Abstract
Abstract The development of monoclonal antibodies (mAbs) counts as one of the major medical steps forward, opening up endless possibilities for research, diagnosis, and treatment of a wide range of various diseases and disorders. Development of both humanized and fully human mAbs was expected to be non-immunogenic, that allow repeated administration without any anti-drug antibodies (ADA) responses. Unfortunately, these expectations have proven to be unrealistic. They fail to completely eliminate mAb immunogenicity and ADA formation; they have reduced the extreme immunogenicity associated with murine origin mAbs, but still, they have shown to induce antibodies that sometimes have an impact on clinical outcomes. Understanding the mechanisms of ADA generation and then factors that may influence the immunogenicity of mAbs will help us design mAbs with lower immunogenic responses. Accurate ADA detection is necessary, to provide sufficient information for patient monitoring and clinical intervention. However, ADA assays for mAb have more challenges as both the analyte and antigen (mAb) are antibodies. This short communication discusses the assessment of mAbs immunogenicity. First, we provided an overview about the development of different generations of mAbs and the immunogenicity developed upon their administration. Additionally, we also provided a conclusive review about various types of methods and their principles that have been used for assays of ADA as well as those developed for the detection of anti-trastuzumab Ab used in our study. Finally, we summarized the results and conclusions observed in our study and discussed future prospects for studying ADA developed against mAbs.
Keywords
PImmunogenicity, Monoclonal Abs, ADA assays, Trastuzumab
Background
Therapeutic monoclonal antibodies (mAb) have the potential to treat a wide range of various diseases and disorders including cancer, chronic autoimmune and inflammatory diseases, allergies, infections, transplantations, and cardiovascular diseases [1]. They have several characteristics that make them useful and attractive options for the treatment of multiple conditions: First, their highly specific binding to their target antigen; Second, the relatively long half-life of a therapeutic mAb unlike most traditional small-molecule medicines that are usually dosed daily, that allow less frequent dosing intervals for mAb (weekly or monthly); Third, the lower risk for interactions with other drugs since mAb catabolism does not involve the cytochrome P450 system [2].
Like almost all therapeutic proteins, mAbs may have immunogenic (IG) responses when administered to patients; they may elicit anti-drug antibody (ADA) responses [3]. The first generation of therapeutic mAbs of murine origin (purified derived mouse Ab), showed high immunogenicity, which limited their efficacy and was associated with severe adverse reactions [4,5]. This resulted in the development of other generations of mAbs including chimeric (replacing murine constant regions with human constant regions); humanized (the variable antigen-binding regions of the murine mAbs were grafted onto a human monoclonal backbone, thus replacing most mouse sequences derived amino acids for human sequences, only the complementarity determining regions (CDRs) of the variable (v) regions of mouse sequence origin remain); fully human antibodies (mAbs completely derived from human sequences) introduced as a therapeutic agent to reduce these IG responses; however, even fully human mAbs were proved to be still immunogenic [6]. This indicates presence of other factors than the murine sequences that determine the immunogenicity of mAbs. Different factors contributing to the risk of immunogenicity of therapeutic proteins include factors related to the nature of the therapeutic proteins as size and structural complexity, sequence variation from endogenous protein, aggregates, post-translational modification (e.g., glycosylation, pegylation), neoepitopes due to denaturation or fragmentation, the adjuvant potential of inactive ingredients and other impurities; factors related to the target disease and population as patient characteristics such as genetic background, comorbidity, tolerance to protein, preexisting immunodeficiency, use of immunosuppressive drugs or chemotherapy; factors related to the treatment regimen as dose, route of administration, frequency of treatment and duration of treatment [1,7].
ADAs are antibodies developed as a result of triggering the humoral immune response against the therapeutic proteins, they can be classified into two main groups according to their action: i) neutralizing ADAs (nADAs) or neutralizing antibodies (NABs), that affect the biological function of the biotherapeutic; ii) non-nADAs, ADAs that do not affect the biological function of the therapeutics. Others classify ADAs into three groups: NABs; non-NABs but have an impact on drug activity; and non-NABs with no impact on drug activity [8]. NABs prevent the therapeutic proteins from acting effectively by preventing them from binding to their pharmacological targets. However, even antibodies that do not directly affect the function of the proteins (also referred to as binding antibodies) can alter drug bioavailability and/or accelerate clearance from circulation [9]. In rare cases, immune responses can be severe, resulting in hypersensitivity reactions and even death [10].
Herceptin (trastuzumab) developed by Genentech Inc. (San Francisco, CA, USA) is the first of such agents which was registered for use in patients with HER2-overexpressing breast cancer; it has been served as a remarkable example of a successful therapy targeting breast cancer [11]. However, the cancer cells develop ways to become resistant during treatment with trastuzumab. Researches describe two types of resistance, either primary resistance (a substantial proportion of patients will not respond to trastuzumab-based regimens) or acquired resistance (also called secondary resistance when patients do respond will often lose clinical benefits). Nevertheless, trastuzumab resistance has been increasingly recognized as a major obstacle in the clinical management of this disease [12]. Many researchers studied and reviewed mechanisms of trastuzumab developing resistance and discussed ways to overcome this developed resistance [13-15]. Like almost all medicines, trastuzumab might cause side effects (fever, nausea, vomiting, infusion reactions, diarrhea, infections, increased cough, headache, fatigue, dyspnea, rash, neutropenia, anemia, and myalgia.), some of them severe [16]. Other serious significant complications of trastuzumab have been reported including its effect on the heart, serious infusion reactions, and pulmonary toxicity [17]. Trastuzumab is often administered with chemotherapy, the mentioned above side effects are associated with chemotherapy too. Cancer patients are usually subjected to different treatment options and can develop adverse reactions that should be considered in the treatment protocols. In addition to all the above-mentioned side effects, trastuzumab as a biological therapeutic agent could induce immunogenic responses which may affect its efficacy as a therapeutic agent. Many studies reported the low immunogenicity of trastuzumab [18-21]. Nearly there were no clinical studies conducted on trastuzumab immunogenicity in Egypt [22].
Methods
Different assay methodologies including ELISA, radioimmunoassay, surface Plasmon resonance, and electrochemiluminescence-based techniques have been used to detect ADAs [23]. Different assays can have an impact on the determined immunogenicity rates and severity, as assays must be sensitive enough to detect low levels of ADA and be able to differentiate between the ADA and the therapeutic proteins including the mAbs. Two types of assays are commonly used for ADA identification: i) immunoreactivity assays that detect binding of ADAs to the therapeutics, and ii) neutralization assays for the identification of NABs [9]. Immunoreactivity assays detect the presence of any antibodies that bind to drugs in serum samples. The two immunoreactivity assay types commonly used to screen for the detection of ADAs are ELISAs (enzyme-linked immunosorbent assays) and RIAs (radio-immunoprecipitation assays). The automated ELISAs are rapid, robust, easy to perform, while RIAs are highly sensitive, but are expensive and require radiolabels [9]. Neutralization assays, in addition to determining the presence of antibodies, the neutralizing potential of assays must also be considered. Neutralizing antibodies (NABs) bind to epitopes within the active sites of the protein and thereby interfere with or neutralize the desired biological activity of a therapeutic. The NABs assays measure biological effects such as indications of cell proliferation, cytokine release, gene expression, and apoptosis. There are two types of NAB assays: i) cell-based, and ii) non-cell-based [24,25]. In cell-based assays, the sample is pre-incubated with the drug then added to cells, and if NABs are present, the assay response on the cells is inhibited because the drug is unable to bind to its target. In our study, we used the cell-based assay as a confirmatory assay to detect antitrastuzumab Ab in the serum sample that showed the highest Ab titer and to determine the neutralizing activity of the detected ADAs. We used three different regimens in loading the drug and serum preparations; the usage of different regimens might indicate the mechanism of neutralizing activity of the detected Ab, whether their effect on a cell line or on the drug itself, as detailed in our previous published work [22]. Non-cell-based NABs assays are typically simple and do not exhibit some of the technical limitations of cell-based assays. It is important to note that there are also instances where NABs are present with no impact on drug affinity and elimination [9]. A common limitation for the neutralization assays is their low drug tolerance levels compared with typical immunoreactivity assays. Consequently, NABs may not be detected in samples that are ADA-positive, since it does not necessarily indicate the absence of NABs. So the true NABs incidence is difficult to determine [6].
The most common three types of ELISA assays for ADA characterization include: i) sandwich ELISA, the main issue with sandwich ELISA is lack of specificity; ii) competitive immunoassay, the main issue with this method no labeled Ag; iii) bridging assay, one of the most commonly used assays [9]. Bridging assays are highly sensitive and widely accepted by both regulators and the industry for the regulatory approval of mAb therapies. Limitations to bridging assays can be summarized as following: fist interference with soluble antigen or pre-existing Abs in plasma, which may cause drug molecules to bridge, resulting in false positives; second interference with a free drug in plasma, is a major issue as the presence of too much free drug can conceal the presence of low levels of ADA, that resulting in false negatives [3]. In our study, we used the Affinity Capture Elution (ACE) assay method for in vitro detection of anti trastuzumab Ab in patients’ sera withdrawn at different points during the treatment course, and also it was used to detect anti trastuzumab Ab developed in mice sera injected with trastuzumab. In case of in vitro detection of anti trastuzumab Ab in patients’ sera, sera were prepared from blood samples collected from patients diagnosed with breast cancer (stages I-IV as classified and categorized according to TNM [tumor, node, and metastasis] stage classification). Blood samples were collected from patients whose tumor biopsies were HER2 positive and under treatment with trastuzumab and were obtained from National Cancer Institute (NCI), Cairo, Egypt. Blood samples were withdrawn from 101 patients and these included: 18 patient’s serum samples withdrawn before the trastuzumab treatment course; 46 patient’s serum samples withdrawn at a single point during trastuzumab treatment course; serum samples of 32 patients withdrawn at 2 different points during trastuzumab treatment course; 5 patient’s serum samples withdrawn after trastuzumab treatment course. Clinical data for all patients included in the study were reviewed and any AEs were recorded. Nearly all patients (100 out of 101) showed no observed AEs during the treatment course except infusion-related reactions (IRRs) like fever, redness, and swelling that were observed in few patients, and it was not known if these reactions were related to trastuzumab or any other combined drugs. Only one female out of 101 included patients, her clinical data showed unresponsiveness for treatment with trastuzumab, and the treatment was stopped after the 2nd dose. In case of in vivo immunogenicity testing of trastuzumab in lab animals, one control group and three test groups injected with different concentrations of trastuzumab were used. All statistical analysis of the results was done using Excel 2010 and validation of methods was done using IBM SPSS Statistics Data Editor.
Challenges of ADA measurement include the following: i) the absence of human ADA standards (positive control) to determine exact concentrations; ii) specificity of the assay, to ensure specific binding for the ADA, differentiate between ADA and drug product; and iii) sensitivity of the assay, to ensure that assay is sensitive enough to detect even low levels of ADA in the presence of an excess of free drug. In our study to overcome these challenges, the following were considered: i) to compensate the absence of human ADA standards, all serum samples obtained from human participants were analyzed first to determine samples’ levels of ADA, the sample that gave the highest level of ADA detected to indicate the strongest positive, this sample was considered as positive control and were run in parallel in each ELISA assay; ii) to ensure specific binding, the ACE assay method was used, non-specific binding was eliminated by the following: first acid treatment of samples to dissociate ADA-free drug complexes followed by neutralization in the presence of solid-phase drug allowing the ADA to be affinity captured. Second, washing away excess free drugs, then ADAs are eluted off with acid and allowed to bind to a fresh solid surface. Detection of the bound ADA was carried out by the addition of biotinylated drug followed by streptavidin- HRP and substrate; iii) to ensure assay sensitivity, ACE assay method is sensitive enough to detect low levels of free ADA as well as it involves the dissociation of ADA-drug complexes with acid treatment; this enables determination of total ADA developed in patient’s serum. Bourdage et al., study results indicate that the affinity capture elution (ACE) assay format is capable of detecting low levels of ADA (ng/ml) in the presence of a 1000-fold excess of free drug [26].
Some advanced techniques have been developed to improve ADA analysis assays with high sensitivity, including Gyrolab, immune-PCR, SQI SquidLite Technology, Immunocapture- LC/MS, and Genaltye Maverick System. But for some of these assays, major limitations still apply [23].
Results
In our study, Kilany et al. [22] the results of measuring ADA developed against trastuzumab (humanized mAb) among Egyptian participants guided us to consider any low levels (<1) of anti-trastuzumab Ab detected in serum samples as artifacts. The assay cutoff point was calculated and it was found to be 1.
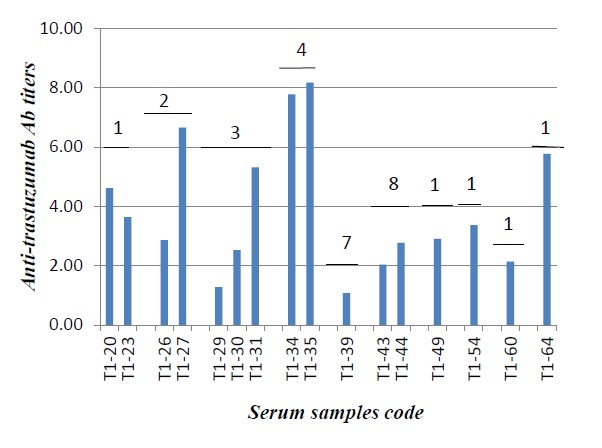
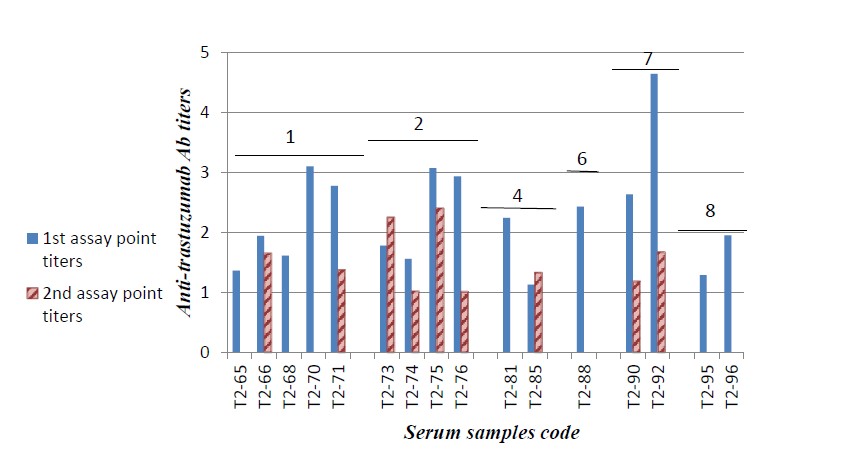
In vitro detection of anti-trastuzumab Ab levels in serum samples withdrawn during trastuzumab treatment course showed that some samples gave variable low levels of antitrastuzumab Ab titers. The levels of anti-trastuzumab Ab detected were lower in the higher administered doses as shown in Figure 1. Most samples showed either a marked decrease or no detectable Ab levels when they were assessed in the 2nd assay point (serum samples withdrawn from the same patient at a different time interval and subjected to analysis) as shown in Figure 2.
Only one serum sample showed markedly high antitrastuzumab Ab level and reviewing her clinical data revealed that the patient with this serum sample experienced negative clinical outcomes that ended by the stop of trastuzumab administration after the 2nd dose. MTT cytotoxicity assay was used as a confirmatory method and to determine the neutralizing activity of anti-trastuzumab Ab detected in this serum sample. Interaction between the Ab and the cell line depends on its degree of association/dissociation from its immunocomplex. In our study dilutions seems to dissociate immunocomplexes as reported by Aliza Kijlstra et al. of that immunocomplex dissociates by dilutions [27]. Also, acid treatment of samples could be a better way for dissociation of such like immunocomplexes that may interfere with ADA neutralizing effect on the cell level bioassays. Different methods that involve the use of acid steps to dissociate ADA-drug immune complexes include ELISA and cell-based neutralizing immunoassays. New methods also describe other procedures used for complexes dissociation in immunogenicity assays [28].
Conclusion
In our study, trastuzumab showed results that indicate its low immunogenicity as a biological therapeutic agent in preclinical and clinical studies. Only one patient showed a high level of Ab that might of an impact on his clinical outcomes. So it is much recommended to take into consideration detection of immunogenicity of mAbs in patients showing unresponsiveness to treatment or any serious adverse events after starting treatment course. In the case of trastuzumab, ADA developed in most patients seems to be transient as they decreased progressively along the treatment course. ADA can be either persistent or transient; transient ADA rarely impacts clinical outcomes and causes adverse events [29]. This could be due to the tolerance developed by the body after the initial administered doses. Low immunogenicity of trastuzumab could also be explained in the view that patients with breast cancer are considered as having a severe disease or immunocompromised patients, so they are with less incidence to induce immunogenic responses [6].
Future Prospectives
With the steep development of new biotherapeutics including the mAbs, immunogenicity testing will continue to grow in the future. The assay methods used for immunogenicity testing are of critical importance and should be accompanied with critical clinical data observations. Recently the increase in reporting the incidence of ADAs development against mAbs is a result of the following three main factors: the development of highly sensitive assays; most ADA assays improve ways for drug-tolerant limit; establishment of regulatory guidance for increasing assay sensitivity as FDA recommendations to increase assay sensitivity. This increase in reported ADA does not mean that these ADAs have an impact on clinical outcomes [6].
It is important in reporting immunogenicity of any therapeutic proteins that the reported data include the following information: not only the presence of ADAs, but the relative amount (titer) is also important and the duration of response (it indicates the transient or persistent type of ADAs developed). Variability of the reported ADA responses even for the same patient populations was attributed to the use of different assays. So, we recommend the presence of a standard assay system for reporting immunogenicity for each therapeutic protein, the assays have to be validated during clinical trials in the drug development stage by regulators. Development of studies that support possible molecular mechanisms of how therapeutic mAbs elicit ADAs is a helpful way to decrease the probability of mAbs to induce ADAs [8,30].
Further researches for studying mAb immunogenicity in different populations is required as still the question “why some patients have the inherit to develop this kind of IG responses than other patients?” could not be answered yet. So, researches that involve the study of biotherapeutics immunogenicity can make steep progress that helps practitioners in selection of appropriate treatment options for various patients’ types taking into consideration the risk-benefit ratio.
References
2. Martinez JM, Hindiyeh N, Anglin G, Kalidas K, Hodsdon ME, Kielbasa W, et al. Assessment of immunogenicity from galcanezumab phase 3 trials in patients with episodic or chronic migraine. Cephalalgia. 2020 Aug;40(9):978-89.
3. Dingman R, Balu-Iyer SV. Immunogenicity of protein pharmaceuticals. Journal of Pharmaceutical Sciences. 2019 May 1;108(5):1637-54.
4. Onda M. Reducing the immunogenicity of protein therapeutics. Current Drug Targets. 2009 Feb 1;10(2):131-9.
5. Harding FA, Stickler MM, Razo J, DuBridge R. The immunogenicity of humanized and fully human antibodies: residual immunogenicity resides in the CDR regions. In:MAbs 2010 May 1; 2(3): 256-265.
6. Hassanein M, A Partridge M, Shao W, Torri A. Assessment of clinically relevant immunogenicity for mAbs; are we over reporting ADA?. Bioanalysis. 2020 Sep;12(18):1325-36.
7. Pimentel FF, Morgan G, Tiezzi DG, de Andrade JM. Development of new formulations of biologics: expectations, immunogenicity, and safety for subcutaneous trastuzumab. Pharmaceutical Medicine. 2018 Oct;32(5):319-25.
8. Vaisman-Mentesh A, Gutierrez-Gonzalez M, DeKosky BJ, Wine Y. The molecular mechanisms that underlie the immune biology of anti-drug antibody formation following treatment with monoclonal antibodies. Frontiers in Immunology. 2020 Aug 18;11:1951.
9. McMaster M, Mohr K, Page A, Closmore A, Towne F, Brooks BD. Epitope characterization of anti-drug antibodies—a tool for discovery and health: an overview of the necessity of early epitope characterization to avoid anti-drug antibodies and promote patient health. Expert Opinion on Biological Therapy. 2021 Jun 3;21(6):705- 15.
10. Hamuro L, Kijanka G, Kinderman F, Kropshofer H, Bu DX, Zepeda M, et al. Perspectives on subcutaneous route of administration as an immunogenicity risk factor for therapeutic proteins. Journal of Pharmaceutical Sciences. 2017 Oct 1;106(10):2946-54.
11. Kunte S, Abraham J, Montero AJ. Novel HER2–targeted therapies for HER2–positive metastatic breast cancer. Cancer. 2020 Oct 1;126(19):4278-88.
12. Valabrega G, Montemurro F, Aglietta M. Trastuzumab: mechanism of action, resistance and future perspectives in HER2-overexpressing breast cancer. Annals of Oncology. 2007 Jun 1;18(6):977-84.
13. Wilken JA, Maihle NJ. Primary trastuzumab resistance: new tricks for an old drug. Annals of the New York Academy of Sciences. 2010 Oct;1210:53.
14. Han M, Gu Y, Lu P, Li J, Cao H, Li X, et al. Exosome-mediated lncRNA AFAP1-AS1 promotes trastuzumab resistance through binding with AUF1 and activating ERBB2 translation. Molecular Cancer. 2020 Dec;19(1):1-8.
15. Derakhshani A, Rezaei Z, Safarpour H, Sabri M, Mir A, Sanati MA, et al. Overcoming trastuzumab resistance in HER2-positive breast cancer using combination therapy. Journal of Cellular Physiology. 2020 Apr;235(4):3142-56.
16. Pivot X, Bondarenko I, Nowecki Z, Dvorkin M, Trishkina E, Ahn JH, et al. Phase III, randomized, double-blind study comparing the efficacy, safety, and immunogenicity of SB3 (trastuzumab biosimilar) and reference trastuzumab in patients treated with neoadjuvant therapy for human epidermal growth factor receptor 2–positive early breast cancer. Journal of Clinical Oncology. 2018 Apr 1;36(10):968-74.
17. Gemmete JJ, Mukherji SK. Trastuzumab (herceptin). American Journal of Neuroradiology. 2011 Sep 1;32(8):1373-4.
18. Hegg R, Pienkowski T, Chen S, Staroslawska E, Falcon S, Kovalenko N,et al. Immunogenicity of trastuzumab intravenous and subcutaneous formulations in the Phase III HannaH study. Annals of Oncology. 2012 Sep 1;23:ix103.
19. Pivot X, Bondarenko I, Nowecki Z, Dvorkin M, Trishkina E, Ahn JH, et al. A phase III study comparing SB3 (a proposed trastuzumab biosimilar) and trastuzumab reference product in HER2-positive early breast cancer treated with neoadjuvant-adjuvant treatment: final safety, immunogenicity and survival results. European Journal of Cancer. 2018 Apr 1;93:19-27.
20. Lamb YN. SB3 (Ontruzant®): a trastuzumab biosimilar. BioDrugs. 2018 Jun;32(3):293-6.
21. Lammers PE, Dank M, Masetti R, Abbas R, Hilton F, Coppola J, et al. Neoadjuvant PF-05280014 (a potential trastuzumab biosimilar) versus trastuzumab for operable HER2+ breast cancer. British Journal of Cancer. 2018 Aug;119(3):266-73.
22. Kilany LA, Gaber AA, Aboulwafa MM, Zedan HH. Trastuzumab immunogenicity development in patients’ sera and in laboratory animals. BMC Immunology. 2021 Dec;22(1):1-5.
23. Partridge MA, Purushothama S, Elango C, Lu Y. Emerging technologies and generic assays for the detection of anti-drug antibodies. Journal of Immunology Research. 2016 Jan 1;2016.
24. Wu B, Chung S, Jiang XR, McNally J, Pedras-Vasconcelos J, Pillutla R, et al. Strategies to determine assay format for the assessment of neutralizing antibody responses to biotherapeutics. The AAPS Journal. 2016 Nov;18(6):1335-50.
25. Carrasco-Triguero M, Yi JH, Dere R, Qiu ZJ, Lei C, Li Y, et al. Immunogenicity assays for antibody–drug conjugates: case study with ado-trastuzumab emtansine. Bioanalysis. 2013 May;5(9):1007- 23.
26. Bourdage JS, Cook CA, Farrington DL, Chain JS, Konrad RJ. An Affinity Capture Elution (ACE) assay for detection of anti-drug antibody to monoclonal antibody therapeutics in the presence of high levels of drug. Journal of Immunological Methods. 2007 Oct 31;327(1-2):10-7.
27. Kijlstra A, Knutson DW, Van Der Lelij A, Van Es LA. Characteristics of soluble immune complexes prepared from oligovalent DNP conjugates and anti-DNP antibodies. Journal of Immunological Methods. 1977 Oct 1;17(3-4):263-77.
28. Jordan G, Pöhler A, Guilhot F, Zaspel M, Staack RF. High ionic strength dissociation assay (HISDA) for high drug tolerant immunogenicity testing. Bioanalysis. 2020 Jun;12(12):857-66.
29. Bian S, Ferrante M, Gils A. Validation of a drug-resistant antiadalimumab antibody assay to monitor immunogenicity in the presence of high concentrations of adalimumab. The AAPS Journal. 2017 Mar;19(2):468-74.
30. Karle AC. Applying MAPPs assays to assess drug immunogenicity. Frontiers in immunology. 2020 Apr 21;11:698.