impacts on relationships. In contrast, procedural discomfort, nervousness, and post-procedure precautions showed weaker associations, suggesting that logistical, social, and financial factors are the main drivers of treatment burden.
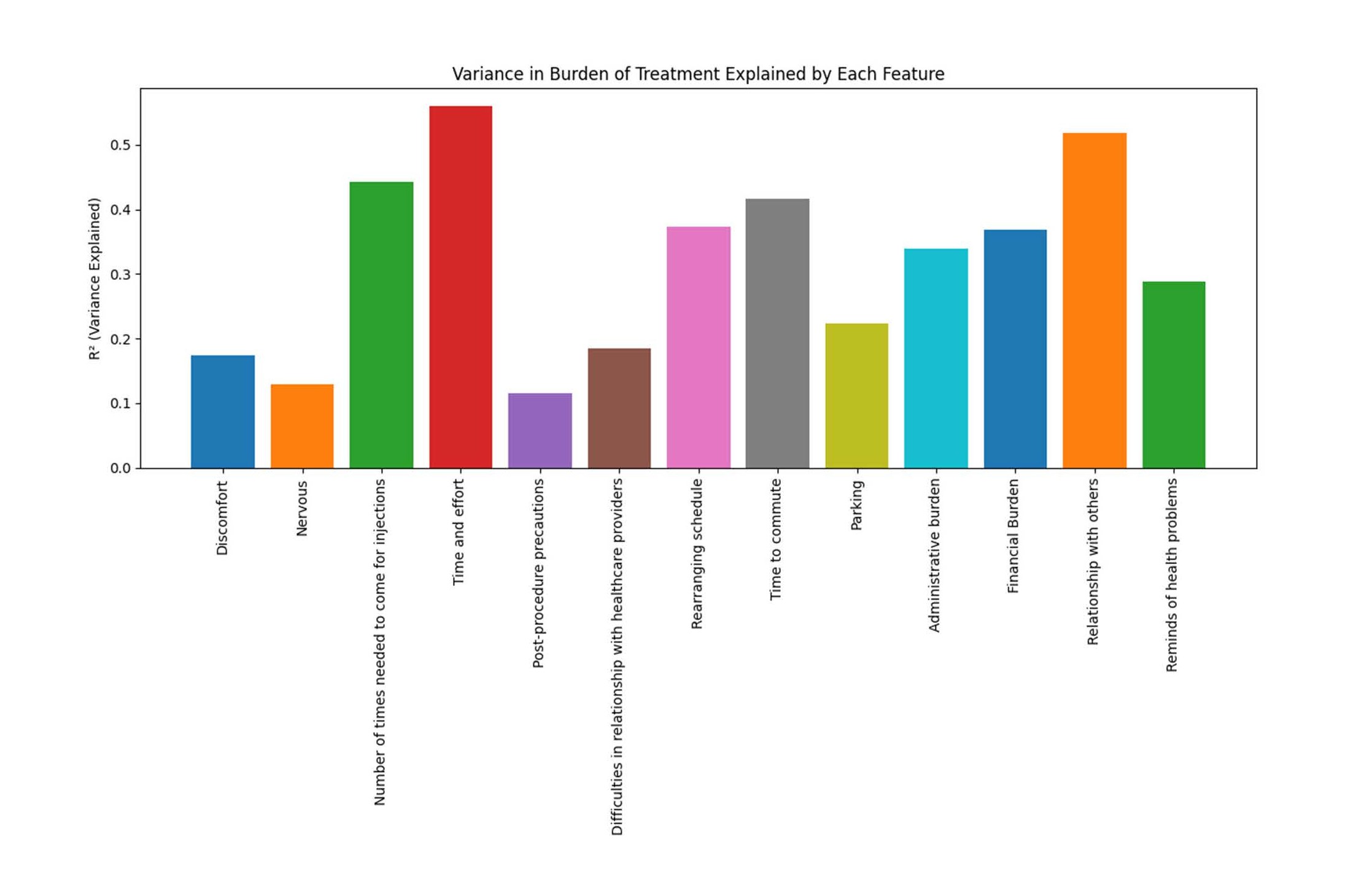
Figure 3. Variance in burden of treatment explained by each variable. Univariate variance explained in burden of treatment by each feature (bar height = R^2). Higher bars indicate stronger associations; bars are not additive and do not imply causation.
Results
A total of 73 participants were enrolled, comprising 38 males (52.1%) and 35 females (47.9%). The age distribution was as follows: 21 participants (28.8%) were aged 80–89 years, 26 (35.6%) aged 70–79, 10 (13.7%) aged 60–69, 9 (12.3%) aged 50–59, 1 (1.4%) aged 40–49, and 1 (1.4%) aged 26–29; one participant did not report their age.
Most participants (54/73, 74%) required assistance with transportation to appointments. Among those who did not require assistance, 14.3% used public transport, 5.7% attended on foot, and 2.8% drove themselves. The mean number of intravitreal injection appointments attended annually was 6.65 (range 2–12). Mean travel time was 54.9 minutes (range up to 120 minutes), and the average out-of-pocket cost per appointment was €22.08 (range €0–€170). One participant reported incurring €170 in taxi fares per visit.
The mean TBQ score was 34.1 (SD 21.71, range 0–82). Ten participants (13.7%) scored ≥59, indicating a high treatment burden. Some participants reported notable pre-procedural anxiety, with one describing difficulty sleeping prior to appointments.
Parking was identified as a major challenge: of 49 respondents, 44 (89.8%) reported difficulties and 26 (53.1%) rated parking as a “very big issue.” Self-reported diagnoses included age-related macular degeneration (39/73, 53.4%), diabetic macular oedema (5/73, 6.9%), retinal vein occlusion (2/73, 2.7%), and neovascular glaucoma (3/73, 4.1%). Twenty-one participants (28.8%) were unsure of their diagnosis.
Commute times were distributed as follows: <10 minutes (9.6%), 20 minutes (8.2%), 30 minutes (13.7%), 30–60 minutes (21.9%), 1–2 hours (31.5%), and >2 hours (13.7%).
Correlation analysis identified commute duration (r=0.400, p<0.001) and difficulty arranging transport (r=0.465, p<0.001) as the strongest predictors of TBQ score. Parking showed a non-significant positive association (r=0.223, p=0.058), age was not correlated (r=–0.024, p=0.842), and cost showed a weak, non-significant association (r=0.124, p=0.538).
Results of post-hoc power analysis may be seen in Table 1.
|
Variable |
Effect size |
p-value |
Power |
|
Age |
f = 0.394 (medium) |
0.050 |
0.72 |
|
Gender |
f = 0.111 (small) |
0.351 |
0.16 |
|
Diagnosis |
f = 0.159 (small) |
0.468 |
0.18 |
|
Treatment frequency |
r = 0.051 (very small) |
0.759 |
0.06 |
|
Travel method |
f = 0.242 (small) |
0.466 |
0.29 |
|
Length of commute |
f = 0.493 (large) |
0.016 |
0.88 |
|
Place of residence |
f = 0.167 (small) |
0.769 |
0.16 |
|
Availability of someone to bring |
f = 0.151 (small) |
0.225 |
0.23 |
|
Difficulty organizing transport |
d = 1.238 (large) |
0.002 |
1.00 |
|
Parking |
r = 0.299 (small) |
0.055 |
0.49 |
|
Exact cost |
r = 0.168 (small) |
0.230 |
0.23 |
|
Summary: Of all variables assessed, length of commute and difficulty organizing transport were both statistically significant and adequately powered, representing the most robust findings. |
|||
Discussion
This study indicates that transportation logistics, specifically commute duration and difficulty arranging transport, are the dominant contributors to treatment burden among patients receiving intravitreal injections at SIVUH. In contrast, demographic factors such as age and gender and financial considerations appeared to have a lesser impact. Parking difficulties emerged as an almost significant factor (r=0.223, p=0.0593), suggesting a larger sample size may have provided a significant result but further investigation is warranted.
The mean TBQ score among participants was 34.1 with a maximum score of 82 and a minimum score of 0. Previous studies have shown that patients with a score of greater than or equal to 59 are at risk of becoming overwhelmed and developing treatment-related burnout [2]. 10/73 participants had a TBQ score greater than or equal to 59 (13.70%). Thus suggesting 13.70% of participants in this study are at risk of treatment-related burnout.
The sample size (n=73) offered moderate statistical power. Larger studies may uncover additional associations and account for potentially complex or non-linear relationships between variables. Although a post-hoc power analysis suggested that the study had adequate power to detect stronger associations, smaller effects may have gone undetected. This underscores the need for larger, multi-center studies.
Further limitations include measurement limitations (e.g. categorical rather than continuous data) and potential response bias due to assistance during questionnaire completion along with the fact that the modified questionnaire was not validated and the absence of a control group or baseline TBQ scores in Irish patients and patients on different treatment regimes. This limits broader comparisons and limits generalizability.
Conversely, the real-time assistance provided to participants may also be listed as a strength of this study which enhanced data completeness and rigorous data standardization during analysis.
Almost one third (28.8%) of patients receiving regular intravitreal injections did not possess a clear understanding of or even the name of the condition for which they were receiving treatment. The uncertainty around diagnosis for such a large portion of patients may reflect a communication gap between clinician and patient and warrants further investigation. Such gaps may contribute to increased treatment burden and possibly affect the perceived value of ongoing therapy. This finding highlights a possible system-level issue in the delivery of ophthalmic care, where high procedural throughput and time constraints may inadvertently limit opportunities for effective patient-clinician dialogue.
All participants were receiving unilateral injections. Notably, patients requiring bilateral treatment often face even greater logistical challenges, attending appointments as frequently as every two weeks. Previous research from St. Michael’s Hospital in Toronto demonstrated that same-day bilateral anti-VEGF injections are safe and well-tolerated, with the potential to reduce burden for both patients and the healthcare system [8]. Fewer appointments could also reduce the carbon footprint associated with treatment, an increasingly important consideration as IVIs become more widespread [9].
A previous Irish study found travel to appointments accounted for 77% of carbon emissions associated with IVIs [10]. The study proposed that longer acting agents offered the greatest future potential for meaningful CO2 reductions. With that said, it would follow that same day injections (thus resulting in fewer appointments) would also result in reduced carbon emissions.
Emerging therapies offer further avenues for burden reduction and reduced carbon emissions. The TENAYA and LUCERNE trials demonstrated that faricimab, administered every 16 weeks, provided outcomes comparable to aflibercept dosed every 8 weeks, highlighting the potential for extended dosing intervals without compromising efficacy [11]. Additionally, aflibercept, administered at an 8mg dose, also offers the potential for significantly extended durations when compared with bevacizumab [12].
This study was compared to other studies in the literature [13,14]. When compared with the QUALITII study (Questionnaire to Assess Life Impact of Treatment by Intravitreal Injections) (Houston, Texas 2021) [12], our study highlighted logistical challenges faced by patients as major TBQ contributors whereas the QUALITII study identified anxiety, pain and recovery time as more impactful factors in terms of treatment burden. An Australian study on the BOT among patients undergoing IVIs for diabetic macular oedema found that direct medical costs and time burden on carers were the main contributors to the BOT [14]. All three studies underscore the importance of patient centered treatment planning, extended dosing intervals, support for transportation and caregiver involvement and patient education. The differences in TBQ contributors may reflect that treatment burden is shaped not only by the procedure itself but by the broader social, economic, cultural and healthcare environment in which treatment occurs. The South Infirmary Victoria University Hospital is the only public center providing IVIs to the Cork and Kerry region (which has a population of 740,000 people). This study highlights the urgent need to establish satellite IVT treatment centers, particularly in the Kerry area.
Conclusion
This study provides valuable insights into the experiences of patients undergoing intravitreal injection therapy at a tertiary center in Ireland. While anti-VEGF treatment offers clear clinical benefits, the associated burden, particularly regarding transportation logistics, is substantial. Commute time and difficulty arranging transport emerged as the strongest predictors of treatment burden, whereas age and financial costs were less influential.
Qualitative responses further underscored the emotional and financial toll, with one patient reporting sleeplessness before appointments and another incurring €170 per visit for transport—highlighting the hidden costs borne by patients.
Addressing this burden will require multifaceted solutions, such as possibly enabling bilateral same-day injections, adopting extended-interval therapies, exploring mobile injection services and improving hospital accessibility through enhanced parking and transport support. As demand for intravitreal injections continues to grow, integrating patient-centered approaches will be essential not only to preserve vision but also to support patient well-being and the sustainability of healthcare delivery.
Conflicts of Interest
Mr. Eamonn O’Connell, Drs. Daniel Coakley and Ann Marie O’Leary declare no conflict of interest related to this work.
Funding Statement
The authors received no funding or sponsorship for the research, authorship, and/or publication of this article.
Acknowledgments
We are grateful to the individuals and organizations whose support shaped the direction and quality of this work. Their guidance, feedback, and patience—whether in the clinic, during data collection, or in the quieter moments of drafting—made it possible to carry this project through to completion. I especially appreciate the participants who generously shared their time and experiences, as well as the colleagues and mentors who offered insight when the path forward felt uncertain.
References
2. Tran VT, Montori VM, Ravaud P. Is My Patient Overwhelmed?: Determining Thresholds for Acceptable Burden of Treatment Using Data From the ComPaRe e-Cohort. Mayo Clin Proc. 2020 Mar;95(3):504–12.
3. Yorston D. Anti-VEGF drugs in the prevention of blindness. Community Eye Health. 2014;27(87):44–6.
4. Deng Y, Qiao L, Du M, Qu C, Wan L, Li J, et al. Age-related macular degeneration: Epidemiology, genetics, pathophysiology, diagnosis, and targeted therapy. Genes Dis. 2021 Feb 27;9(1):62–79.
5. Martin DF. Evolution of Intravitreal Therapy for Retinal Diseases-From CMV to CNV: The LXXIV Edward Jackson Memorial Lecture. Am J Ophthalmol. 2018 Jul;191:xli–lviii.
6. Ambati J, Fowler BJ. Mechanisms of age-related macular degeneration. Neuron. 2012 Jul 12;75(1):26-39.
7. Chandra P, Welch S, Oliver GF, Gale J. The carbon footprint of intravitreal injections. Clin Exp Ophthalmol. 2022 Apr;50(3):347–9.
8. Juncal VR, Francisconi CLM, Altomare F, Chow DR, Giavedoni LR, Muni RH et al. Same-Day Bilateral Intravitreal Anti-Vascular Endothelial Growth Factor Injections: Experience of a Large Canadian Retina Center. Ophthalmologica. 2019;242(1):1–7.
9. Ong AY, Birtel J, Charbel Issa P. Greener intravitreal injections: a narrative review. Eye (Lond). 2024 Oct;38(15):2874–9.
10. Power B, Brady R, Connell P. Analyzing the Carbon Footprint of an Intravitreal Injection. J Ophthalmic Vis Res. 2021 Jul 29;16(3):367–76.
11. Heier JS, Khanani AM, Quezada Ruiz C, Basu K, Ferrone PJ, Brittain C, et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): two randomised, double-masked, phase 3, non-inferiority trials. Lancet. 2022 Feb 19;399(10326):729–40.
12. Veritti D, Sarao V, Di Bin F, Lanzetta P. Pharmacokinetic and Pharmacodynamic Rationale for Extending VEGF Inhibition Increasing Intravitreal Aflibercept Dose. Pharmaceutics. 2023 May 6;15(5):1416.
13. McClard CK, Wang R, Windham V, Munoz J, Gomez S, Fried S, et al. Questionnaire to Assess Life Impact of Treatment by Intravitreal Injections (QUALITII): Development of a patient-reported measure to assess treatment burden of repeat intravitreal injections. BMJ Open Ophthalmol. 2021 Apr 7;6(1):e000669.
14. Spooner KL, Guinan G, Koller S, Hong T, Chang AA. Burden Of Treatment Among Patients Undergoing Intravitreal Injections For Diabetic Macular Oedema In Australia. Diabetes Metab Syndr Obes. 2019 Sep 19;12:1913–21.