Abstract
To date, neutropenia and agranulocytosis related to TNF-α inhibitors have been discussed infrequently in the literature. In the current paper, a narrative review of the literature was performed on anti-TNF-α inhibitors, including infliximab, adalimumab, etanercept, golimumab, and certolizumab, using the PubMed database of the US National Library of Medicine. The review was restricted to autoimmune and auto-inflammatory diseases or other orphan diseases. In these conditions, transitory Grade 1-2 neutropenia (absolute blood neutrophil count [NC] between 1.5 to 1 x 109/L and NC between1 to 0.5 x 109/L, respectively) related to TNF-α inhibitors are relatively common. Grade 3-4 neutropenia (NC between 0.5 to 0.1 x 109/L and NC <0.1 x 109/L, respectively), or agranulocytosis ( NC = 0.5 x 109/L + fever) with clinical manifestations related to sepsis, is less common, with only a few case reports to date for the majority of TNF-α inhibitors. Neutropenia should be managed depending on clinical severity, with temporary or permanent discontinuation or reduction in dose of the drug, switching from one drug to another of the same or another TNF-α inhibitor class, broad-spectrum antibiotics in case of sepsis, and hematopoietic growth factors (G-CSF) in the more severe cases.
Keywords
Neutropenia, Agranulocytosis, Idiosyncratic, Biotherapy, Anti-TNF-α agent, Infliximab, Adalimumab, Etanercept, Golimumab, Certolizumab, Princeps, Biosimilar, Autoimmune disease, Auto-inflammatory disorder, Systemic vasculitis, Orphan disease, Hematopoietic growth factor, G-CSF
Introduction
Drug-induced severe neutropenia, defined as an absolute neutrophil count (NC) ≤ 0.5 x 109/L or a complete lack of neutrophils in circulating blood, is a potentially severe complication that has been related to most classes of drugs [1]. For the majority of drugs, the risk is likely to be very small. For medications such as antithyroid drugs, ticlopidine, clozapine, cotrimoxazole (trimethoprimsulfamethoxazole), sulfasalazine, methimazole, and dipyrone, the risk may be higher [2,3]. Although the pathogenesis is not yet fully clear, direct toxicity to the myeloid cell line and immune-mediated destruction are the main reported mechanisms [4].
In recent years, several reports have been published regarding drug-induced neutropenia and agranulocytosis [1-5]. However, only one of these papers includes specific data on biotherapies, agents that are increasingly being used, particularly in the context of autoimmune and auto-inflammatory disorders and other orphan diseases [5]. These drugs are frequently used in internal medicine, rheumatology, and gastroenterology, particularly the TNF-alpha (α) inhibitors tocilizumab (interleukin 6 inhibitor) and rituximab (anti-CD20 agents) [6-9]. To our knowledge, TNF-α inhibitors have rarely been described as the cause of severe neutropenia or even agranulocytosis [5].
Thus, we have decided to carry out a review on this topic, specifically focused on TNF-α inhibitors.
Search Strategy
A literature search was performed using the PubMed database of the US National Library of Medicine. We searched for articles published between January 2010 and February 2020 using the following keywords or associations: “biotherapy-induced neutropenia”, “biotherapy-induced agranulocytosis”, “TNF-alphainduced neutropenia” and “TNF-alpha-induced agranulocytosis”. Restrictions included: English-, Spanish-, or French-language publications; human subjects; and type of publication as clinical trials, review articles, or guidelines. We limited our research to the utilization of TNF-α inhibitors in autoimmune and auto-inflammatory disorders, systemic vasculitis, or orphan diseases including rheumatoid arthritis (RA), systemic lupus erythematous (SLE), Sjögren’s syndrome, Still’s disease, Behçet’s disease, giant cell arteritis, Crohn’s disease, ulcerative colitis, psoriatic arthritis, granulomatosis with polyangiitis, and genetic fevers. Two senior researchers from our work group reviewed all the abstracts.
American Society of Hematology educational books, textbooks of hematology and internal medicine, and information gleaned from international meetings were also used. Two senior researchers from our work group reviewed all texts related to our present subject of research.
Criteria of Causality
Most but not all cases of neutropenia occur as a result of exposure to drugs, either chemotherapy (“chemotherapy neutropenia”) or other drugs (“idiosyncratic neutropenia”) [10,11]. Several biotherapies have been implicated in the occurrence of idiosyncratic neutropenia and agranulocytosis (for review see [5]). This is the case for the different TNF-α inhibitors. Table 1 presents the criteria for assessing causality for implicating a class of drugs, here TNF-α inhibitors, in the etiology of neutropenia.
| - Onset of neutropenia or agranulocytosis during treatment or within 7 days after exposure to the drug, with a complete recovery in absolute neutrophil count (≥ 1.5 × 109/L) within 1 month of discontinuing the drug |
| - Recurrence of neutropenia or agranulocytosis upon re-exposure to the drug (theoretically the gold method but ethically questionable) |
| - Exclusion criteria: history of congenital neutropenia or immune-mediated neutropenia, recent infectious disease (particularly recent viral infection), recent chemotherapy and/or radiotherapy and/or biotherapy, and existence of an underlying hematological disease |
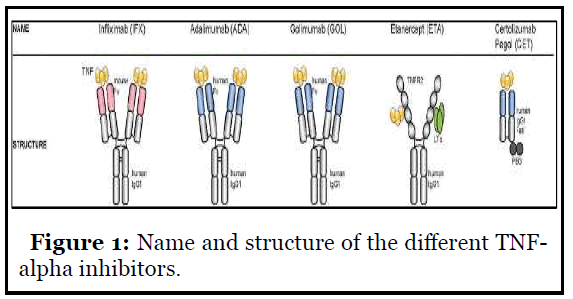
All TNF-α inhibitors have been implicated in the occurrence of this side effect, especially infliximab and adalimumab, but also etanercept, golimumab, and certolizumab, whether for the native molecule or for the biosimilar (Figure 1).
Differential Diagnosis
In adults, the differential diagnosis of neutropenia with a NC ≤ 1.5 x 109/L includes a limited number of conditions [11]. Table 2 presents the main conditions. In cases with NC ≤ 0.5 x 109/L, neutropenia has been shown to be attributable to drugs in 70 to 90% of cases [10].
| - Normal variations: Ethnic and familial neutropenia |
| - Splenic sequestration: Cirrhosis and portal hypertension (alcoholism), Gaucher’s disease |
| - Nutritional deficiencies: Cobalamin and folate deficiencies, copper deficiency, cachexia (Kwashiorkor) |
| - Infections: Bacterial (typhoid fever, brucellosis, tuberculosis, rickettsia, severe sepsis), viral (Epstein- Barr virus, cytomegalovirus, human immunodeficiency virus, hepatitis virus, rubella, parvovirus B19), protozoal and fungal (histoplasmosis, leishmaniasis, malaria) |
| - Other drugs taken: especially ticlopidine, clozapine, sulfasalazine, cotrimoxazole (trimethoprimsulfamethoxazole, methimazole, deferiprone, dipyrone |
| - Immune neutropenia: Isolated autoimmune neutropenia, collagen vascular autoimmune disease (systemic lupus erythematosus, rheumatoid arthritis or Felty’s syndrome), T γ-delta lymphocytosis |
| - Hematological disease: Myelodysplasia, pure white blood cell aplasia and red cell aplasia, Marchiafava-Michelli disease |
| - Primary congenital or chronic neutropenia: Familial and nonfamilial cyclic neutropenia |
In the setting of autoimmune and auto-inflammatory diseases, neutropenia may be related to several factors:
- The biological phenotype of several such diseases (e.g., seropositive and destructive RA, Felty’s syndrome, SLE, Sjögren’s syndrome), particularly in chronic neutropenia;
- Concomitant infections, often severe in frail patients or in cases of severe refractory heavily pre-treated disease, or in association with opportunistic infections;
- Previous or concomitant administration of immunosuppressive agents such as methotrexate, cyclophosphamide, or azathioprine;
- Nutritional deficiencies (e.g., B9 vitamin in cases of methotrexate therapy) [5,11].
Thus in practice, the diagnosis of neutropenia related to biotherapy becomes a diagnosis of exclusion.
In the presence of biotherapy-induced neutropenia, the potential hematological impact of the chemical drugs should be considered, including:
- nonsteroidal anti-inflammatory drugs;
- glucocorticoids;
- disease-modifying anti-rheumatic drugs (DMARDs), particularly hydroxychloroquine, penicillamine, and sulfasalazine;
- Immune-modulator or suppressive agents such as methotrexate, leflunomide, azathioprine, cyclophosphamide;
- Other drugs frequently used in internal medicine and rheumatology such as colchicine and dapsone;
- Previous administration of rituximab, with late onset neutropenia related to this anti-CD20 agent [5].
Incidence
To our knowledge, the rate of TNF-α inhibitor-induced neutropenia has been comparable to that of neutropenia associated with other commonly prescribed DMARDs, such as methotrexate and leflunomide, with a neutropenia rate between 2.8 to 15.3% (Table 3) [5,13]. This incidence includes mainly Grade 1 and Grade 2 neutropenia (NC from 1.5 to 1 x 109/L and NC from 1 to 0.5 x 109/L, respectively) [5].
| Study | Biotherapy | Incidence |
|---|---|---|
| Espinoza’s retrospective study; rheumatic diseases, mainly rheumatoid arthritis (72%); n=499 [14] |
Infliximab, abatacept, or tocilizumab |
2.8% to 18.6% (2.8% for infliximab) |
| Hastings’s retrospective cohort study; mainly rheumatoid arthritis (81.2%), ankylosing spondylitis (10.4%) and psoriatic arthritis (8.4%); n=367 [13] |
Etanercept (72.8%), infliximab (18.7%), adalimumab (8.4%) |
12.5% to 14.9% |
| Rajakulendran’s study; rheumatoid arthritis; n=133 [15] |
Etanercept (63%), infliximab (19.3%), adalimumab (17.6%) |
13.1% to 15.3% (mean 14.3%) |
In a retrospective cohort study, Espinoza et al. reported at least one neutropenic episode in 52 of their 499 patients (10.4%) [14]. These patients were treated by intravenous infliximab, abatacept, or tocilizumab for rheumatic diseases, mainly RA (72%). In this study, tocilizumab was more commonly associated with neutropenia than abatacept (18.6% versus 3.8%) or infliximab (18.6% versus 2.8%) (all p<0.001).
In a recent study, Hastings et al. reported a neutropenia rate between 12.5 and 14.9% among 367 patients receiving TNF-α inhibitors [13]. In another study, Rajakulendran et al. also reported a 14.3% rate of idiosyncratic neutropenia in 133 patients with RA, without any other obvious cause other than TNF-α treatment [15]. In autoimmune or auto-inflammatory diseases, Grade 3 and Grade 4 neutropenia (NC between 0.5 to 0.1 x 109/L and NC ≤0.1 x 109/L, respectively) or agranulocytosis (NC ≤ 0.5 x 109/L + fever or clinical manifestations of infection) are more rarely reported with biotherapy, particularly with TNF-α inhibitors [5].
A neutropenia rate of 1 to 2% has been reported especially in cases treated with rituximab therapy, with late onset neutropenia, and alemtuzumab [16,17]. Only a few case reports of Grade 3 and Grade 4 neutropenia have been reported to date with TNF-α inhibitors, tocilizumab therapy, and interleukin-1 inhibitors (for review see references [5,18]).
Risk Factors and Predisposing Conditions
Being able to predict neutropenia is the holy grail for clinicians. In this regard, Hastings et al. performed a retrospective cohort study examining the association between baseline demographics, clinical features, medications used, development of neutropenia, and behavior of neutrophil counts upon anti-TNF-α therapy [13]. Their study included 367 patients given TNF-α inhibitors, mainly for RA (n=298, 81.2%). Of these patients, 69 (18.8%) had at least one episode of neutropenia during TNF-α inhibitor treatment. In their study, patients with neutropenia exhibited significantly lower baseline blood neutrophil count levels (4.2 x 109/L; 95% CI: 3.8, 4.6 x 109/L), and a previous neutropenia history related to DMARD therapy increased the risk of neutropenia upon receiving TNF-α inhibitors (hazard ratio 2.97; 95% CI: 1.69-5.25). The most significant predictor of developing neutropenia was a history of prior neutropenia when receiving a previous DMARD, given that these patients were three times more likely to develop neutropenia, especially during the first 3 months following treatment initiation [5,13]. In addition, a significant drop in mean NC was observed following 2 weeks of TNF-α inhibitor therapy, suggesting a bolus effect generated by the intravenous delivery of this drug.
Pathogenesis
The mechanisms of the neutropenia related to biotherapies are not clear [4,5]. However, an essential mechanism for TNF-α inhibitor-induced neutropenia relies on the impact of drugs that serve as haptens and sensitize neutrophils or neutrophil precursors, resulting in immune-mediated peripheral destruction [5,13]. In addition, induced circulating anti-neutrophil antibodies are likely to cause increased peripheral destruction, which is often seen in some viral infections. Consistent with this, Sebastian et al. reported two cases of severe neutropenia in patients with Crohn`s disease following treatment with TNF-α inhibitors [19].
In all of these cases, positive granulocyte bound antibodies (GBA) and neutrophil specific CD16 bound antibodies (e.g. anti-neutrophil antigen [NA]) were detected, underscoring the involvement of an immune mechanism in neutropenia [5,19]. Ten weeks after infliximab infusion, blood NC, GBA, and anti-NA assay spontaneously returned to normal.
Clinical Manifestations
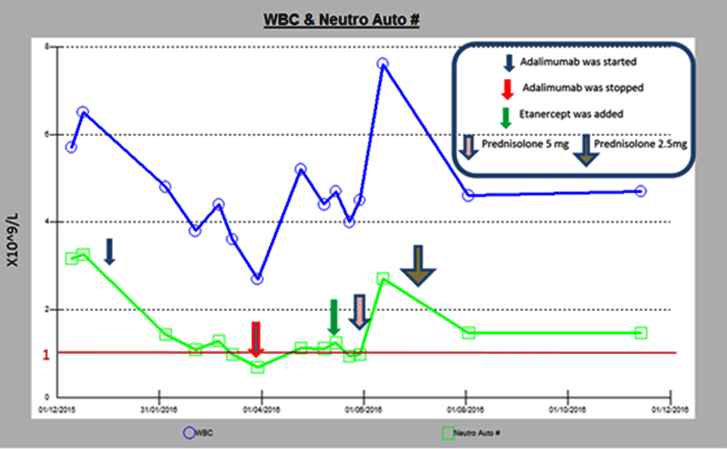
Unlike other drugs, only a minority of patients treated with TNF-α inhibitors develop a neutropenia that is symptomatic or results in a sepsis [5,13]. To our knowledge, the mild nature of the infection (fever, painful swallowing, gingival pain, skin abscesses, sore throats, and otitis) has not yet been explained. The majority of neutropenias reported with biotherapies is Grade 1 and Grade 2 and is diagnosed early (Figure 2). However, most of those patients are heavily pretreated and have severe refractory autoimmune or auto-inflammatory disorders.
Exceptional cases of severe infections have nevertheless been reported, especially with rituximab, but also with TNF-α inhibitors, and more rarely with alemtuzumab and interleukin-1 inhibitors (for review see references [5,13,16-18]). In the aforementioned Hasting et al. study (n=298), only 6% of the studied neutropenic patients later developed serious infections secondary to neutropenia induced by TNF-α inhibitors [13].
The first case of the development of agranulocytosis two weeks after a single dose of infliximab was reported in 2005 [21]. Favalli et al. [21], described agranulocytosis (NC ≤ 0.5 x 109/L) in a 20-year-old Caucasian male affected by enteropathic (Crohn’s disease) HLA-B27 negative spondyloarthropathy, successfully treated with infliximab. After the second infliximab infusion, he was found to have a severe transient neutropenia. A bone marrow needle aspirate showed a normal tri-lineage differentiation. Other well-documented cases have since been reported [22-24].
Montane et al. reported the case of a 50-year-old man with ankylosing spondylitis who developed neutropenia after treatment of etanercept after an initial infliximab infusion as first line therapy, with two positive rechallenges [23].
Guiddir et al. reported the first case series of four newborn patients with severe neutropenia born to mothers treated for ulcerative colitis with infliximab during pregnancy (including the third trimester) [22]. The newborns presented with severe neutropenia at birth, which was subsequently complicated by skin infections. The newborns’ blood NCs returned to the normal range within 8 to 14 weeks, at which time infliximab could not be detected in the blood.
The question of whether neutropenia induced by TNF-α inhibitors represents an individual drug adverse effect or a class adverse effect is not currently settled [5,13]. Nevertheless, no case of severe neutropenia has been reported with the newer anti-TNF-α agents golimumab and certolizumab.
Prognosis and Mortality Rate
Over the past twenty years, the mortality rate for idiosyncratic chemical drug-induced neutropenia was 10-16% in European studies [1-3]. This is likely due to improved recognition, management, and treatment of the condition [1]. Grade 3 and Grade 4 neutropenia are the most likely to cause death, as in oncology, where the severity of neutropenia has a documented impact on prognosis [1,3].
To date, no robust data are available with biotherapies in the context of autoimmune or auto-inflammatory diseases (for review see reference [5]). This is due to the low number of documented cases available and the small number of patients in series.
To our knowledge, only two well-documented cases of death have been reported with Grade 3 and Grade 4 neutropenia secondary to the use of rituximab and alemtuzumab biotherapy [25,26]. To our knowledge, no death has been directly related to TNF-α inhibitors.
Management
In the setting of TNF-α inhibitor therapy, only a minority of induced neutropenia cases are considered severe (Grade 3 and Grade 4). Typically these cases appear to be transient and self-limited [5,13]. Thus, the management of neutropenic episodes caused by TNF-α inhibitor consists of the following:
- Continuation of the original TNF-α inhibitor in cases of Grade 1 neutropenia with strict monitoring;
- Temporary cessation of the original drug and reinstatement once neutrophil count has returned to normal level for Grade 2 neutropenia;
- Switching to an alternative agent, while definitively stopping the biotherapy, in cases of Grade 3-4 neutropenia or severe sepsis [5,13].
The transient and moderate depth of neutropenia in most cases might explain why most of the patients with a prior TNF-α inhibitor-induced neutropenia may be able to be re-treated with the same biotherapeutic agent [5].
In the study by Rajakulendran et al., 84.2% of the 133 patients continued to receive their initial anti-TNF-α therapy, with only one temporary cessation [15]. In this study, one Infliximab-receiving patient had recurrent episodes of neutropenia that were managed by means of temporary cessation. Subsequently, however, the patient was switched to etanercept, with no further neutropenia episodes since. Another patient was switched from etanercept to adalimumab, without further problems. In the Hasting et al. study, no new neutropenia episodes were described once patients were switched to another anti-TNF-α molecule [13].
In cases with sepsis, empiric, broad-spectrum antibacterial therapy is generally the best option, but the specific antibiotic used may need to be adapted depending on the nature of the sepsis, the clinical status of the patient, local patterns of antibiotic resistance, and previous antibiotic use [5]. In the setting of biotherapyinduced neutropenia, this is even more important as patients are often fragile with more severe forms of the disease, many of whom have been heavily pre-treated [5,13]. The impact of immune impairment in autoimmune and auto-inflammatory disorders on infection risk is not fully understood.
At present, the only recommended preventive measures consist of hepatitis B and C vaccination, a Listeria-free diet, tuberculosis screening and prophylaxis, and annual papillomavirus screening for all biotherapies [6,13].
Table 4 includes a checklist and recommendations for the management of neutropenia associated with TNF-α administration.
In drug-induced neutropenia, the successful use of the hematopoietic growth factors, particularly granulocytecolony stimulating factor (G-CSF), has been previously reported [1,27]. G-CSF was found to be useful in shortening the time to blood count recovery without inducing any significant toxic or adverse effects, particularly in patients with poor prognostic factors [1,2]. In the setting of Grade 3 and Grade 4 neutropenia, several authors have reported successful treatment using G-CSF, while others have observed blood cell count recovery after a simple discontinuation of the incriminated agent [5,13].
| • Comply with the indications of TNF-a inhibitor |
| • Observe the precautions for the use of TNF-a inhibitor (dosage) |
| • Look for active or latent infection (tuberculosis, hepatitis B and C) |
| • Check that the usual vaccinations are up to date |
| • Initiate broad-spectrum antibiotic therapy for fever or any sepsis in a neutropenic patient, adapted depending on the nature of the sepsis, the clinical status of the patient, local patterns of antibiotic resistance, and previous antibiotic use |
| • Consider the use of hematopoietic growth factor in cases of severe neutropenia (Grade 3 and 4 neutropenia and agranulocytosis) |
| • Empiric, broad-spectrum antibacterial therapy |
| • Evaluate the immunological status related to the history and/or concomitant use of other immunomodulatory and immunosuppressive agents (e.g., cyclophosphamide, methotrexate, cyclosporine, rituximab) |
| • Consider the immunological status of the autoimmune or auto-inflammatory disease (e.g., systemic lupus erythematosus, vasculitis) |
| • Evaluate the general condition of the patient, his or her age, and co-morbidities (e.g., renal failure, diabetes mellitus, chronic heart failure, COPD) |
Conclusions
Today, drug-induced neutropenia remains a potentially serious adverse event. Knowledge of the commonlyimplicated agents and a high index of suspicion are essential in diagnosis. This is particularly important in the setting of autoimmune and auto-inflammatory disorders or in orphan disorders where the causes of neutropenia can be multiple. Transitory Grade 1 and Grade 2 neutropenia related to several biotherapies are relatively common, with an incidence of 10 to 15% with TNF-α inhibitors.
Grade 3 and Grade 4 neutropenia or agranulocytosis and clinical manifestations related to sepsis are the exception, with only a few published case reports to date.
Nevertheless, physicians must be vigilant in identifying drug-induced neutropenia because early detection can decrease the severity and prevent mortality if the drug is discontinued.
Conflicts of Interest
No sources of funding were used to assist in the preparation of this manuscript. The authors have no conflicts of interest that are directly relevant of the content of this manuscript. E. Andrès is the recipient of several grants from different laboratories: Novartis, BMS, Pfizer, Léo Pharma, Boehringer, Ferring, Chugai, Servier, Amgen, Roche and Air Liquide, but these sponsors had no part in the research or writing of the present manuscript.
References
2. Andersohn F, Konzen C, Garbe E. Non-Chemotherapy Drug-Induced Agranulocytosis: A Systematic Review of Case Reports: 108. Pharmacoepidemiology and Drug Safety. 2007 Jul;16.
3. Andrès E, Mourot-Cottet R, Maloisel F, Severac F, Keller O, Vogel T, et al. Idiosyncratic drug-induced neutropenia and agranulocytosis. QJM: An International Journal of Medicine. 2017 May 1;110(5):299-305.
4. Johnston A, Uetrecht J. Current understanding of the mechanisms of idiosyncratic drug-induced agranulocytosis. Expert Opinion on Drug Metabolism & Toxicology. 2015 Feb 1;11(2):243-57.
5. Andrès E, Lorenzo Villalba N, Zulfiqar AA, Serraj K, Mourot-Cottet R, Gottenberg JE. State of Art of Idiosyncratic Drug-Induced Neutropenia or Agranulocytosis, with a Focus on Biotherapies. Journal of Clinical Medicine. 2019 Sep;8(9):1351.
6. Sibilia J, Solignac M. The experience of anti-TNF-alpha registries and observatories. InAnnales de Dermatologie et de Venereologie 2010 Apr 1 (Vol. 137, No. 4 Suppl, pp. 18-21).
7. Scott LJ. Tocilizumab: A Review in Rheumatoid Arthritis. Drugs. 2017; 77: 1865-79.
8. Schioppo T, Ingegnoli F. Current perspective on rituximab in rheumatic diseases. Drug Design, Development and Therapy. 2017;11:2891.
9. Jacobs I, Petersel D, Isakov L, Lula S, Sewell KL. Biosimilars for the treatment of chronic inflammatory diseases: a systematic review of published evidence. BioDrugs. 2016 Dec 1;30(6):525-70.
10. Andersohn F, Bronder E, Klimpel A, Garbe E. Proportion of drug-related serious rare blood dyscrasias: estimates from the Berlin case-control surveillance study. American Journal of Hematology. 2004 Nov;77(3):316-8.
11. Palmblad J, Dufour C, Papadaki HA. How we diagnose neutropenia in the adult and elderly patient. Haematologica. 2014 Jul;99(7):1130.
12. Benichou C, Solal PC. Standardization of definitions and criteria for causality assessment of adverse drug reactions. Drug-induced blood cytopenias: report of an international consensus meeting. Nouvelle Revue Francaise d’hematologie. 1991;33(3):257-62.
13. Hastings R, Ding T, Butt S, Gadsby K, Zhang W, Moots RJ, et al. Neutropenia in patients receiving anti– tumor necrosis factor therapy. Arthritis Care & Research. 2010 Jun;62(6):764-9.
14. Espinoza F, Le Blay P, Combe B. Biologic diseasemodifying antirheumatic drug (bDMARD)-induced neutropenia: a registry from a retrospective cohort of patients with rheumatic diseases treated with 3 classes of intravenous bDMARD. The Journal of Rheumatology. 2017 Jun 1;44(6):844-9.
15. Rajakulendran S, Gadsby K, Allen D, O’Reilly S, Deighton C. Neutropenia while receiving anti-tumour necrosis factor treatment for rheumatoid arthritis. Annals of the Rheumatic Diseases. 2006 Dec 1;65(12):1678-9.
16. Salmon JH, Cacoub P, Combe B, Sibilia J, Pallot- Prades B, Fain O, et al. Late-onset neutropenia after treatment with rituximab for rheumatoid arthritis and other autoimmune diseases: data from the AutoImmunity and Rituximab registry. RMD open. 2015 Jun 1;1(1):e000034.
17. Baker D, Giovannoni G, Schmierer K. Marked neutropenia: significant but rare in people with multiple sclerosis after alemtuzumab treatment. Multiple Sclerosis and Related Disorders. 2017 Nov 1;18:181-3.
18. Shovman O, Shoenfeld Y, Langevitz P. Tocilizumabinduced neutropenia in rheumatoid arthritis patients with previous history of neutropenia: case series and review of literature. Immunologic Research. 2015 Feb 1;61(1- 2):164-8.
19. Sebastian S, Ashton K, Houston Y, Diggory TM, Dore P. Anti-TNF therapy induced immune neutropenia in Crohns disease-report of 2 cases and review of literature. Journal of Crohn’s and Colitis. 2012 Jul 1;6(6):713-6.
20. Hanan HA, Osamah AA, Mosaab AM, Nori R. A Case of Anti TNF Induced Leukopenia Responding to Small Dose of Prednisolone in Patient with Ankylosing Spondylitis. Journal of Rheumatology and Arthritic Diseases. 2017; 2(4): 1-2.
21. Favalli EG, Varenna M, Sinigaglia L. Drug-induced agranulocytosis during treatment with infliximab in enteropathic spondyloarthropathy. Clinical and Experimental Rheumatology. 2005 Mar 1;23(2):247-50.
22. Guiddir T, Frémond ML, Triki TB, Candon S, Croisille L, Leblanc T, de Pontual L. Anti–TNF-α Therapy May Cause Neonatal Neutropenia. Pediatrics. 2014 Oct 1;134(4):e1189-93.
23. Montané E, Sallés M, Barriocanal A, Riera E, Costa J, Tena X. Antitumor necrosis factor-induced neutropenia: a case report with double positive rechallenges. Clinical Rheumatology. 2007 Sep 1;26(9):1527-9.
24. Ottaviani SB, Cerf-Payrastre I, Kemiche F, Pertuiset E. Adalimumab-induced neutropenia in a patient with rheumatoid arthritis. Joint, Bone, Spine: Revue du Rhumatisme. 2009 May;76(3):312.
25. Yiannopoulou KG, Papadimitriou D, Anastasiou AI, Siakantaris M. Neutropenia with fatal outcome in a multiple sclerosis patient 23 days after alemtuzumab infusion. Multiple Sclerosis and Related Disorders. 2018 Jul 1;23:15-6.
26. Ahmadi F, Dashti-Khavidaki S, Khatami MR, Lessan- Pezeshki M, Khalili H, Khosravi M. Rituximab-related lateonset neutropenia in kidney transplant recipients treated for antibody-mediated acute rejection. Experimental and Clinical Transplantation. 2017 Aug;15(4):414-9.
27. Andrès E, Maloisel F, Zimmer J. The role of haematopoietic growth factors G-CSF and GM-CSF in the management of drug-induced agranulocytosis. British Journal of Haematology. 2010;150:3-8.