Abstract
Functionally limiting health conditions have a high rate of prevalence worldwide and incur a significant amount of economic burden. Physical activity (PA) can prevent the onset of these conditions and alleviate economic burden by reducing symptoms, but a large portion of these individuals do not engage in health enhancing PA. Consumer wearable physical activity monitors (WPAM) are tools that have become increasingly popular within the past few years and could provide a means to improve PA levels for individuals with health conditions that cause functional limitations. This review reports on the validity of PA outcomes, feasibility and utility, and intervention/promotion effectiveness for consumer WPAM in functionally limited clinical populations. 2250 records from January 2018 to July 2021 were retrieved from PubMed, Web of Science, SPORTDiscus and CINAHL with 656 records being duplicates and 23 records passing a full-text article review. Studies included within the review looked at individuals with osteoarthritis, rheumatoid arthritis, axial spondyloarthritis, multiple sclerosis, Parkinson’s disease, ischemic stroke and peripheral arterial disease. The most popular brand of consumer WPAM was Fitbit. Validation studies for consumer WPAM were primarily focused on step counts showing overestimations for daily step counts and over- and under-estimations occurring within shorter time durations depending on step cadence. Wrist worn WPAM are the most feasible for functionally limited clinical populations with widespread utilization for associating clinically relevant outcomes with PA levels but they have limited validation to confirm their accuracy and precision in measurement. Interventions included used a mixture of a WPAM and other behavior change techniques to improve PA levels for clinical populations and show promising effectiveness. Future work is warranted on determining the validity of PA outcomes from WPAM determined to be feasible in select clinical populations and creating interventions looking at which features of a consumer WPAM intervention promote PA.
Keywords
Physical Activity, Activity Monitor, Fitbit, Arthritis, Multiple Sclerosis, Parkinson’s, Stroke
Introduction
The prevalence of many musculoskeletal and cardiovascular diseases that cause limitations in physical functioning has increased globally [1] with age-adjusted rates of incidence rising for many of these diseases [2-5]. In the United States alone, chronic health conditions that limit physical function have an estimated economic impact of $200 billion per year [6]. There is evidence suggesting regular physical activity (PA) and exercise can alleviate symptoms and help prevent the onset of chronic conditions that cause functional limitations [7-11]. However, it has been observed in many epidemiological studies that individuals with chronic health conditions are significantly less active than their healthy counterparts [12,13]. For example, data from the Osteoarthritis Initiative in the United States show only 44.1% of men and 22.2% of women who are at risk or have knee osteoarthritis meet the 2018 Physical Activity Guidelines for Americans [14]. Given this, there is an enormous public health opportunity for investigating ways to increase PA in populations that have chronic health ailments that impact their level of physical functioning.
Wearable technology for health has been a growing market and social trend [15,16] that represents one avenue for measuring PA, increasing PA levels, and measuring clinically relevant outcomes. For measuring PA, consumer wearable physical activity monitors (WPAM) are low-cost devices that allow users to self-monitor changes in PA, which allow them to be easily adopted as facilitators of PA behavior change [17]. Previous research has been done in reviewing the literature of consumer WPAM for their validity and effectiveness as behavior change tools [18,19]. This mini-review aims to extend the previous research of consumer WPAM as a tool for measuring and promoting PA by reviewing the literature focused on clinical populations with functional limitations. Specifically, in this review we will examine the validity, feasibility and utility of use, and intervention/promotion effectiveness of WPAM for individuals with musculoskeletal, cardiovascular and neurological health conditions, and provide future recommendations to progress this line of scientific investigation.
Methods
A small-scale systematic review was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines [20]. Records were considered eligible for review if they met the following criteria: 1) used a consumer WPAM as a tool to measure a PA outcome, 2) recruited participants aged 18 years or older that were not receiving in or outpatient care, and 3) recruited participants that were either diagnosed or self-reported as having a musculoskeletal, cardiovascular or neurological condition that is associated with less physical functioning, including osteoarthritis (OA), rheumatoid arthritis (RA), multiple sclerosis (MS), Parkinson’s disease (PD), ischemic stroke and peripheral arterial disease (PAD). Records obtained were grouped by health condition. As there is no clear definition of what constitutes a consumer WPAM as opposed to a research-grade WPAM, such as an ActiGraph (AG) GT3X+ or activPAL (AP) 4, online marketplaces and product descriptions were searched to determine if a WPAM was marketed towards a consumer. Four databases were searched (PubMed Central, Web of Science, SPORTDiscus and CINAHL) with the following search terms: (arthritis OR “rheumatoid arthritis” OR osteoarthritis OR “multiple sclerosis” OR “ms” OR parkinsons OR parkinson’s OR “parkinsons disease” OR “parkinson’s disease” OR stroke OR “cerebral palsy” OR fibromyalgia OR “post-polio syndrome” OR “spinal cord injury” OR “traumatic brain injury” OR neurodegenerative ) AND ( “physical activity” OR “exercise” OR “sedentary behavior” OR sedentary OR “step count” OR “energy expenditure” OR intensity ) AND ( “activity monitor” OR “activity tracker” OR fitbit OR jawbone OR garmin OR nokia OR misfit OR sensewear OR pedometer OR accelerometer OR “wearable device” OR “wearable” OR “smart device” OR “consumer” OR “apple watch” ) NOT ( child OR children OR youth OR infant OR athlete ). Filters used were a publication date between January 1st, 2018 and July 20th, 2021 and the article being written in English.
After search results were received, a simple R script was written to 1) combine PubMed results with abstracts and 2) remove duplicates of records by comparing lowercased titles between records with special symbols removed. Titles and abstracts of records were then screened by authors JM and TG independently. A record was excluded if it did not meet the eligibility criteria, only included an abstract, was a proceedings paper from an academic meeting, was a case study, or systematic review. If a record was reporting on a re-analysis or a secondary analysis of a study that took place before 2018, it was only included if the study took place after January 1st, 2015 in order to focus on new research in the field. Studies included in prior systematic reviews were searched as another method for identifying potential records. If a record’s title or abstract mentioned the use of an accelerometer, a sensor typically used to estimate PA, within the abstract without a brand or if it was unclear a consumer WPAM was used to assess a PA outcome, the full-text article for the record was sought for retrieval and then evaluated to determine if eligibility criteria were met. If there was still uncertainty by a reviewer on the eligibility of a record, authors JM, TG and SJS reached a consensus on the eligibility of the record. Records that met the eligibility criteria then had the full-text articles read entirely by authors JM and TG for further screening. Once records were fully screened, the following data was retrieved from the articles: purpose and/or hypothesis of the study, participant size and characteristics, study design, consumer WPAM used, and PA outcome measure from WPAM. Included records were then grouped into one of three categories based on the study purpose/ hypothesis: validation of WPAM PA estimates, feasibility & utility of WPAM within observational studies and WPAM intervention use for PA behavior change as either the motivational tool for change or as the measurement tool for change.
Results
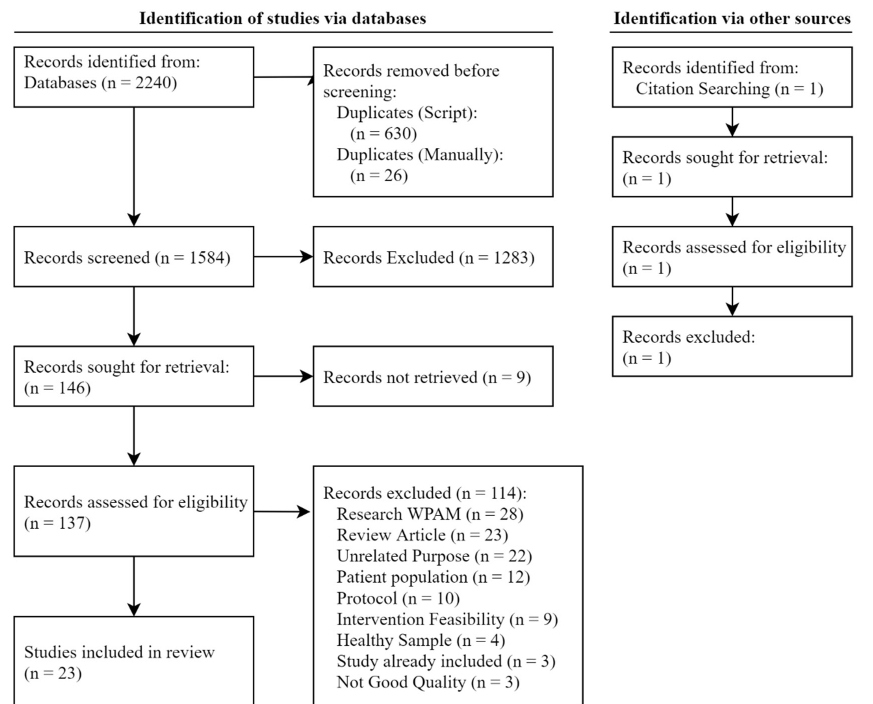
A flowchart of identification, screening and inclusion is shown in Figure 1. 2250 records were retrieved from database searching with 656 records being duplicates. 146 records passed the screening process with 23 records passing a full-text article review. Out of the 23 studies, 7 were focused on validating PA measures from a consumer WPAM, 13 explored the feasibility of implementing a WPAM for a specific population and utilizing a WPAM within observational studies to determine associations related to PA and 3 focused on using a WPAM for PA tracking and behavior change within an intervention. Table 1 provides characteristics of included studies and Table 2 lists the PA outcomes, price and features for consumer WPAMs included in the review.
Figure 1. Modified Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Flow diagram of screening process. WPAM: Wearable Physical Activity Monitor.
| Validation | Semanik et al. [25] | Arthritis (n = 35)Illinois, USA | Fitbit Flex | Daily time in Light and MVPA compared to AG GT3X+ hip. | -37.7 minute bias for light intensity; 18.7 minute bias for MVPA.Under and overestimations increase as criterion estimate increases. |
| Collins et al. [26] | Osteoarthritis (n = 15)Massachusetts, USA | Fitbit Charge 2 | ICC of step counts against AG GT3X+ hip and % bias of daily step counts | ICC = 0.602.39% bias for step counts (5,084 steps from criterion); | |
| Sedentary time to AG GT3X+ hip. | 37% bias for sedentary time (2.1 hours criterion) | ||||
| Block et al. [28] | Multiple Sclerosis (n = 61)California, USA | Fitbit Flex | Absolute step count to manual counts during 2MWT; | No significant bias reported although the Fitbit Flex overestimated. | |
| (n = 36) | Fitbit Flex Fitbit Flex 2 | Flex 2 and Flex daily step counts to AG GT3X | Fitbit Flex2: Significant bias of808 steps/day.Fitbit Flex: Significant bias of873 steps/day. | ||
| Lai et al. [29] | Parkinson’s (n = 31)Alabama, USA | Fitbit OneFitbit Charge 2Garmin Vivosmart 3 | Absolute step counts over a indoor course for 6 minutes to manual counts | ICC ≥ 0.97 for both Fitbit Oneand Garmin Vivosmart 3.ICC of 0.47 for Fitbit Charge HR 2. | |
| Absolute step counts during treadmill walking for 6 minutes to manual counts | Fitbit One: ICC of 0.98Fitbit Vivosmart 3: ICC of 0.67Fitbit Charge HR 2: ICC of 0.27 | ||||
| Lamont et al. [30] | Parkinson’s (n = 33)Queensland, Australia | Fitbit Charge HRGarmin Vivosmart | Absolute step counts over 2-minute walking tests at a self-selected pace, 60, 80, 100, 120and 140 steps/minute to AP | Both Monitors: APE < 3.0% and ICCs ≥ 0.88 at self- selected pace.Both Monitors: APE ≥ 37.2% &ICC = 0.36 at 60 steps/minute.Fitbit Charge HR: APE of 3.5- 17.6% & ICC of 0.18-0.37 forother cadences.Garmin Vivosmart: APE < 5.0% & ICC of 0.68-0.89 forother cadences | |
| Absolute step counts during a 500m outdoor walking course (slopes, grass, stairs, crowds of people) at a self-selected pace to AP | Fitbit Charge HR: APE = 1.5% & ICC = 0.94Garmin Vivosmart: APE = 1.9% & ICC = 0.97 | ||||
| Wendel et al. [31] | Parkinson’s (n = 35)Massachusetts, USA | Fitbit Surge Fitbit ZipJawbone Up Move Jawbone Up 2 |
Absolute step counts during a 2-minute walk test at a comfortable and fast pace to manual counts | Fitbit Zip: ICCs ≥ 0.90 & Bias≤ 2.15 stepsComfortable Pace: Lowest ICCs & Bias seen for Surge and Up 2 (0.38, 0.10 & -14.28,-4.00 steps, respectively).Fast Pace: Lowest ICCs & Bias seen for Surge and Up 2 (0.13,-0.02 & -23.03, -7.56 steps,respectively). | |
| Absolute step counts during a household simulated course and obstacle negotiation course to manual counts | Household: ICCs ≤ 0.17 & Bias≥ -22.76 steps for all WPAMsObstacle: Fitbit monitors had an ICC of 0.41-0.58 & Bias of-4.27 to -6.39 steps. Jawbone monitors had an ICC of 0.05 & Bias of -4.88 to -12.61 steps | ||||
| Duclos et al. [32] | Stroke (n = 17) Quebec, Canada |
Fitbit One(non-paretic leg ankle) | Absolute step counts during 6-minute walk test, and circuit inside mall to manual counts. | APE = 0.50% | |
| (n = 13) | Fitbit One (hip) | Absolute step counts during 6-minute walk test, and circuit inside mall to manual counts. | APE = 2.67% | ||
| Feasibility | Beukenhorst et al. [34] | Osteoarthritis (n = 18) Manchester, UK |
Huawei Watch 2 | 1 interview at baseline, 1 interview after wearing WPAM for 90 days. Interviews were semi-structured anddeveloped from techno- utopian and critical approaches towards self- tracking. |
Baseline Interviews: Motivation for participation revolved around yearning to learn more about relationship between PA and pain. Concern about operating smartwatch was present. End Interviews: Smartwatch was easy to use. Battery life and step counts restarting after a recharge were causes for frustration. There was enthusiasm for recording pain levels using smartwatch. |
| Manini et al. [35] | Osteoarthritis (n = 19) Florida, USA |
Samsung Gear S3 | Focus groups following a semi-structured interview format consisting of questions related to impressions of smartwatch technology, ecological momentary assessment approach from smartwatch appon patient-reported outcomes (PRO)s and potential future improvements. | A desire for customizing when to be notified for PRO outcomes and how to respond(i.e. more detailed information than app allowed) appeared as a theme. Recommendation for assessments to occur throughout the day and then a summary assessment at the end of the day. |
|
| Jacquemin et al. [36] | Rheumatoid Arthritis; Axial Spondyloarthritis (n = 177) Paris, France |
Withings Activité Pop Watch | Adherence to wearing WPAM over 90 days. | WPAM worn 88% of days, 78.5% still wore smartwatch at end of study. | |
| (n = 171) | Acceptability questionnaire, inquiring about discomfort, security and utilityof smartwatch, administered at the end of wearing WPAM for 3 months. | Out of a score of 10 (10 = Totally acceptable), a mean score of 8.5 was answered for acceptability of smartwatch. 63% said they will continue to wear the watch most of the time after the study end and 63% thought the watchallowed them to increase their PA. 17% needed help with the watch and 30% thought the watch underestimated PA. |
|||
| Fortune et al. [37] | Multiple Sclerosis (n = 15)Southeast England | Yamax SW-200 Digiwalker | One-to-one semi- structured interviews on engagement with PA and perspectives of WPAM. | Themes: A raised consciousness of PA levels, step goals are not abstract anymore, step counts were motivational for increasing PA. However, the accuracy of the WPAM was questioned and when it was inaccurate, demoralizing for participants.WPAM placement on hipcaused difficulties for usability. | |
| Block et al. [38] | Multiple Sclerosis (n = 79)California, USA | Fitbit Flex | Valid days (at least 128 steps) of wearing WPAM for 12 months. | 3 valid weeks (3 valid days or more) of average daily step counts per month.Participants with longer Timed-Up-And-Go times, greater disability and more pain only had one valid week of data per month. | |
| Pradhan et al. [39] | Parkinson’s (n = 30)Washington, USA | Fitbit Charge HR | Questions regarding the effort to learn how to use WPAM, satisfaction from using WPAM,and if WPAM provided motivation to be more active at the end of a 14- day wear period. | On a scale from 1 to 5 (5 = easy to use), an average score of4.3 was reported for effort onlearning WPAM use.On a scale of 1 to 5 (5 = verysatisfied), an average score of4.1 was reported on WPAM satisfaction.66% of individuals reported increased motivation to be active. | |
| Katzan et al. [40] | Stroke (n = 15)Ohio, USA | Fitbit Charge HR | Participant adherence (days with ≥ 100 steps) during a 90-day wear period | Participants wore WPAM 83.6% of time. | |
| Elmagboul et al. [41] | Gout Flares (n = 33)Alabama, USA | Fitbit Charge 2 | WPAM compliance in 4 categories: “Compliant wear with sleep” = Worn 80% of 1440 minutes, “Compliant wear without sleep” = 80% of 960 minutes, “No health tracker data”= no sensor data and “Partial wear” = all other patterns.A minute of wear time is defined as any minute where heart rate, step count or sleep data was available. | Out of 6572 days of collected data, 3978 days met a compliant wear pattern.Of 3978 days, 68% were classified as Compliant wear with sleep, 7% as Complaint wear without sleep and 25% as Partial wear. | |
| Utility | Jacquemin et al. [36] | Rheumatoid Arthritis; Axial Spondyloarthritis(n = 157) Paris, France |
Withings Activite Pop Watch | Comparison of step counts over 90-day wear period betweenrheumatoid arthritis and axial spondyloarthritis participants. | No significant differencewas found between the two clinical populations over the 3 months. |
| Description of PA levels for participants over 90 days. | Partitioning the participants into 3 clusters of homogeneous activity (low, moderate and high), 54.1% of participants had a low activity level, 42.7% had a moderate activity level and 3.2% had a high activity level. | ||||
| Bauer et al. [42] | Multiple Sclerosis (n = 38) Innsbruck, Austria |
Beurer AS 80 | Association between WPAM-defined activitytime and 25-hydroxyvitamin-D3 levels over a 14-day wear period. |
A weak correlation (0.221) was found between WPAM-defined activity time and vitamin D levels. | |
| Ryan et al.[43] | Multiple Sclerosis (n = 52)England | Yamax SW-200 Digiwalker | Associations between 6-minute walk testdistance, walking ability assessed from Twelve Item Multiple Sclerosis Walking Scale and average daily steps over 6 days. | An increase of 10 meters during the 6-minute walk test was associated with a significant increase of 130 steps/day and a 1 point increase on the Twelve Item Multiple Sclerosis Walking Scale was associated witha significant increase of 87steps/day. | |
| Pradhan et al. [39] | Parkinson’s (n = 30)Washington, USA | Fitbit Charge HR | Associations between average daily step counts, 10-meter walk times, balance and disease severity over 14 days. | There was a moderate correlation of step counts with self-selected and fast paces for the 10-meter walk (-0.60 and-0.64, respectively).A low significant correlation was found with balance scores (0.38) and no correlation was found with disease severity. | |
| Elmagboul et al. [41] | Gout Flares (n = 33)Alabama, USA | Fitbit Charge 2 | Associations of average daily step counts with days of gout flares and days without gout flares. | A non-significant decrease of 396 steps/day were seen on days of gout flares compared to days without gout flares. | |
| Sasaki et al. [44] | Stroke (n = 22)Hy?go Prefecture,Japan | Fitbit One | Association between average daily steps with EuroQoL 5-dimension 3 level health utility scores. | A significant positive correlation of 0.466 was found between average step counts and health utility score. | |
| Kanai et al.[45] | Stroke (n = 50)Hy?go Prefecture,Japan | Fitbit One | Associations between average daily steps and MVPA to EuroQoL5-dimension 3 level health utility scores over 7 days. | Multiple linear regression analyses showed the health utility score was significantly associated with an increase of steps but not an increase in MVPA. | |
| Lazaridou et al. [46] | Fibromyalgia (n = 107) Massachusetts, USA |
Fitbit Flex | Associations between daily step counts, pain intensity and pain catastrophizingassociations among daily pain symptoms, catastrophizing, and physical activity in patients with FM | Significant bivariate correlations were found between average state pain catastrophizing, pain intensity and step counts | |
| Intervention | Li et al. [47] | Osteoarthritis (n = 51) British Columbia, Canada |
Fitbit Flex 2 | Mean daily MVPA over a 7-day period assessed by SenseWear Mini | A significant mean increase of 13.1 minutes in MVPA was observed in the primary group compared to a delayed intervention group. |
| Ellis et al. [48] | Parkinson’s (n = 44) Massachusetts, USA |
Fitbit Zip Omron HJ-113 |
Mean daily step counts and time spent in moderate intensity stepping over a 7-day period assessed by StepWatch. | A non-significant increase of102.6 steps was observed for the intervention group. The increase was not significantly different from the active control group.A non-significant increaseof 17.4 minutes in moderate intensity PA was observed for the intervention group. The increase was not significantly different from the active control group. | |
| McDermott et al. [49] | Peripheral Arterial DiseaseIllinois, Minnesota & New York, USA | Fitbit Zip | Change in 6-minute walk distance and average total step counts/day as assessed by an AG monitor. | A non-significant increase of5.5 meters was observed for the intervention group. The increase was not significantly different from the usual care group.A non-significant decrease of 494 steps/day was observed for the intervention group. The decrease was not significantly different from the usual care group. | |
| Study Category | Author | Population | Device(s) | Main Outcomes | Results |
|---|---|---|---|---|---|
| AG: Actigraph; ICC: Intra-class Correlation Coefficient; AP: activPAL; APE: Absolute Percentage Error; PA: Physical Activity | |||||
| Brand (Website) | Monitor Name (Type) | Sensor(s) | Battery Life | Attachment | Dimensions (height x width x depth) (cm) | Market Price ($) | PAOutcomes | Water Resistance |
|---|---|---|---|---|---|---|---|---|
| Beurer (https:// www.beurer.com) | AS 80 (AT) | Not Reported | Not Reported | Wrist with band | 25.4 x 1.8 x 1.1 | Unavailable | Steps Calories DistanceActive Minutes Sleep | Splash Proof |
| Fitbit (https://www.fitbit.com) | Charge HR (AT) | Triaxial acceler- ometer Altimeter Optical Sensor (photoplethys- mography) | 5 days | Wrist with band | Not Reported | Unavailable | Steps Calories Distance Heart Rate FloorsActive Minutes Sleep | Splash Proof |
| Charge 2 (AT) | Triaxial acceler- ometer Altimeter Optical Sensor (photoplethys- mography) | 5 days | Wrist with band | Not Reported | Unavailable | Steps Calories Distance Heart Rate FloorsActive Minutes Sleep | Splash Proof | |
| Flex (AT) | Triaxial accelerometer | Up to 5 days | Wrist with band | 14-20.9 x 1.4cm | Unavailable | Steps Calories DistanceActive Minutes Sleep | Water- resistant | |
| Flex 2 (AT) | Triaxial accelerometer | Up to 5 days | Wrist with band | 1.1 width | Unavailable | Steps Calories DistanceActive Minutes Sleep | Water resistant up to 50 meters | |
| One (AT) | Triaxial accelerometer Altimeter | Up to 10 days | Clip | 4.8 x 1.9 x 1.0cm | Unavailable | Steps Calories Distance Sleep | ||
| Surge (AT) | Triaxial Acceler- ometer Altimeter Triaxial gyro- scopeTriaxial magne- tometerGPSOptical Sensor (photoplethys- mography) | 7 days; 10 hours with GPS | Wrist with band | 1.0 x 3.5 cm | Unavailable | Steps Calories Distance Heart Rate FloorsActive Minutes Sleep | Splash Proof | |
| Zip (AT) | Triaxial Accelerometer | 6 months | Clip (pocket, waist) | 3.8 x 2.8 x 1.0 | Unavailable | Steps Calories Distance | Splash Proof | |
| Garmin (https:// www.garmin.com) | Vivosmart (AT) | Not Reported | Up to 3 days | Wrist | Not Reported | Unavailable | Steps Calories Distance Heart RateActive Minutes Sleep | Water resistant Up to 10 meters |
| Vivosmart 3 (AT) | Not Reported | Up to 5 days | Wrist | 1.9 x 1.0 x 2.0cm | $119.99 | Steps Calories Distance Heart Rate FloorsActive Minutes Sleep | Water resistant up to 50 meters | |
| Huawei (https:// consumer.huawei. com/) | Watch 2 (SW) | Not Reported | Not Reported | Wrist with band | Not Reported | Unavailable | Steps Calories Distance Heart Rate | Water resistant up to 1.5 meters |
| Jawbone (https:// www.jawbone.com) | Up 2 (AT) | Triaxial Accelerometer | Not Reported | Wrist with band | Not reported | Unavailable | Steps | |
| Up Move (AT) | Triaxial Accelerometer | 9.21 x 3.18 in | Clip | Not reported | Unavailable | Steps | ||
| Withings (https:// www.withings.com) | Activite Pop Watch (AT) | Triaxial Accelerometer | 8 months | Wrist with band | 3.7 diameter | Unavailable | Steps Calories Distance Sleep | Water resistant up to 50 meters |
| Omron (https:// omronhealthcare. com) | HJ-113(Pedometer) | Pedometer | 6 months | Clip (pocket, waist) | 6.7 x 4.8 x 1.6 | Unavailable | Steps Calories Distance | None |
| Samsung (https:// www.samsung. com) | Gear S3 (AT) | GPSAccelerometer Barometer Gyro Sensor Heart Rate Sensor Ambient Light Sensor | Up to 3 days | Wrist | 4.6 x 4.9 x 1.3 | Unavailable | Steps Calories Distance Heart RateActive Minutes | Water resistant up to 1.5 meters |
| Yamax (https:// www.yamax.co.uk) | SW 200 Digi- walker (AT) | Pedometer | Approx. 3 years | Clip (waist) | 0.5 x 0.38 x0.14 cm | $26.62 | Steps | Not Reported |
| AT: Activity Tracker, SW: Smartwatch | ||||||||
Discussion
The purpose of this mini-review is to synthesize the research within the past few years on the validity, feasibility and utility of use and intervention/promotion effectiveness of consumer WPAM in functionally limited clinical populations. Understanding the synthesis of results within this review requires knowledge on the complex nature of PA behavior, sensors included in WPAM that are used to estimate PA, estimates of PA outcomes that are outputted from these sensors and goldstandard methods for measuring these outcomes. These topics have been reviewed in detail previously [18,21-23]. Briefly, PA constitutes “any bodily movement produced by the contraction of skeletal muscles that results in energy expenditure (EE)” [24]. This bodily movement is complex and can be broken down into many different components such as frequency of movement, intensity of movement, mode of movement, and duration of movement. Sensors, such as accelerometers, altimeters, gyroscopes and magnetometers, are used to measure these components of PA for estimating EE-related outcomes. These EErelated outcomes include calories spent during a duration of PA, steps per day, time spent in physiological postures (sitting vs. standing) and time spent in different PA intensity categories. Sedentary, light, moderate, vigorous and moderate-to-vigorous physical activity (MVPA) are standard intensity categories with time spent in each category having implications for health. Research-grade WPAM are typically used for their accuracy in estimating these EE-related outcomes, ability to download data directly from the monitoring device and transparency in data algorithms whereas consumer WPAM are convenient, low-cost measuring tools focused primarily on assessing and promoting PA with industry or manufacturer propriety algorithms.
Validity of consumer wearable physical activity monitors in functionally limited clinical populations
Minutes within an intensity category: Two studies included within this review examined consumer WPAM estimated minutes within a PA intensity category while most studies were focused on steps. Semanik et al. [25] focused on office employees living with chronic knee symptoms to determine the validity of average daily time spent in different PA intensity categories from the Fitbit Flex compared with research grade AG GT3X+ estimations. Specifically, time spent in light intensity activity and bouts of 10-minutes or more in moderate, vigorous and MVPA were compared. Bland-Altman plots indicated that the Fitbit Flex underestimated light intensity minutes when AG estimates were low and overestimated light intensity minutes when AG estimates were high. For MVPA, the Fitbit Flex systematically overestimated minutes with the magnitude of overestimation increasing as AG estimations increased. Collins et al. [26] looked at a similar population for determining the validity of PA intensity minutes from the Fitbit Charge 2 compared to a research-grade AG GT3X+ estimates for individuals with knee OA. Results showed that the Fitbit Charge 2 overestimated average daily sedentary time by 37% (2.1 hours).
Average steps per day: Collins et al. [26] also compared average daily step counts between the two monitors and found the Fitbit Charge 2 to overestimate steps per day by as much as 39% (1,648 steps). An interclass correlation coefficient (ICC) of 0.602 was also seen where it is generally considered that ICC “values less than 0.5, between 0.5 and 0.75, between 0.75 and 0.9, and greater than 0.90 are indicative of poor, moderate, good, and excellent reliability, respectively”. [27] Another study that compared average step counts per day, this time looking at Fitbit Flex and Fitbit Flex 2 estimates for people with MS to a research-grade AG GT3X+, found that both of the consumer WPAM overestimated steps per day. The Fitbit Flex overestimated an average of 873 steps per day while the Fitbit Flex 2 overestimated 808 steps per day with the magnitude of overestimation for both monitors increasing for individuals with higher Expanded Disability Status Scale scores [28]. This study also compared total step counts from the Fitbit Flex to directly observed handtallied step counts during a 2-minute walk test and found no systematic bias. This result from a short duration walk contradicts the results on the validity of WPAM estimates for average steps accumulated during the course of a day. This discrepancy is likely due to the total day values being compared with a research grade WPAM and not being compared to a criterion standard hand-tallied count.
Total steps within short durations: Further exploring the validity of consumer WPAM step counts in shorter time durations, Lai et al. [29] compared consumer WPAM total step counts during timed walks to manually tallied criterion step counts for people with PD. The authors found the Fitbit One to be the most accurate and precise when participants were asked to walk for 6 minutes over normal ground and on a treadmill at their self-selected pace. Looking at shorter walking durations, Lamont et al. [30] had participants with PD, while wearing a Fitbit Charge HR and Garmin Vivosmart, perform six 2-minute walks at their self-selected pace and at cadences of 60, 80, 100, 120 and 140 beats per minute. Using step count estimates from a research-grade AP as the criterion, both the Fitbit Charge HR and Garmin Vivosmart had an average percent error ≤ 3% but had greater average percent errors during the walks at pre-determined cadences. Further exploring the accuracy of step counts for people with PD, Wendel et al. [31] looked at the accuracy of consumer WPAMs to manually tallied criterion steps while participants walked in simulated house and obstacle courses. No WPAM had an ICC above 0.17 for the household course while the Fitbit Zip had an ICC of 0.58. In our review, only one included study looked at the accuracy of a consumer WPAM to manually tallied criterion step counts for post-stroke individuals in a variety of walking settings [32]. The authors found that a Fitbit Zip placed on the non-paretic ankle for individuals were accurate in a laboratory setting but errors increased within a mall circuit that included stairs and ramps.
Summary: A majority of validation studies in the past couple of years have been focused on step counts estimates from consumer WPAMs, showing them to differ in accuracy depending on whether total step counts within a laboratory setting or average daily step counts are the outcome of interest. No studies looking at the validity of average daily step counts have used a true criterion or goldstandard measure, or a comparison measure that has been shown to be accurate and precise compared with a goldstandard criterion measure. For example, the researchgrade WPAM StepWatch, which has shown to be the most accurate in estimating step counts compared to direct observation [33], could be used in lieu of research-grade monitors for naturalistic or field-based daily validation studies. Alternatively, comparing the StepWatch to a research-grade WPAM in clinical populations with functional limitations would extend the utility of these monitors to be used as comparison standards to consumer WPAMs.
Feasibility and utility of implementing consumer wearables in clinical populations
Feasibility: In the past few years, a number of qualitative and mixed-method studies have looked at the feasibility of implementing WPAMs for clinical populations. Beukenhorst et al. [34] conducted semistructured interviews with 26 older individuals who wore a Huawei Watch 2 smartwatch for 3 months every day. An app developed for the study was also installed on the watch and asked participants questions relating to pain and knee OA symptoms. Themes that arose from the interviews were the ease of using the smartwatch, motivation to see a relationship between their pain levels and activity levels (determined by step count), and doubts about the smartwatch’s battery life and step count accuracy. Beukenhorst and colleagues also found the median daily wear time over the 90 days to be approximately 11 hours and that the watch was worn for 73% of the study period [34]. Manini et al. [35] also developed an app for the Samsung Gear S3 smartwatch that asked older individuals with knee OA about pain throughout the day. Instead of having individuals wear the smartwatch for a period of time, focus groups were set up with participants where the smartwatch + app was shown to the group first and questions were asked afterwards about their thoughts on the watch. Themes that arose from the focus groups were a desire for customizing how the watch would alert them and the ability to provide more detailed information than the questions provided on the smartwatch app. For individuals with RA and/or axial spondyloarthritis, the ActConnect study gave participants a Withings Activité Pop watch to wear every day for 3 months [36]. From 177 participants, the activity monitor was worn for a mean time of 79 days (88%) over the 90-day period. From a questionnaire asked at the end of the period, 97% of participants considered daily use of the watch acceptable and 63% of participants even considered keeping some sort of activity tracker on after the end of the study. Focusing on people with MS, Fortune et al. [37] purposefully recruited 15 participants after the end of a randomized control trial to be part of semi-structured interviews. Purposeful recruitment was done to get a wide range of experiences (e.g. high and low activity engagement, different life commitments, gender) during the interview. Themes that arose from the interview regarding their experience of the hip-worn Yamax SW200 Digiwalker were increased activity awareness through the pedometers digital display, objective numeric feedback from a monitor that leads to increased motivation, and placement difficulties that led through decreased confidence in the accuracy of pedometer readings.
Looking at quantitative measures of feasibility for consumer WPAMs, Block et al. [38] examined weartime compliance during the FITRiMS study in which 95 individuals with MS were recruited to wear the Fitbit Flex every day for one year. Of the 79 individuals that were retained to the end of the study, an average of 3 valid weeks of average daily step counts were available each month in which a valid week consisted of 3 or more valid days and a valid day consisted of 128 steps or more. In other clinical populations, Pradhan et al. [39] saw that 30 individuals with PD found the Fitbit Flex easy to use for 14 full days with 66% of individuals stating increased motivation. Katzan et al. [40] found that 15 participants, whom were required to wear the Fitbit Charge HR after hospital discharge for ischemic stroke, wore the WPAM for 83.6% of a 90-day period and Elmagboul et al. [41] saw that a Fitbit Charge HR 2 was worn for 60.5% of the time, determined from when there was available heart rate, step count or sleep data in a day, out of an average of 6572 days for individuals who experienced gout flares.
Utility: Consumer WPAMs have also been utilized to measure prospective and cross-sectional associations of PA with functional disability and other chronic conditions for clinical populations. The ActConnect study explored factors associated with lower PA in individuals with RA and axial spondyloarthritis using a smartwatch [36]. For people with MS, a Beurer AS 80 and Yamax SW 200 Digiwalker were used to determine cross sectional associations of serum vitamin D levels and activity capacity with daily PA levels [42,43]. For individuals who experienced gout flares, were in the early stages of PD, were stroke survivors, and experience fibromyalgia, step counts and time spent in MVPA from a Fitbit device worn for ≥ 7 days was used to measure PA levels [39,41,44-46].
Summary: Qualitative and quantitative measures of feasibility and utilization in observational studies for determining PA associations have shown there is a great interest in using wrist-worn WPAMs to measure PA levels. Only one hip-worn consumer WPAM included has been examined for feasibility and utilized for a functionally limited clinical population. This follows the trend of wristworn monitors increasing in popularity these past few years, with many new monitors being able to function as a smartwatch to tell time, answer calls and connect with smartphones for increased functionality. Despite the shift in using more wrist worn consumer WPAM, stemming from ease of wear, high compliance and their dual functionality, there is limited research on their validity for measuring PA outcomes. As discussed previously, the majority of validation work in this area has been focused on older consumer WPAM and not on newer smart watch WPAM technology
Intervention/promotion use of consumer wearables in clinical populations
Interventions included: Despite the increasing use of consumer WPAMs for measuring associations of PA with clinically relevant outcomes for individuals with a functionally limiting condition, there is a paucity of research in the past few years in determining the effectiveness of interventions for community-dwelling individuals who have health conditions impacting their physical functioning. Within the limited work completed in this area, Li et al. [47] conducted a 12-week multifaceted randomized control trial in individuals with knee OA. 51 individuals were randomized to an immediate group and delayed group in which the delayed group received the intervention 14 weeks after group allocation. The intervention included 4 biweekly phone calls from physical therapists to monitor progression in achieving SMART goals, a Fitbit Flex 2 to track PA and a web-based app developed for the study that interacted with the Flex 2 to monitor progress towards goals. After 8 weeks of the intervention, physical therapists stopped counseling with participants for weeks 9-12. Difference in time spent performing MVPA, measured by a SenseWear Mini WPAM for 7 days at week 13, between the immediate and delayed group was the primary outcome. Results showed that the immediate group had significantly increased their PA by 13.1 more minutes of MVPA per day compared with the delayed group.
In another study focusing in PD patients, Ellis et al. [48] recruited 51 individuals to participate in a 12-month randomized control trial that involved a mHealth group and an active control group to see if increases in daily step counts improved walking capacity and health-related quality of life. Both groups were given a consumer WPAM to track activity levels (Fitbit Zip and Omron HJ-113 for mHealth and active control group, respectively) with the mHealth group having more accessible communication with a physical therapist and action plans through dedicated app developed for the iPad. Results showed that there was no significant difference in step counts or time spent in MVPA between and within groups. However, individuals within the mHealth group who achieved less than 7500 steps/day at baseline did see a significant increase of 763 steps/day and 55.7 minutes of moderate intensity activity which was not seen in the active control group. As for walking capacity and health-related quality of life, only the mHealth group saw significant improvements.
Finally, McDermott et al. [49] recruited 200 individuals with peripheral arterial disease (PAD) for a 9-month randomized control trial to improve 6-minute walking test (6MWT) distance, Patient-Reported Outcomes Measurement Information System (PROMIS) scores, and total average steps per day. The intervention was homebased and included behavioral change techniques that followed participant feedback from focus groups, social cognitive theory concepts and a Fitbit Zip consumer WPAM. Results showed no significant improvement in 6MWT distance or step counts within the intervention group or compared to the usual care group, but there was a significant improvement in PROMIS pain interference scores for the usual care group. The authors noted that total day estimates from the consumer WPAM may have influenced participants to increase overall activity whereas the intervention was focused on increasing bouts of walking throughout the day. They point to previous literature showing that increasing overall activity level does not increase walking endurance for individuals with PAD.
Summary: The few studies included have used a consumer WPAM in conjunction with other behavior change techniques, such as direct communication with physical therapists. Results from these reviewed interventional studies show promising results for individuals with knee OA and PD but not for individuals with PAD. As the number of interventions focused on community-dwelling clinical populations within the past few years is small, there is a need to increase research in this area to fully explore the ability of consumer WPAM to be used as a tool to promote and increase PA behavior and health outcomes.
Recommendations for Future Research
The authors suggest possible avenues of research that can be explored for the use of consumer WPAMs for clinical populations.
• Coinciding with Strath et al. [18], the consumer WPAM industry should reach out to researchers specialized in objective measurements of PA to improve estimates of PA from consumer WPAMs.
• Validation studies would benefit from following a framework similar to the one suggested by Keadle et al. [23]. Specifically, the use of gold-standard measures as the criterion should be used while validating a consumer WPAM from a laboratory setting to more free-living settings in clinical populations.
• To our knowledge there has been no study to determine the accuracy and precision of step count estimations from research-grade WPAMs in free-living settings for individuals with functional limitations. As daily step counts from consumer WPAMs have been a PA outcome in many of the studies within this review and included validation studies used a research-grade WPAM in lieu of an established criterion, further work is needed in validating research-grade WPAMs before they can be used as a criterion for validating daily step counts from consumer WPAMs.
• The feasibility of smartwatches and apps developed for them have been researched for select clinical populations. Determining the validity of PA outcomes from smartwatches and utilizing them more in observational and interventional studies is an area of research that has yet to have been fully explored.
• Features specific to consumer WPAMs that promote behavior change should be examined in functionally limited clinical populations. This will allow researchers and the consumer WPAM industry to determine what features are most effective for behavior change.
Conclusion
The use of consumer wearables for clinical populations is an area of research that will continue to grow. Fitbit monitors have been researched in all manners for a variety of clinical populations while smartwatches are beginning to see more utilization in observational and validation studies. With the smartwatch market expected to grow from $20.64 billion in 2019 to a projected $96.31 billion by 2027 [50], there is great potential for smartwatches to be included in future studies involving clinical populations. Regardless, the continued use of consumer WPAM for measuring PA outcomes and improving health-related factors for clinical populations continues to be a growing field with many exciting possibilities for use.
Conflicts of Interest
The results in this report do not constitute endorsement by the authors for the products described in the paper. The authors have no financial conflicts of interest with any of the monitor manufacturers and have received no research funding from these companies.
Funding Statement
Strath is supported by a grant from the National Institutes of Health (grant no. NIH R01CA215318).
Acknowledgements
The authors would like to thank Avery Bayer for his assistance in cataloging the characteristics of consumer wearables.
References
2. Safiri S, Kolahi AA, Smith E, Hill C, Bettampadi D, Mansournia MA, et al. Global, regional and national burden of osteoarthritis 1990-2017: a systematic analysis of the Global Burden of Disease Study 2017. Ann Rheum Dis. 2020;79(6):819-28.
3. Safiri S, Kolahi AA, Hoy D, Smith E, Bettampadi D, Mansournia MA, et al. Global, regional and national burden of rheumatoid arthritis 1990-2017: a systematic analysis of the Global Burden of Disease study 2017. Ann Rheum Dis. 2019;78(11):1463-71.
4. Dorsey ER, Elbaz A, Nichols E, Abd-Allah F, Abdelalim A, Adsuar JC, et al. Global, regional, and national burden of Parkinson’s disease, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17(11):939-53.
5. Wallin MT, Culpepper WJ, Nichols E, Bhutta ZA, Gebrehiwot TT, Hay SI, et al. Global, regional, and national burden of multiple sclerosis 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016.Lancet Neurol. 2019;18(3):269-85.
6. Ma VY, Chan L, Carruthers KJ. Incidence, Prevalence, Costs, and Impact on Disability of Common Conditions Requiring Rehabilitation in the United States: Stroke, Spinal Cord Injury, Traumatic Brain Injury, Multiple Sclerosis, Osteoarthritis, Rheumatoid Arthritis, Limb Loss, and Back Pain. Arch Phys Med Rehabil. 2014;95(5):986- 95.
7. Bull FC, Al-Ansari SS, Biddle S, Borodulin K, Buman MP, Cardon G, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020;54(24):1451-62.
8. Carty C, van der Ploeg HP, Biddle SJH, Bull F, Willumsen J, Lee L, et al. The First Global Physical Activity and Sedentary Behavior Guidelines for People Living With Disability. J Phys Act Health. 2021;18(1):86-93.
9. Motl RW, Mowry EM, Ehde DM, LaRocca NG, Smith KE, Costello K, et al. Wellness and multiple sclerosis: The National MS Society establishes a Wellness Research Working Group and research priorities. Mult Scler J. 2018;24(3):262-7.
10. Geenen R, Overman CL, Christensen R, Asenlof P, Capela S, Huisinga KL, et al. EULAR recommendations for the health professional’s approach to pain management in inflammatory arthritis and osteoarthritis. Ann Rheum Dis. 2018;77(6):797-807.
11. Regnaux JP, Davergne T, Palazzo C, Roren A, Rannou F, Boutron I, et al. Exercise programmes for ankylosing spondylitis. Cochrane Database Syst Rev. 2019(10):84.
12. Barker J, Byrne KS, Doherty A, Foster C, Rahimi K, Ramakrishnan R, et al. Physical activity of UK adults with chronic disease: cross-sectional analysis of accelerometermeasured physical activity in 96 706 UK Biobank participants. Int J Epidemiol. 2019;48(4):1167-74.
13. Brawner CA, Churilla JR, Keteyian SJ. Prevalence of Physical Activity Is Lower among Individuals with Chronic Disease. Med Sci Sports Exerc. 2016;48(6):1062-7.
14. Chang AH, Song J, Lee J, Chang RW, Semanik PA, Dunlop DD. Proportion and associated factors of meeting the 2018 Physical Activity Guidelines for Americans in adults with or at risk for knee osteoarthritis. Osteoarthritis Cartilage. 2020;28(6):774-81.
15. Thompson WR. Worldwide Survey of Fitness Trends for 2021. ACSMS Health Fit J. 2021;25(1):10-9.
16. Wearable Technology Market Size & Share | Industry Analysis Allied Market Research [Available from: https://www.alliedmarketresearch.com/wearable-technologymarket. (Accessed 22 September 2021).
17. Patel MS, Asch DA, Volpp KG. Wearable Devices as Facilitators, Not Drivers, of Health Behavior Change. JAMA-J Am Med Assoc. 2015;313(5):459-60.
18. Strath SJ, Rowley TW. Wearables for Promoting Physical Activity. Clin Chem. 2018;64(1):53-63.
19. Gal R, May AM, van Overmeeren EJ, Simons M, Monninkhof EM. The Effect of Physical Activity Interventions Comprising Wearables and Smartphone Applications on Physical Activity: a Systematic Review and Meta-analysis. Sports Med-Open. 2018;4:15.
20. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLos Med. 2021;18(3):15.
21. Booth BM, Mundnich K, Feng TT, Nadarajan A, Falk TH, Villatte JL, et al. Multimodal Human and Environmental Sensing for Longitudinal Behavioral Studies in Naturalistic Settings: Framework for Sensor Selection, Deployment, and Management. J Med Internet Res. 2019;21(8):28.
22. Butte NF, Ekelund U, Westerterp KR. Assessing Physical Activity Using Wearable Monitors: Measures of Physical Activity. Med Sci Sports Exerc. 2012;44:S5-S12.
23. Keadle SK, Lyden KA, Strath SJ, Staudenmayer JW, Freedson PS. A Framework to Evaluate Devices That Assess Physical Behavior. Exerc Sport Sci Rev. 2019;47(4):206- 14.
24. Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100(2):126-31.
25. Semanik P, Lee J, Pellegrini CA, Song J, Dunlop DD, Chang RW. Comparison of Physical Activity Measures Derived From the Fitbit Flex and the ActiGraph GT3X+in an Employee Population With Chronic Knee Symptoms. ACR Open Rheumatol. 2020;2(1):48-52.
26. Collins JE, Yang HY, Trentadue TP, Gong YS, Losina E. Validation of the Fitbit Charge 2 compared to the ActiGraph GT3X+in older adults with knee osteoarthritis in free-living conditions. PLoS One. 2019;14(1):14.
27. Koo TK, Li MY. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J Chiropr Med. 2016;15(2):155-63. Available from doi: 10.1016/j.jcm.2016.02.012
28. Block VJ, Zhao C, Hollenbach JA, Olgin JE, Marcus GM, Pletcher MJ, et al. Validation of a consumer-grade activity monitor for continuous daily activity monitoring in individuals with multiple sclerosis. Mult Scler J Exp Transl Clin. 2019;5(4):10.
29. Lai B, Sasaki JE, Jeng B, Cederberg KL, Bamman MM, Motl RW. Accuracy and Precision of Three Consumer-Grade Motion Sensors During Overground and Treadmill Walking in People With Parkinson Disease: Cross-Sectional Comparative Study. JMIR Rehabil Assist Technol. 2020;7(1):e14059.
30. Lamont RM, Daniel HL, Payne CL, Brauer SG. Accuracy of wearable physical activity trackers in people with Parkinson’s disease. Gait Posture. 2018;63:104-8.
31. Wendel N, Macpherson CE, Webber K, Hendron K, DeAngelis T, Colon-Semenza C, et al. Accuracy of Activity Trackers in Parkinson Disease: Should We Prescribe Them? Phys Ther. 2018;98(8):705-14.
32. Duclos NC, Aguiar LT, Aissaoui R, Faria C, Nadeau S, Duclos C. Activity Monitor Placed at the Nonparetic Ankle Is Accurate in Measuring Step Counts During Community Walking in Poststroke Individuals: A Validation Study. Pm&R. 2019;11(9):963-71.
33. Toth LP, Park S, Springer CM, Feyerabend MD, Steeves JA, Bassett DR. Video-Recorded Validation of Wearable Step Counters under Free-living Conditions. Med Sci Sports Exerc. 2018;50(6):1315-22.
34. Beukenhorst AL, Howells K, Cook L, McBeth J, O’Neill TW, Parkes MJ, et al. Engagement and Participant Experiences With Consumer Smartwatches for Health Research: Longitudinal, Observational Feasibility Study. Jmir Mhealth and Uhealth. 2020;8(1):12.
35. Manini TM, Mendoza T, Battula M, Davoudi A, Kheirkhahan M, Young ME, et al. Perception of Older Adults Toward Smartwatch Technology for Assessing Pain and Related Patient-Reported Outcomes: Pilot Study. Jmir Mhealth and Uhealth. 2019;7(3):13.
36. Jacquemin C, Servy H, Molto A, Sellam J, Foltz V, Gandjbakhch F, et al. Physical Activity Assessment Using an Activity Tracker in Patients with Rheumatoid Arthritis and Axial Spondyloarthritis: Prospective Observational Study. Jmir Mhealth and Uhealth. 2018;6(1):15.
37. Fortune J, Norris M, Stennett A, Kilbride C, Lavelle G, Victor C, et al. Pedometers, the frustrating motivators: a qualitative investigation of users’ experiences of the Yamax SW-200 among people with multiple sclerosis. Disabil Rehabil. 2020:7.
38. Block VJ, Bove R, Zhao C, Garcha P, Graves J, Romeo AR, et al. Association of Continuous Assessment of Step Count by Remote Monitoring With Disability Progression Among Adults With Multiple Sclerosis. JAMA Netw Open. 2019;2(3):15.
39. Pradhan S, Kelly VE. Quantifying physical activity in early Parkinson disease using a commercial activity monitor. Parkinsonism Relat Disord. 2019;66:171-5.
40. Katzan I, Schuster A, Kinzy T. Physical Activity Monitoring Using a Fitbit Device in Ischemic Stroke Patients: Prospective Cohort Feasibility Study. Jmir Mhealth and Uhealth. 2021;9(1):14.
41. Elmagboul N, Coburn BW, Foster J, Mudano A, Melnick J, Bergman D, et al. Physical activity measured using wearable activity tracking devices associated with gout flares. Arthritis Res Ther. 2020;22(1):9.
42. Bauer A, Lechner I, Auer M, Berger T, Bsteh G, Di Pauli F, et al. Influence of physical activity on serum vitamin D levels in people with multiple sclerosis. PLoS One. 2020;15(6):9.
43. Ryan JM, Stennett AM, Peacock S, Baker G, Norris M. Associations between activity and participation in adults with multiple sclerosis: a cross sectional study. Physiotherapy. 2019;105(4):453-60.
44. Sasaki S, Kanai M, Shinoda T, Morita H, Shimada S, Izawa KP. Relation between health utility score and physical activity in community-dwelling ambulatory patients with stroke: a preliminary cross-sectional study. Top Stroke Rehabil. 2018;25(7):475-9.
45. Kanai M, Izawa KP, Kubo H, Nozoe M, Mase K, Shimada S. Association of Health Utility Score with Physical Activity Outcomes in Stroke Survivors. Int J Environ Res Public Health. 2021;18(1):9.
46. Lazaridou A, Paschali M, Schreiber K, Galenkamp L, Berry M, Paschalis T, et al. The association between daily physical exercise and pain among women with fibromyalgia: the moderating role of pain catastrophizing. Pain Rep. 2020;5(4):6.
47. Li LC, Feehan LM, Xie H, Lu N, Shaw CD, Gromala D, et al. Effects of a 12-Week Multifaceted Wearable- Based Program for People With Knee Osteoarthritis: Randomized Controlled Trial. Jmir Mhealth and Uhealth. 2020;8(7):16.
48. Ellis TD, Cavanaugh JT, DeAngelis T, Hendron K, Thomas CA, Saint-Hilaire M, et al. Comparative Effectiveness of mHealth-Supported Exercise Compared With Exercise Alone for People With Parkinson Disease: Randomized Controlled Pilot Study. Phys Ther. 2019;99(2):203-16.
49. McDermott MM, Spring B, Berger JS, Treat-Jacobson D, Conte MS, Creager MA, et al. Effect of a Home-Based Exercise Intervention of Wearable Technology and Telephone Coaching on Walking Performance in Peripheral Artery Disease The HONOR Randomized Clinical Trial. JAMA-J Am Med Assoc. 2018;319(16):1665-76.
50. Smartwatch Market Size, Share & Industry Growth | Analysis - 2027 [Available from: https://www. alliedmarketresearch.com/smartwatch-market. (Accessed 22 September 2021).