Abstract
Objectives: Hypertension in pregnancy is one of the leading causes of maternal mortality and rapid treatment is critical. Treatment of emergent hypertensive crises in pregnancy is limited to only three medications, one of which is labetalol. Current literature recommends a daily maximum intravenous labetalol dose of 300 mg per day. However, this maximum dose has not been studied in pregnancy where higher doses may be required.
Study Design: This is a retrospective study of Obstetric patients from 12 hospitals from October 2010 to May 2018. Patients >18 years old were included and stratified into those receiving ≤ 300 mg labetalol and >300 mg in a 24-hour period.
Main Outcome Measures: Primary outcomes included maternal hypotension and bradycardia. A secondary outcome was fetal bradycardia.
Results: A total of 238 women met criteria: 208 patients in the ≤ 300mg labetalol group and 30 patients in the >300 mg labetalol group. Demographics were not different between groups except for a higher rate of cesarean delivery in the >300 mg group (63.3%). Unadjusted outcomes showed no differences between the >300 mg group and the ≤ 300 mg labetalol group in maternal hypotension (3.3% vs. 5.8%; p>0.999) but an increased rate of maternal bradycardia in the >300 mg group (30% vs. 13.5%; p=0.029). After adjusting for confounding factors there was no association seen between labetalol dose and adverse events (p=0.316). There was no difference in fetal bradycardia between the two groups (0% vs. 2.9%; p>0.999).
Conclusions: Doses of intravenous labetalol >300 mg were not associated with increased rates of maternal hypotension or bradycardia or fetal bradycardia.
Keywords
Antihypertensive, Beta-blocker, Hypertension, Hypertensive emergency, Obstetrics
Introduction
Medical management of hypertension in pregnancy is indicated for severe range blood pressures. This is diagnosed with either systolic blood pressure (SBP) ≥ 160 mm Hg and/or diastolic blood pressure (DBP) ≥110 mm Hg on two occasions at least 4 hours apart [1]. When this diagnosis is established, fast-acting anti-hypertensive medications can be utilized for acutely severe range blood pressures. The goal of medications is not to normalize blood pressures, but rather to reduce them just below the severe range (<160/110 mmHg) so as to avoid hypoperfusion to the fetus and woman [1].
Not all anti-hypertensive medications are safe in pregnancy, but three well-studied medications that are used first line in pregnancy include labetalol, nifedipine, and hydralazine. Multiple studies have been performed comparing the effectiveness between these medications [1-4]. Labetalol is a mixed alpha/beta adrenergic antagonist that is frequently given intravenously to patients experiencing hypertensive crises or severe range blood pressures in the acute care setting. The main adverse events for labetalol include maternal hypotension and bradycardia. Maternal hypotension can then lead to hypo-perfusion in the fetus, resulting in fetal bradycardia [5]. For hypertension in pregnancy, the American College of Obstetrics and Gynecology (ACOG) Task Force outlines the steps for use of intravenous (IV) labetalol in the setting of acutely severe blood pressures that persist for 15 minutes or more [2]. However, depending on the patient and the control of her hypertensive disorder, this same algorithm may need to be used later within a 24-hour period.
This becomes limiting when the labetalol manufacturer recommends a maximum dose of 300 mg IV labetalol in a 24- hour period. This parameter was set by original trials where the maximum dose of labetalol used was 300 mg and is not backed up by any other data [6]. To date, no published research studies have examined the safety of high dose IV labetalol use in pregnancy. The most recently published study included 188 patients, and showed that doses of labetalol greater than 300 mg rarely caused clinically significant hemodynamic compromise. However, this study excluded pregnant women [7]. Our study aims to address this gap in the literature by looking at the safety of this practice with a large sample of pregnant women who received high dose labetalol. Our objective is to compare the acute incidence of adverse events in pregnant women who received >300 mg vs. ≤ 300 mg IV labetalol in a 24-hour period.
Methods
This is a retrospective, multicenter study conducted at 12 hospitals that are part of the Trinity Health network, a national Catholic Health System. The Institutional Review Board of St. Joseph Mercy Ann Arbor approved the study to include the records of patients admitted to a Trinity Health hospital from October 1, 2010 through May 31, 2018.
Patients were included in the study if they were pregnant, aged 18 years or older, had an inpatient admission, and had received at least a total of 300 mg of IV labetalol hydrochloride during their entire admission. Exclusion criteria included receipt of concomitant IV anti-hypertensive medications in addition to labetalol. These were defined as IV metoprolol, hydralazine, nicardipine, or nitroglycerine.
Data scientists employed by Trinity Health accessed patient records. Data included basic demographic information such as gender and age, past medical history, body mass index (BMI), and admitting diagnosis. We also obtained the inpatient medication list, vital signs, hospital length of stay, mode of delivery, and fetal heart rate.
Our primary outcome was the rate of maternal hypotension, defined as SBP less than 90 mm Hg, and maternal bradycardia, defined as HR less than 60 beats per minute (bpm). The secondary outcome was the rate of fetal bradycardia, defined as fetal HR less than 110 bpm.
Each patient had the maximum dose of administered IV labetalol in a 24-hour time frame manually calculated. This time frame started when the patient began receiving IV labetalol and finished either a) 28 hours after the onset (to include the 24 hours plus a 4-hour wash out period) or b) the occurrence of an adverse event including maternal hypotension or bradycardia or fetal bradycardia. Women that received ≤ 300 mg IV labetalol (“low-dose labetalol”) in the time period were put into one group, and women that received >300 mg IV labetalol (“high-dose labetalol”) were put into another group. The frequency of maternal hypotension, maternal bradycardia, or fetal bradycardia was then analyzed and compared between the two groups.
Baseline demographic and clinical characteristics between high-dose labetalol and low-dose labetalol groups were summarized using means (standard deviation) for continuous variables and N (percentage) for categorical variables. Difference in the variables between the two groups were compared by two sample t-test or chi square test, wherever appropriate. Generalized Estimating Equations were used to estimate the association between labetalol dose (“high” versus “low”) and the incidence of binary outcomes. This model adjusts for baseline characteristics that are considered as known and suspected confounders: age, BMI, mode of delivery, and use of oral anti-hypertensive agents. All statistical tests were two-sided. A p-value less than 0.05 was considered statistically significant. All analyses were performed in R (Version 3.5.2).
Results
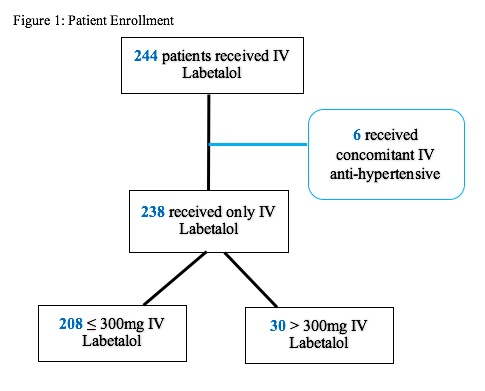
We identified 244 women who received more than 300 mg IV labetalol hydrochloride during their entire hospital admission. Six women were excluded for receiving a concomitant IV antihypertensive, resulting in 238 women. 208 of these women received ≤ 300mg IV (“low dose group”) and the remaining 30 women received >300 mg (“high dose group”) of IV labetalol in 24 hours (Figure 1).
Figure 1. Patients enrolled in the study using exclusion criteria of concomitant intravenous anti-hypertensives, and then dividing based on dosage of intravenous labetalol received in 24 hours.
Baseline demographics such as race, smoking status, age, BMI, gravity, parity, maternal baseline heart rate, maternal baseline SBP, and hospital length of stay were all similar between groups (Table 1). One statistically significant difference between the two groups was regarding mode of delivery, with a higher percentage of low dose labetalol patients having a vaginal delivery whereas a higher percentage of high dose labetalol patients had a cesarean delivery (p=0.045). In the low dose labetalol group, 59 of 208 (28%) women had a vaginal delivery and 118 (56%) had a cesarean section. In contrast, 19 of the 30 (63%) women in the high dose labetalol group had a cesarean section and only 3 (10%) had a vaginal delivery. Gestational age in both the high and low dose labetalol group was 34.2 weeks. The only other statistically significant difference in demographics was regarding baseline diastolic blood pressure (DBP). The low dose labetalol group had a baseline DBP of 99.709 whereas the high dose labetalol group was significantly lower, with a DBP baseline of 93.607 (p=0.027).
| Variable | ≤ 300 mg IV labetalol (N=208) | >300 mg IV labetalol (N=30) | p-value |
| Race, No (%) | 0.174 | ||
| Black | 92 (44.2%) | 15 (50%) | |
| White | 88 (42.3%) | 8 (26.7%) | |
| Other/Declined | 28 (13.5%) | 7 (23.3%) | |
| Delivery Type, No (%) | 0.045 | ||
| Cesarean | 118 (56.7%) | 19 (63.3%) | |
| Vaginal | 59 (28.4%) | 3 (10%) | |
| Other | 31 (14.9%) | 8 (26.7%) | |
| Smoking Status, No (%) | 0.98 | ||
| Current | 14 (6.7%) | 2 (6.7%) | |
| Former | 29 (13.9%) | 3 (10%) | |
| Never | 151 (72.6%) | 23 (76.7%) | |
| Unknown | 14 (6.7%) | 2 (6.7%) | |
| Age, years SD | 30.2 (6.2) | 31.8 (5.7) | 0.176 |
| BMI, kg/m2 ± SD | 38.6 (18.6) | 44.6 (43.4) | 0.462 |
| Baseline SBP (mmHg) | 174.1 (21.4) | 169.1 (24.1) | 0.312 |
| Baseline DBP (mmHg) | 99.7 (13.5) | 93.6 (12.9) | 0.027 |
| Baseline HR | 84.1 (15.1) | 81.1 (14.8) | 0.329 |
| Maternal Gravity | 2.3 (2.1) | 2.8 (2.9) | 0.435 |
| Maternal Parity | 0.83 (1.5) | 0.92 (1.8) | 0.811 |
| Hospital Total LOS Days | 6.6 (6.2) | 6.0 (3.6) | 0.475 |
| Hospital LOS prior to starting IV labetalol (Hours) | 14.7 (54.3) | 15.2 (40.5) | 0.953 |
| SDP: Systolic Blood Pressure; DBP: Diastolic Blood Pressure; HR: Heart Rate; LOS: Length of Stay | |||
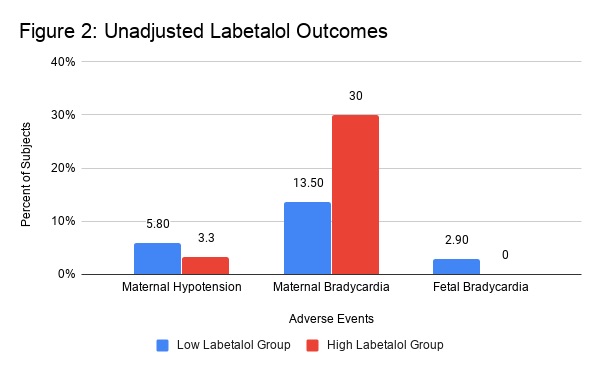
Looking at unadjusted outcomes of maternal hypotension, maternal bradycardia, and fetal bradycardia, there was no statistically significant difference in rates of maternal hypotension or fetal bradycardia between the high and low dose labetalol groups (Table 2, Figure 2). The primary outcome of maternal hypotension occurred in 12 women (5.8%) in the low dose labetalol group and 1 woman (3.3%) in the high dose labetalol group. Without adjusting for confounders, patients in the high dose labetalol group had a significantly higher incidence rate for maternal bradycardia, with 9 of the 30 (30%) women in the high dose group experiencing bradycardia as opposed to 28 of the 208 women in the low dose group (13.5%) (p=0.29).
| Variable | ≤ 300 mg IV labetalol (N=208) | >300 mg IV labetalol (N=30) | p-value |
| Maternal Hypotension, No (%) | 12 (5.8%) | 1 (3.3%) | >0.999 |
| Maternal Bradycardia, No (%) | 28 (13.5%) | 9 (30%) | 0.029 |
| Fetal Bradycardia, No (%) | 3 (2.9%) | 0 (0%) | >0.999 |
Figure 2. Unadjusted outcomes (percentiles) of adverse events compared between the high and low dose labetalol groups.
However, after adjusting for confounders (Table 3), the high dose labetalol group did not have an association with maternal bradycardia (OR 1.774; 95% CI 0.578-5.442; p=0.316). Increases in maternal age as well as BMI were found to be associated with a higher incidence of maternal bradycardia. Every oneyear increase in age conveyed a 10.2% increased risk (p=0.009) and every BMI increase of 1 conveyed an additional risk of 1.3% (p=0.013). This association was not found when looking at maternal hypotension. Finally, three subjects of the low dose labetalol group experienced fetal bradycardia, in contrast to none in the high dose labetalol group.
| Covariate | Odds Ratio (OR) | 95% CI for OR | p-value |
| >300 mg IV labetalol | 1.78 | (0.58-5.44) | 0.316 |
| Age | 1.10 | (1.03-1.19) | 0.009 |
| BMI | 1.01 | (1.00-1.02) | 0.013 |
| Cesarean Delivery | 2.16 | (0.82-5.68) | 0.120 |
| PO Anti-hypertensive | 0.55 | (0.23-1.34) | 0.187 |
Discussion
Doses of intravenous labetalol >300 mg administered in a 24-hour period were not associated with adverse maternal or fetal events in our study population. Our primary outcome of maternal hypotension and bradycardia did not show any difference in rates between the group receiving >300 mg IV labetalol versus the group receiving ≤ 300 mg IV labetalol during our study timeframe. Our secondary outcome of fetal bradycardia did not show an association with receiving >300 mg IV labetalol. This information is an important first step in analyzing the safety profile of a commonly used antihypertensive in the pregnant population. While it does not conclusively mean that providers should start administering >300 mg IV labetalol in a 24-hour period, it does suggest that current recommendations may be too restrictive.
There were a few limitations to the study. One is the fact that this is a retrospective review and we are limited to what patient information we could extract, including gestational age at time of labetalol administration as well as timing of labetalol administration in correlation to timing of delivery. Additionally, our sample size was also smaller than we anticipated.
Additionally, our sample size was small for drawing strong statistical associations but given that is an infrequent clinical event to administer >300 mg of labetalol our multicenter design allowed us to capture a relatively large sample size. This is especially true regarding fetal data, because not all patients had fetal information available to analyze. We were unable to extract data regarding fetal tracing categories and therefore do not know variability and presence or absence of accelerations and decelerations. Instead, fetal heart rate was used to assess fetal well-being, but this may not always accurately represent fetal outcomes. Having a larger study could possibly reveal further trends that were not discovered from our analyses. Finally, our patients received concomitant agents; however, these data were captured and were addressed as either exclusion criteria or a confounder during our analysis.
A strength to our study is that it is a multi-center study, and therefore more generalizable to the pregnant population. Our study also analyzed patients that received at least 300 mg IV labetalol during their entire hospital admission. This was done because if there truly was going to be a difference between labetalol groups, we wanted to be able to see it. Our robust data set gave us the exact dosing of labetalol and the titrations were known, making our results more accurate. Additionally, all adverse events of hypotension and bradycardia were selflimiting and no rescue therapy was needed among patients that did experience these side effects.
Cardiovascular disease is a primary cause of maternal morbidity and mortality, and hypertensive emergencies are a subgroup of this. With increasing national focus on ways to decrease maternal morbidity and mortality, more protocols are created to standardize management of high-risk obstetric patients. Therefore, it is important to understand the safety profile of the medications that are administered and what risks may or may not be associated with such treatments. The current IV labetalol algorithm as recommended by ACOG in hypertensive emergency is to begin with 20 mg, followed by 40 mg, and then 80 mg. This gives a total of 140 mg in the span of as little as 30 minutes, and is close to half of the current maximum daily dose. If there is allowance for higher doses of labetalol within this algorithm, it could potentially permit further blood pressure control while avoiding the shortening of a patient’s labor progression.
Further prospective studies are needed regarding labetalol administration and safety. Future directions would involve collecting data on the patient’s gestational age, singleton versus multiple gestation pregnancy, and postpartum patients. Including neonatal information such as cord blood pH, glucose levels, and NICU admissions would also be beneficial. This opens doors for further investigation regarding the appropriate daily dosing of IV labetalol and options for management in a hypertensive emergency.
Acknowledgements
The authors would like to thank the St. Joseph Mercy Department of Obstetrics and Gynecology, Ann Arbor, MI as well as Caleb Scheidel, MS, of Methods Consultants Ann Arbor, MI for assistance in this research.
Conflict of Interest
The authors report no conflict of interest.
References
2. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstetrics and Gynecology. 2013;122(5):1122-1131.
3. Committee on Obstetric Practice. Committee opinion no. 623: emergency therapy for acute-onset, severe hypertension during pregnancy and the postpartum period. Obstetrics and Gynecology. 2015;125(2):521-525.
4. Easterling T. Pharmacologic management of hypertension in Pregnancy. Seminars in Perinatology. 2014;38(8):487-495.
5. Bateman BT, Patorno E, Desai RJ, Seely EW, Mogun H, Maeda A, et al. Late Pregnancy Beta Blocker Exposure and Risks of Neonatal Hypoglycemia and Bradycardia. Pediatrics. 2016;138(3):e20160731.
6. Trandate (labetalol hydrochloride) Injection. NDA 19425/S-021. Prometheus Laboratories, Inc. 2010 [cited 2022 June 6].
7. Hecht JP, Mahmood SM, Brandt MM. Safety of high-dose intravenous labetalol in hypertensive crisis. Am J Health-Syst Pharm. 2019; 68:286-93.