Abstract
Objective: Evaluate the prevalence of anemia in term and preterm pregnancies and compare maternal and perinatal outcomes among groups.
Methods: Secondary analysis of Brazilian Multicenter Study on Preterm Birth (EMIP). Cross sectional study on preterm births, with sample of term births to evaluate risk factors and comparisons. Current analysis compared prevalence of anemia in term and preterm births and among their types (spontaneous preterm birth (sPTB), preterm premature rupture of membranes (pPROM), or provider initiated preterm birth (piPTB)) and between women with anemia in both groups, correlating maternal and neonatal findings. Multivariate analysis to identify conditions that independently associated to anemia in term and preterm groups was performed.
Results: Study included 3716 preterm and 1048 term births with hemoglobin level data. The prevalence of anemia was 33.23% and 27.74% in preterm and term pregnancies respectively. Anemia was higher among the pPROM group (36.3%, p=0.029). Less than 8 schooling years (p=0.003), maternal age <19 years old (p=0.047), non-white skin color (p<0.001) and children under 5 years (p<0.001) were associated with anemia and PTB. In this group, anemia was associated with adverse perinatal outcomes, including need of ventilatory support (p=0.003), sepsis (p=0.006), endocrine dysfunction (p=0.001), small for gestational age newborns (p<0.001), 5th minute Apgar score <7 (p=0.001), and neonatal death (p=0.002). Multivariate analysis showed association between living area and anemia in term group (p=0.001), urinary tract infection and anemia in sPTB and piPTBgroup (p<0.001), neonatal morbidity (p=0.001), inadequate number of prenatal care visits (p=0.009) and anemia in pPROM group.
Conclusion: Anemia was associated with poor maternal education, children below 5 years, late onset of prenatal care and less than six medical visits. Also, it is more prevalent in preterm births, especially among cases of pPROM and could be either associated to its cause or consequence, worsening an unfavorable condition.
Keywords
Anemia, Pregnancy, Preterm birth, Antenatal care, Adverse neonatal outcomes
Background
Prematurity is a major health problem and the main cause of neonatal morbidity and mortality worldwide [1,2]. The World Health Organization (WHO) estimates an annual incidence of 15 million preterm deliveries worldwide. In Brazil, it is reported to be between 9.9% and 12.3% [1,2], accounting for major health costs and burden. The main underlying causes of preterm delivery are spontaneous preterm birth (sPTB), preterm premature rupture of membranes (pPROM) and provider-initiated preterm birth (piPTB) due to either adverse maternal and/or fetal conditions [1-3], with estimated rates of 40-45%, 25-30% and 30-35% respectively [3].
Physiopathology of preterm birth is not fully understood and is described as multifactorial, associated to social and environmental conditions that can be hardly identifiable. It is estimated that around 50% of these births remain without a defined cause after childbirth [2,3]. Risk factors for prematurity include maternal malnutrition, short interpregnancy interval (6 months or less), previous preterm birth, infections, smoking and anemia [1-3]. Also, multiple pregnancy and conditions that can affect fetal well-being such as preeclampsia, placental dysfunction and fetal growth restriction are responsible for increasing prematurity rates in the group of piPTB [4].
Anemia in pregnancy is defined based on WHO’s criterion of hemoglobin level below 11g/dL [5,6]. The WHO estimates a prevalence of 41.8% of anemia in pregnant women, but it varies in low- and high-income countries [1,3,5,6]. Several studies showed anemia as a risk factor for prematurity, pPROM, cesarean delivery, low birth weight, high neonatal morbidity, maternal blood transfusion and infections, gestational hypertension and preeclampsia [5-10].
As preterm birth is highly associated with neonatal morbidity and mortality and also with the presence of anemia, this secondary analysis of a national multicentric study is intended to evaluate the prevalence of and factors associated with anemia among cases of preterm and term births and its association with poor maternal and perinatal outcomes.
Methods
The present study is a secondary analysis of the Brazilian Multicenter Study on Preterm Birth (EMIP). The study was reviewed and approved by the National Council for Ethics in Research (CONEP) and by the Institutional Review Board of each participating center (#0564.1.146.000- 09). All women included in the study signed an individual informed consent after understanding the conditions of the study.
The study protocol and complete methods have been previously published elsewhere [1,11]. Briefly, the EMIP study was conducted in 20 referral obstetric hospitals from the three most populated Brazilian regions: Southeast, Northeast and South. It was a prospective cross-sectional study that screened 33,740 births from April 2011 to July 2012, enrolling all cases of preterm birth and their infants. Then, causes of preterm births were separately described. In addition, it enrolled an intentional sample of term births randomly identified immediately after a preterm one.
For the main study, sample size was calculated based on the Brazilian preterm birth prevalence at the time, reported as 6.5% in 2006 with allocation of at least 1054 subjects in each group to allow for all the planned analyses [2]. Information was collected from an interview with the women during admission for childbirth and through review of their medical records until 60 days if the neonate remained hospitalized [1,11].
For this secondary analysis, women with no information about anemia and hemoglobin levels in their medical records were excluded. The remaining women were divided in two groups, according to the presence of anemia, and analysis performed for preterm (before 37 weeks), term (37 weeks or more) and comparing both conditions. Anemia was defined as hemoglobin level below 11g/dL anytime during pregnancy, according to the WHO definition [4].
The prevalence of anemia was described in both term and preterm groups, and among different types of PTB (sPTB, pPROM and piPTB). We compared maternal and perinatal outcomes of preterm and term births with and without anemia, plus, only for women with anemia, term and preterm births.
The variables under study included sociodemographic characteristics (maternal age, urban or rural area, region of the country (Southeast, Northeast, South), skin color as a proxy for ethnicity, marital status, years of schooling, previous morbidity, previous obstetric history (other children under 5 years old, previous cesarean section, interpregnancy interval , previous preterm birth, previous newborn under 2500g), adequacy of prenatal care (evaluated through onset of prenatal care at first trimester or later, number or prenatal care visits, gestational weight gain, initial body mass index, use of alcohol or drugs). We also assessed the presence of urinary tract infection, vaginal bleeding, occurrence of fetal morbidity), route of childbirth, and neonatal outcomes as birth weight, 5th minute Apgar score <7, fetal death, neonatal ventilatory support, neonatal morbidity, intraventricular hemorrhage and neonate’s condition at discharge.
Statistical analysis for categorical variables was performed with Chi-Square test or Fisher’s exact test where appropriate, while for continuous variables Mann Whitney test was performed with. Then, a multivariate analysis was run to evaluate whether any variables/ conditions were independently associated with anemia in term and preterm births. For that, logistic regression was performed. We considered p-value of <0.05 as statistically significant. Data analyses were performed using SAS (Statistical Analysis System for windows, version 9.4. SAS Institute Inc, 2002-2008, Cary, NC, USA).
Results
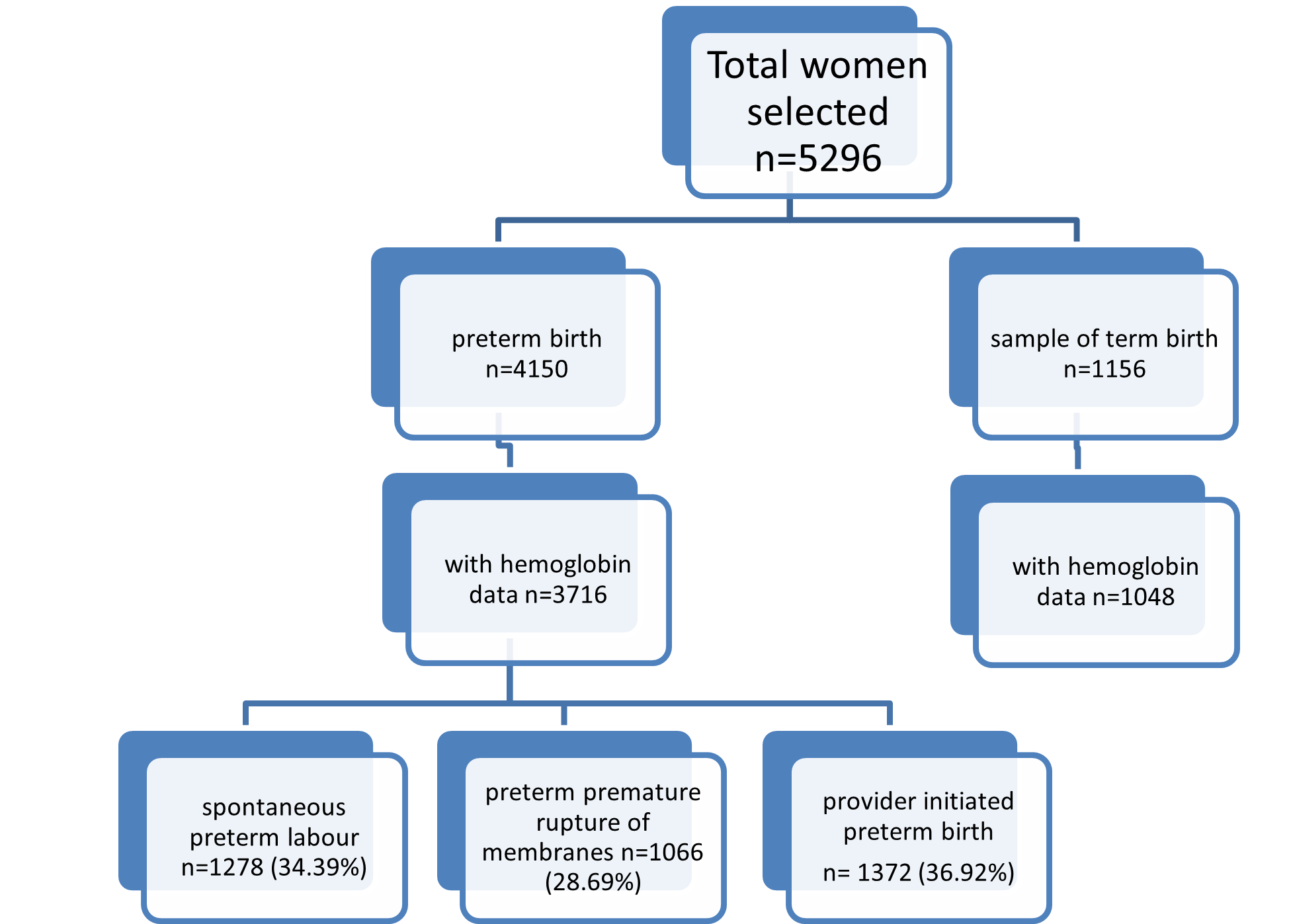
The EMIP study identified 4150 preterm births (≤37 weeks gestation) during data collection and selected a sample of 1156 term births as a control group. For our current secondary analysis, we excluded 434 (10.46%) and 108 (9.42%) cases from preterm and term groups respectively, due to lack of information on the hemoglobin level for identifying anemia. Among all preterm births, 1278 women (34.39%) had spontaneous preterm birth (sPTB), 1066 (28.69%) preterm premature rupture of membranes (pPROM) and 1372 women (36.92%) had a provider-initiated preterm birth (piPTB) (Figure 1).
Figure 1. Flowchart of included cases in the study.
The overall prevalence of anemia in preterm births was 33.23% while in term births was 27.74% (p=0.01). Frequency of anemia was significantly higher among the pPROM group (36.3%, p=0.029). Among spontaneous preterm births the frequency of anemia was 32.79% and in the piPTB group it was 31.27%.
Table 1 shows the maternal characteristics according to gestational age at birth and the presence of anemia. Age ≤19 years (p=0.047), non-white skin color (p=0.001), living in the Northeast region (p=0.001), ≤8 schooling years (p=0.003), history of morbidity (p=0.003) and having children under 5 years old (p=0.001), were significantly associated with anemia and preterm birth. Among the group of term childbirth, a significant association with anemia was found for women living in urban area (p=0.001) and with non-white skin color (p=0.015). Furthermore, comparing only the women with anemia, between preterm and term pregnancies, living in the Southeast region (p=0.026), ≤8 schooling years (p=0.001), ≤12 months interpregnancy interval (p=0.021), previous preterm birth (p=0.001) and previous low birth weight (p=0.001) were associated with preterm deliveries.
| Maternal profile | Gestational age at birth | ||||||
|---|---|---|---|---|---|---|---|
| < 37 weeks | ≥ 37 weeks | p | |||||
| Anemia yes n (%) | Anemia no n (%) | p | Anemia yes n (%) | Anemia no n (%) | p | Anemia Yes <37 x ≥ 37 |
|
| Maternal age (years) | 1235 | 2481 | 0.047 | 288 | 750 | 0.559 | 0.292 |
| ≤19 | 276 (22.3) | 472 (19.0) | 58 (20.1) | 130 (17.3) | |||
| 20-34 | 786 (63.6) | 1626 (65.5) | 197 (68.4) | 535 (71.3) | |||
| ≥35 | 173 (14.0) | 383 (15.4) | 33 (11.5) | 85 (11.3) | |||
| Housing area | 1231 | 2471 | 0.239 | 287 | 746 | 0.001 | 0.113 |
| Rural | 103 (8.4) | 236 (9.6) | 16 (5.57) | 94 (12.6) | |||
| Urban | 1128 (91.6) | 2235 (90.5) | 271 (94.4) | 652 (87.4) | |||
| Region | 1235 | 2481 | 0.001 | 288 | 750 | 0.65 | 0.026 |
| Southeast | 703 (56.9) | 1388 (55.9) | 143 (49.6) | 392 (52.3) | |||
| Northeast | 399 (32.3) | 725 (29.2) | 117 (40.6) | 296 (39.5) | |||
| South | 133 (10.8) | 368 (14.8) | 28 (9.7) | 62 (8.3) | |||
| Skin color | 1235 | 2481 | <0.001 | 288 | 750 | 0.015 | 0.064 |
| White | 493 (39.9) | 1181 (47.6) | 98 (34.0) | 317 (42.3) | |||
| Other | 742 (60.1) | 1300 (52.4) | 190 (65.9) | 433 (57.7) | |||
| Marital status | 1235 | 2481 | 0.067 | 288 | 750 | 0.817 | 0.081 |
| With a partner | 940 (76.1) | 1954 (78.8) | 233 (80.9) | 602 (80.3) | |||
| Without a partner | 295 (23.9) | 527 (21.2) | 55 (19.1) | 148 (19.7) | |||
| Schooling (years) | 1229 | 2433 | 0.003 | 287 | 737 | 0.257 | 0.001 |
| ≤8 | 530 (43.1) | 912 (37.5) | 91 (31.7) | 274 (37.2) | |||
| 9-12 | 609 (49.6) | 1305 (53.6) | 172 (59.9) | 408 (55.4) | |||
| >12 | 90 (7.3) | 216 (8.9) | 24 (8.4) | 55 (7.5) | |||
| Morbid history | 1125 | 2344 | 0.001 | 272 | 715 | 0.289 | 0.665 |
| No | 887 (78.8) | 1829 (78.0) | 216 (79.4) | 589 (82.4) | |||
| High blood pressure | 71 (6.3) | 171 (7.3) | 12 (4.4) | 33 (4.6) | |||
| Diabetes | 14 (1.2) | 25 (1.1) | 2 (0.7) | 7 (0.9) | |||
| Blood Disease | 23 (2.0) | 14 (0.6) | 6 (2.2) | 5 (0.7) | |||
| Other | 130 (11.6) | 305 (13.0) | 36 (13.2) | 81 (11.3) | |||
| Children under 5 years | 1234 | 2479 | 288 | 749 | |||
| Yes | 368 (29.8) | 573 (23.1) | <0.001 | 85 (29.5) | 193 (25.8) | 0.222 | 0.981 |
| No | 866 (70.2) | 1906 (79.9) | 203 (70.5) | 556 (74.2) | |||
| Previous C-section | 1234 | 2481 | 288 | 750 | |||
| Yes | 276 (22.4) | 546 (22.0) | 0.803 | 66 (22.9) | 153 (20.4) | 0.373 | 0.840 |
| No | 958 (77.6) | 1935 (77.9) | 222 (77.1) | 597 (79.6) | |||
| Inter-pregnancy interval | 732 | 1443 | 161 | 423 | |||
| ≤12 months | 74 (10.11) | 124 (8.6) | 0.245 | 7 (4.4) | 24 (5.7) | 0.523 | 0.021 |
| >12 months | 658 (89.9) | 1319 (91.4) | 154 (95.6) | 399 (94.3) | |||
| Previous PTB* | 1230 | 2475 | 288 | 750 | |||
| Yes | 242 (19.7) | 473 (19.1) | 0.682 | 23 (7.9) | 64 (8.5) | 0.775 | <0.001 |
| No | 988 (80.3) | 2002 (80.9) | 265 (92.0) | 686 (91.5) | |||
| Previous LBW | 1224 | 470 | 288 | 743 | |||
| Yes | 208 (16.9) | 410 (16.6) | 0.762 | 20 (6.9) | 53 (7.1) | 0.915 | <0.001 |
| No | 1016 (83.0) | 2060 (83.4) | 268 (93.1) | 690 (92.9) | |||
| *PTB: preterm birth | |||||||
Gestational characteristics associated with preterm birth and the presence of anemia includes late onset of prenatal care (p=0.001), <6 prenatal care visits (p=0.007), being pre-pregnancy underweight or normal weight (p=0.001), use of drugs before or during pregnancy (p=0.037), urinary tract infection (p=0.001) and vaginal bleeding (p=0.001), as shown in Table 2. Comparing only the women with anemia, the absence of prenatal care (p=0.001), <6 prenatal care visits (p=0.001), gestational weight gain ≤7 (p=0.001), urinary tract infection (p=0.005), vaginal bleeding (p=0.001) and cesarean section (p=0.0026) were associated with preterm.
| Gestational profile | Gestational age at birth | ||||||
|---|---|---|---|---|---|---|---|
| <37 weeks | ≥37 weeks | p | |||||
| Anemia yes n (%) | Anemia no n (%) | p | Anemia yes n (%) | Anemia no n (%) | p | Anemia Yes <37 x ≥37 |
|
| Prenatal care | 1235 | 2481 | 0.001 | 288 | 750 | 0.564 | 0.001 |
| Yes | 1190 (96.4) | 2444 (98.5) | 288 (100) | 747 (99.6) | |||
| No | 45 (3.6) | 37 (1.5) | 0 (0) | 3 (0.4) | |||
| Onset of prenatal care |
1009 | 2161 | 0.001 | 255 | 664 | 0.006 | 0.443 |
| First trimester | 620 (61.5) | 1453 (67.2) | 150 (58.8) | 454 (68.4) | |||
| Second/third trimester | 389 (38.6) | 708 (32.8) | 105 (41.2) | 210 (31.6) | |||
| Adequacy of number of prenatal care visits* |
1061 | 2265 | 0.007 | 273 | 700 | 0.052 | <0.001 |
| Adequate (≥6) | 538 (50.7) | 1291 (57.0) | 199 (72.9) | 551 (78.7) | |||
| Inadequate (<6) | 523 (49.3) | 974 (43.0) | 74 (27.1) | 149 (21.3) | |||
| Weight gain in pregnancy | 1077 | 2221 | 0.495 | 263 | 768 | 0.577 | <0.001 |
| ≤ 7 kg | 367 (34.1) | 713 (32.1) | 53 (20.2) | 148 (21.8) | |||
| 8-12 kg | 363 (33.7) | 759 (34.2) | 91 (64.6) | 211 (31.1) | |||
| >12 kg | 347 (32.2) | 749 (33.7) | 119 (45.3) | 319 (47.1) | |||
| Initial body mass index | 1090 | 2170 | <0.001 | 261 | 666 | 0.352 | 0.982 |
| <18.5 kg/m2: under- weight | 111 (10.2) | 156 (7.2) | 25 (9.6) | 50 (7.51) | |||
| 18.5–24.99 kg/m2: normal |
631 (57.9) | 1160 (53.5) | 152 (58.2) | 372 (55.9) | |||
| 25-29.99 kg/m2: over- weight | 222 (20.4) | 501 (23.1) | 55 (21.1) | 144 (21.6) | |||
| ≥30 kg/m2: obesity | 126 (11.6) | 353 (16.3) | 29 (11.1) | 100 (15.1) | |||
| Alcohol drinking | 1226 | 2464 |
0.576 |
286 | 745 | 0.141 | 0.176 |
| Yes (often) | 16 (1.3) | 27 (1.10) | 7 (2.45) | 8 (1.07) | |||
| No or few times | 1210 (98.7) | 2437 (98.9) | 279 (97.6) | 737 (98.9) | |||
| Use of drugs | 1235 | 2481 |
0.037 |
288 | 75 | 0.204 | 0.179 |
| Never used | 1162 (94.1) | 2379 (95.9) | 274 (95.1) | 730 (97.3) | |||
| Used before pregnancy | 38 (3.1) | 59 (2.4) | 11 (3.8) | 16 (2.1) | |||
| Used during pregnancy | 35 (2.8) | 43 (1.7) | 3 (1.0) | 4 (0.5) | |||
| Urinary tract infection | 1223 | 2468 |
<0.001 |
288 | 748 | 0.614 | 0.005 |
| Yes | 560 (45.8) | 895 (36.3) | 106 (36.8) | 288 (38.5) | |||
| No | 663 (54.2) | 1573 (63.7) | 182 (63.2) | 460 (61.5) | |||
| Anemia (patient report) | 1206 | 2444 |
<0.001 |
286 | 746 | <0.001 | 0.195 |
| Yes | 683 (56.6) | 362 (14.8) | 112 (39.2) | 138 (18.5) | |||
| No | 523 (43.4) | 2082 (85.2) | 174 (60.8) | 608 (81.5) | |||
| Hb value | 88 | 334 |
<0.001 |
22 | 92 | 0.199 | |
| >9.0 mg/dL | 79 (89.8) | 334 (100) | 22 (100) | 92 (100) | |||
| <9.0 mg/dL | 9 (10.2) | 0 (0) | 0 | 0 | |||
| Vaginal bleeding | 1057 | 2067 |
<0.001 |
244 | 621 | 0.071 | <0.001 |
| Yes | 185 (17.5) | 248 (12.0) | 9 (3.69) | 43 (6.92) | |||
| No | 872 (82.5) | 1819 (88.0) | 235 (96.3) | 578 (93.1) | |||
| Fetal morbidity | 1151 | 2312 |
0.222 |
267 | 701 | 0.523 | <0.001 |
| Malformation | 56 (4,9) | 150 (6.49) | 3 (1.1) | 9 (1.3) | |||
| Fetal growth restriction | 114 (9.9) | 243 (10.5) | 3 (1.1) | 19 (2.7) | |||
| Other | 93 (8.1) | 171 (7.4) | 13 (4.9) | 33 (4.7) | |||
| No | 888 (77.1) | 1748 (75.6) | 248 (92.9) | 640 (91.3) | |||
| Delivery route | 1235 | 2481 |
0.238 |
288 | 750 | 0.802 | 0.002 |
| Vaginal | 518 (41.9) | 1107 (44.6) | 147 (51.0) | 393 (52.4) | |||
| Forceps/vacuum | 19 (0.5) | 26 (0.7) | 10 (3.5) | 34 (4.5) | |||
| Cesarean | 683 (55.3) | 1312 (52.8) | 128 (44.4) | 314 (41.9) | |||
| Vaginal + cesarean | 15 (1.2) | 36 (1.5) | 3 (1.0) | 9 (1.2) | |||
Table 3 shows perinatal outcomes according to gestational age at birth and the presence of anemia. In the preterm group, the presence of anemia was significantly associated with the need of ventilatory support (p=0.003), sepsis (p=0.006) and endocrine dysfunction (hypoglycemia) (p=0.001). This last one was the only association found between term delivery and anemia (p=0.012). In the analysis of the anemia group, newborns classified as small for gestational age were more frequent in the preterm (25.41%) than term (1.74%) births. Furthermore, association of anemia and prematurity was found for: 5th minute Apgar score <7 (p=0.001), fetal death (p=0.002), endotracheal intubation at birth (p=0.001), use of surfactant (p=0.001), ventilatory support (p=0.001), sepsis (p=0.001), respiratory distress (p=0.001), intraventricular hemorrhage (p=0.001), pneumonia (p=0.027), and neonatal death (p=0.001).
| Neonatal Outcomes | Gestational age at birth | ||||||
|---|---|---|---|---|---|---|---|
| <37 weeks | ≥37 weeks | p | |||||
| Anemia yes n (%) | Anemia no n (%) | p | Anemia yes n (%) | Anemia no n (%) | p | p anemia yes <37x ≥37 weeks |
|
| Birth weight | 1232 | 2467 | 0.108 | 288 | 750 | 0.076 | <0.001 |
| ≤1500 g | 243 (19.7) | 524 (21.2) | 0 (0) | 1 (0.1) | |||
| 1501 to 2500 g | 656 (53.3) | 1223 (49.6) | 10 (3.5) | 50 (6.7) | |||
| >2500 g | 333 (27.0) | 720 (29.2) | 278 (96.5) | 699 (93.2) | |||
| Adequacy of weight for gestational age | 1232 | 2467 | 0.268 | 288 | 750 | 0.249 | <.0001 |
| Small | 313 (25.4) | 656 (26.6) | 5 (1.7) | 27 (3.6) | |||
| Adequate | 906 (73.5) | 1771 (71.8) | 232 (80.6) | 604 80.5) | |||
| Large | 13 (1.1) | 40 (1.6) | 51 (17.7) | 119 (15.9) | |||
| Apgar score 5o min <7 | 1227 | 2439 | 0.768 | 287 | 743 | 0.194 | <.0001 |
| Yes | 116 (9.5) | 238 (9.8) | 0 (0.0) | 6 (0.8) | |||
| No | 1111 (90.6) | 2201 (90.2) | 287 (100) | 737 (99.2) | |||
| Fetal death | 1235 | 2481 | 0.232 | 288 | 750 | 0.002 | |
| Yes | 40 (3.2) | 100 (4.0) | 0 (0) | 0 (0) | |||
| No | 1195 (96.8) | 2381 (95.9) | 288 (27.7) | 750 (72.2) | |||
| Endotracheal intubation at birth | 1181 | 2338 | 0.919 | 270 | 678 | 0.946 | <.0001 |
| Yes | 191 (16.2) | 375 (16.0) | 5 (1.9) | 13 (1.9) | |||
| No | 990 (83.8) | 1963 (83.9) | 265 (98.2) | 665 (98.1) | |||
| Surfactant use | 1159 | 2307 | 0.637 | 262 | 671 | 0.483 | <.0001 |
| Yes | 186 (16.1) | 356 (15.4) | 1 (0.4) | 1 (0.2) | |||
| No | 973 (83.9) | 1951 (84.6) | 261 (99.6) | 670 (99.9) | |||
| Fetal Malformation | 1166 | 2315 | 0.942 | 265 | 674 | 0.361 | 0.0002 |
| Yes | 128 (10.9) | 256 (11.1) | 9 (3.4) | 32 (4.8) | |||
| No | 1038 (89.0) | 2059 (88.9) | 256 (96.6) | 642 (95.3) | |||
| Ventilatory support | 1178 | 2341 | 0.003 | 268 | 680 | 0.348 | <0.0001 |
| Yes | 645 (54.8) | 1158 (49.5) | 19 (7.1) | 61 (8.9) | |||
| No | 533 (45.3) | 1183 (50.5) | 249 (92.9) | 619 (91.0) | |||
| Any neonatal morbidity | 1181 | 2336 | 0.001 | 268 | 682 | 0.874 | <0.0001 |
| Yes | 876 (74.2) | 1585 (67.9) | 61 (22.8) | 152 (22.3) | |||
| No | 305 (25.8) | 751 (32.2) | 207 (77.2) | 530 (77.7) | |||
| Sepsis | 839 | 1518 | 0.006 | 60 | 149 | 0.161 | <0.0001 |
| Yes | 273 (32.5) | 413 (27.2) | 2 (3.3) | 14 (9.4) | |||
| No | 566 (67.5) | 1105 (72.8) | 58 (96.7) | 135 (90.6) | |||
| Respiratory distress | 865 | 1572 | 0.457 | 62 | 149 | 0.448 | <0.0001 |
| Yes | 661 (76.4) | 1180 (75.1) | 31 (50.0) | 66 (44.3) | |||
| No | 204 (23.6) | 392 (24.9) | 31 (50.0) | 83 (55.7) | |||
| Pulmonary air leak | 827 | 1483 | 0.767 | 60 | 146 | 1.00 | 0.162 |
| Yes | 35 (4.2) | 59 (3.9) | 0 (0.0) | 2 (1.4) | |||
| No | 792 (95.8) | 1424 (96.0) | 60 (100) | 144 (98.6) | |||
| Intraventricular Hemorrhage | 688 | 1192 | 0.867 | 53 | 109 | 0.010 | |
| Yes | 68 (9.9) | 115 (9.7) | 00 | 0 | |||
| No | 620 (90.1) | 1077 (90.4) | 53 (100) | 109 (100) | |||
| Endocrine dysfunction | 857 | 1539 | 0.001 | 62 | 146 | 0.012 | 0.166 |
| Yes | 235 (27.4) | 335 (21.8) | 12 (19.4) | 11 (7.5) | |||
| No | 622 (72.6) | 1204 (78.2) | 50 (80.7) | 135 (92.5) | |||
| Necrotizing enterocolitis | 852 | 1535 | 0.141 | 62 | 146 | 0.251 | |
| Yes | 28 (3.3) | 35 (2.2) | 0 (0) | 1 (0.7) | |||
| No | 824 (96.7) | 1500 (97.7) | 62 (100) | 145 (99.3) | |||
| Pneumonia | 852 | 1543 | 0.388 | 62 | 146 | 1.000 | 0.027 |
| Yes | 59 (6.9) | 93 (6.1) | 0 (0) | 2 (1.3) | |||
| No | 793 (93.1) | 1450 (93.9) | 62 (100) | 144 (98.6) | |||
| Oxygentherapy at 28 days | 851 | 1541 | 0.729 | 62 | 145 | 0.013 | |
| Yes | 77 (9.1) | 133 (8.6) | 0 (0) | 1 (0.7) | |||
| No | 774 (90.9) | 1408 (91.4) | 62 (100) | 144 (99.3) | |||
In the multivariate analysis (Table 4), for the term group, housing area was the only significant association found. For the preterm group, the factors associated to anemia include: urinary tract infection in sPTB and piPTB groups (p<0.001), neonatal morbidity (p=0.001), adequacy of prenatal care visits (p=0.009) and children under 5 years old in pPROM group.
| Model/ Variable | OR | IC 95% p/RP | p |
| Model 1: Term pregnancies [n=594] | |||
| Housing area-urban | 2.71 | 1.12 to 6.54 | 0.02 |
| Model 2: Preterm- sPTB (<37 weeks) [n= 735] | |||
| - Skin color- non-white | 1.50 | 1.10 to 2.05 | 0.007 |
| - UTI | 1.48 | 1.09 to 2.03 | 0.01 |
| Model 3: Preterm- piPTB [n=745] | |||
| - UTI | 2.19 | 1.57 to 3.04 | <0.01 |
| - Vaginal bleeding | 2.19 | 1.39 to 3.44 | <0.01 |
| Model 4: Preterm- pPROM [n=610] | |||
| - any neonatal morbidity | 1.66 | 1.13 to 2.42 | 0.001 |
| - Inadequate number of prenatal care visits | 1.52 | 1.08 to 2.15 | 0.009 |
| - children under 5 years old | 1.48 | 1.00 to 2.20 | 0.048 |
| UTI: Urinary Tract Infection; pPROM: preterm Premature Rupture of Membranes Independent variables entering models: housing area, maternal age, region of the country, skin color, morbid history, children under 5 years, prenatal care, onset of prenatal care, adequacy of number of prenatal care visits, initial body mass index, use of drugs, urinary tract infection, vaginal bleeding, neonatal ventilatory support, any neonatal morbidity. | |||
Discussion
The overall prevalence of anemia in our study was 31.70%, with 27.74% in term deliveries and significantly higher, 33.23%, among preterm deliveries. Anemia is a public health problem worldwide, especially in low and middle-income countries [11-13] and it is associated with worse maternal and perinatal outcomes, including increased maternal and perinatal death, preterm birth, cesarean section, preeclampsia and low birth weight [14-16]. WHO’s national estimates report anemia prevalence of 41.8% [4], higher than our results.
Furthermore, our study presented higher prevalence of anemia then reported in a study in Lagos, Nigeria in 2020, that was 20.0% [17], higher than a multicenter Chinese study that found an overall prevalence of anemia of 23.5% [18], higher than a study conducted in Jerusalem with 10.5% of anemia [19] and higher than a large retrospective study in British Columbia that found anemia in 12.8% of its records [20]. The difference in findings throughout studies may represent the population analyzed as having anemia is more prevalent in low-income countries and in regions with poor antenatal care [21]. A Brazilian study with adolescents showed an overall prevalence of anemia of 41% [22]. This higher prevalence may be due to the additional risk factors related to pregnancy in adolescents as higher need of iron intake [23] and nutritional deficiencies [24]. Also, our study was conducted in referral obstetric hospitals in the three most populous Brazilian regions, so the prevalence of anemia could have been higher as the most challenging and severe cases are treated in these hospitals.
Our results also found association with maternal age and the presence of anemia in preterm deliveries, as it was more frequent in pregnant adolescents. This is in agreement with the Chinese study that found higher prevalence of anemia in women under 20 [17]. In addition, positive association for preterm birth and anemia was evidenced in non-white women, living in the Northeast region, schooling years less than 8, presence of children below 5 years old, onset of prenatal care at second or third trimester and less than six prenatal care visits. These findings are consistent with literature as anemia is associated with poor access to antenatal care and low socio-economic condition [5,17,18]. In our country, non-white skin color and the Northeast region are associated with lower income regions and therefore associated with anemia [25].
Regular antenatal care is an important intervention that may avoid many maternal adverse complications, by the identification of risk factors, early diagnosis and surveillance, including anemia and its consequences, as deviations from the usual can be early detected and treated. Late onset of prenatal care was a finding associated with anemia in both term and preterm deliveries. It may be related to the fact that iron supplementation wasn’t long enough to recover at least the expected dilutional anemia and a chronic history of malnutrition and self-care. As a universal recommendation, prophylactic iron should be prescribed during gestation throughout postpartum [4].
Anemic women had high cesarean section rates in our study (especially among preterm deliveries) and such procedure can worsen hemoglobin level leading to other complications. Also, in the preterm group, fetal growth restriction was more frequent, and anemia could be partially responsible for the restriction. Previous preterm birth and previous low birth weight newborn were also more frequent in the preterm group. Moreover, anemia increases the risk for postpartum hemorrhage, worsening an already deficient hemoglobin level [10].
Considering factors independently associated to anemia, living in urban area was associated to anemia in term pregnancies. This could most likely be a consequence of the great majority of the population being urban, but also could be worsen by nutritional habits in urban centers where availability of food with low nutritional quality is higher. Differently, a study in China found that rural population is at greater risk for anemia [18], however studies correlate anemia with family income and schooling years, being poor education and low economic conditions associated to anemia [5,18,26]. Urinary tract infection was associated with anemia in sPTB and piPTB. It might have been the trigger of spontaneous preterm birth and could deteriorate maternal clinical condition, as it is an already known risk factor for prematurity, independently the gestational age of the infection [27]. In the sPTB, non-white skin color was also associated with prematurity, probably due to less access to health care. Having other child below 5 years old, any neonatal morbidity and inadequate numbers of antenatal care visits were associated with anemia and pPROM, suggesting that antenatal care is quite important to follow the women and identify alterations that can lead to complications. Studies suggest screening for infections, nutritional adequacy, iron supplementation and a periodic antenatal visit to check maternal health and early detection of possible pathologic conditions [20,28].
It is important to point out that this study has limitations as it was based on a secondary analysis of a major multicentric study. As a consequence, some variables were difficult to standardize and it was hard sometimes to define the cases based on past medical records. The main study (EMIP) was a multicenter cross-sectional study that was conducted in 20 referral obstetric hospitals and was the largest Brazilian study in prematurity so far [1]. One of its findings was that anemia is a risk factor for prematurity, what was an important point for further analysis.
Prematurity is enhancing worldwide and is a complex situation as the goals in order to reduce its rates are difficult to reach [29,30]. WHO estimates almost 15 million preterm deliveries every year (11.1% of all births) increasing costs in public and private healthcare [4]. In our study, prematurity was divided into three etiologies: premature rupture of membranes, spontaneous premature birth and provider-initiated preterm birth. The frequency of anemia in each group was 36.3%, 32.79% and 31.27% respectively. In all the three situations, anemia might be related to the cause of prematurity, however could also be worsen as a consequence of reduced time for iron supplementation. Overall, anemia was more prevalent among preterm birth, what is in agreement with the study in India [5] and the retrospective cohort studied in British Columbia that found an Odds Ratio of 2.44 for preterm birth and unspecified anemia severity [20].
Besides, prematurity costs millions to healthcare and is strongly associated with neonatal morbidity and mortality [20,31,32] with rates that have not decreased despite the knowledge and efforts to reduce its prevalence [30]. The improvement of maternal and neonatal intensive care is a situation that tends to persist and enables women with several morbidities to control the underlying condition and pursue pregnancy and neonates delivered in a very preterm gestational age to survive, sustaining high rates of preterm birth, especially medically indicated.
Our study highlighted the high prevalence of anemia and prematurity in our setting and could bring light to further studies considering the association of anemia and preterm birth and the importance of additional studies to evaluate the topic and improve maternal healthcare aiming the reduction in neonatal morbidity and mortality rates due to prematurity.
Acknowledgements
Brazilian Multicenter Study on Preterm Birth study group
Sergio T Marba, Ruth Guinsburg, Francisco E Martinez, Vilma Zotarelli, Lucio T Gurgel, Rodolfo C. Pacagnella, Samira M. Haddad, Francisco E Feitosa, George N Chaves, Ana M Porto, Isabela C Coutinho, Antonio C Barbosa Lima, Elias F Melo Jr, Débora F Leite, Melania M Amorim, Adriana S O Melo, Fabiana O Melo, Marília G Martins, Marynea V Nunes, Cláudio S Paiva, Moises D Lima, Djacyr M Freire, Edson G Tristão, Denis J Nascimento, Carlos A Menezes, Marcelo Aquino, Janete Vettorazzi, Cintia E Senger, Augusta M B Assumpção, Marcela A F Guedes, Maria E L Moreira, Vera T Borges, Nelson L Maia Filho, Jacinta P Mathias, Eduardo Souza, Ana C P Zamarian, Silvana M Quintana, Patrícia P S Melli, Fátima A Lotufo, Kaliane Uzilin, Elvira A Zanette, Carla B Andreucci, Tenilson A Oliveira, Laércio R Oliveira, Marcos A N Santos, Nelson Sass, Mirian R F Silveira, Pedro R Coutinho, Luciana Siqueira.
References
2. Passini R, Tedesco RP, Marba ST, Cecatti JG, Guinsburg R, Martinez FE, et al. Brazilian multicenter study on prevalence of preterm birth and associated factors. BMC Pregnancy and Childbirth. 2010 Dec;10(1):1-7.
3. Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. The Lancet. 2008 Jan 5;371(9606):75-84.
4. Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. The Lancet. 2012 Jun 9;379(9832):2162-72.
5. Kant S, Kaur R, Goel AD, Malhotra S, Haldar P, Kumar R. Anemia at the time of delivery and its association with pregnancy outcomes: A study from a secondary care hospital in Haryana, India. Indian Journal of Public Health. 2018 Oct 1;62(4):315-8.
6. Suryanarayana R, Chandrappa M, Santhuram AN, Prathima S, Sheela SR. Prospective study on prevalence of anemia of pregnant women and its outcome: A community based study. Journal of Family Medicine and Primary Care. 2017 Oct;6(4):739-43.
7. Beckert RH, Baer RJ, Anderson JG, Jelliffe-Pawlowski LL, Rogers EE. Maternal anemia and pregnancy outcomes: a population-based study. Journal of Perinatology. 2019 Jul;39(7):911-9.
8. Levy A, Fraser D, Katz M, Mazor M, Sheiner E. Maternal anemia during pregnancy is an independent risk factor for low birthweight and preterm delivery. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2005 Oct 1;122(2):182-6.
9. Stevens GA, Finucane MM, De-Regil LM, Paciorek CJ, Flaxman SR, Branca F, et al. Nutrition Impact Model Study Group. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and nonpregnant women for 1995–2011: a systematic analysis of population-representative data. The Lancet Global Health. 2013 Jul 1;1(1):e16-25.
10. Parks S, Hoffman MK, Goudar SS, Patel A, Saleem S, Ali SA, et al. Maternal anaemia and maternal, fetal, and neonatal outcomes in a prospective cohort study in India and Pakistan. BJOG: An International Journal of Obstetrics & Gynaecology. 2019 May;126(6):737-43.
11. Reveiz L, Gyte GM, Cuervo LG, Casasbuenas A. Treatments for iron?deficiency anaemia in pregnancy. Cochrane Database of Systematic Reviews. 2011(10).
12. Balarajan Y, Ramakrishnan U, Özaltin E, Shankar AH, Subramanian SV. Anaemia in low-income and middle-income countries. The Lancet. 2011 Dec 17;378(9809):2123-35.
13. Shander A, Goodnough LT, Javidroozi M, Auerbach M, Carson J, Ershler WB, et al. Iron deficiency anemia— bridging the knowledge and practice gap. Transfusion Medicine Reviews. 2014 Jul 1;28(3):156-66.
14. Vural T, Toz E, Ozcan A, Biler A, Ileri A, Inan AH. Can anemia predict perinatal outcomes in different stages of pregnancy? Pakistan Journal of Medical Sciences. 2016 Nov;32(6):1354-9.
15. Ali AA, Rayis DA, Abdallah TM, Elbashir MI, Adam I. Severe anaemia is associated with a higher risk for preeclampsia and poor perinatal outcomes in Kassala hospital, eastern Sudan. BMC Research Notes. 2011. Dec;4(1):1-5.
16. Amoakoh-Coleman M, Klipstein-Grobusch K, Agyepong IA, Kayode GA, Grobbee DE, Ansah EK. Provider adherence to first antenatal care guidelines and risk of pregnancy complications in public sector facilities: a Ghanaian cohort study. BMC Pregnancy and Childbirth. 2016 Dec;16(1):1-0.
17. Ajepe AA, Okunade KS, Sekumade AI, Daramola ES, Beke MO, Ijasan O, et al. Prevalence and foetomaternal effects of iron deficiency anaemia among pregnant women in Lagos, Nigeria. PlOS One. 2020 Jan 23;15(1):e0227965.
18. Lin L, Wei Y, Zhu W, Wang C, Su R, Feng H, et al. Prevalence, risk factors and associated adverse pregnancy outcomes of anaemia in Chinese pregnant women: a multicentre retrospective study. BMC Pregnancy and Childbirth. 2018 Dec;18(1):1-8.
19. Drukker L, Hants Y, Farkash R, Ruchlemer R, Samueloff A, Grisaru-Granovsky S. Iron deficiency anemia at admission for labor and delivery is associated with an increased risk for Cesarean section and adverse maternal and neonatal outcomes. Transfusion. 2015 Dec;55(12):2799-806.
20. Smith C, Teng F, Branch E, Chu S, Joseph KS. Maternal and perinatal morbidity and mortality associated with anemia in pregnancy. Obstetrics and Gynecology. 2019 Dec;134(6):1234-44.
21. Peña-Rosas JP, De-Regil LM, Garcia-Casal MN, Dowswell T. Daily oral iron supplementation during pregnancy. Cochrane Database of Systematic Reviews. 2015(7).
22. Pinho-Pompeu M, Surita FG, Pastore DA, Paulino DS, Pinto e Silva JL. Anemia in pregnant adolescents: impact of treatment on perinatal outcomes. The Journal of Maternal- Fetal & Neonatal Medicine. 2017 May 19;30(10):1158-62.
23. Sekhar DL, Murray-Kolb LE, Kunselman AR, Weisman CS, Paul IM. Differences in risk factors for anemia between adolescent and adult women. Journal of Women’s Health. 2016 May 1;25(5):505-13.
24. Ochola S, Masibo PK. Dietary intake of schoolchildren and adolescents in developing countries. Annals of Nutrition and Metabolism. 2014;64(Suppl. 2):24-40.
25. Miranda VI, Santos IS, Silveira MF, Silveira MP, Pizzol TD, Bertoldi AD. Validity of patient-reported anemia and therapeutic use of iron supplements during pregnancy: 2015 Pelotas (Brazil) birth cohort. Cadernos de Saude Publica. 2018 Sep 3;34(6):e00125517.
26. Kumari S, Garg N, Kumar A, Guru PK, Ansari S, Anwar S, et al. Maternal and severe anaemia in delivering women is associated with risk of preterm and low birth weight: A cross sectional study from Jharkhand, India. One Health. 2019 Dec 1;8:100098.
27. Baer RJ, Nidey N, Bandoli G, Chambers BD, Chambers CD, Feuer S, et al. Risk of Early Birth among Women with a Urinary Tract Infection: A Retrospective Cohort Study. American Journal of Perinatology Reports. 2021 Jan;11(01):e5-14.
28. Medley N, Vogel JP, Care A, Alfirevic Z. Interventions during pregnancy to prevent preterm birth: an overview of Cochrane systematic reviews. Cochrane Database of Systematic Reviews. 2018(11).
29. Blencowe H, Cousens S, Chou D, Oestergaard M, Say L, Moller AB, et al. Born too soon: the global epidemiology of 15 million preterm births. Reproductive Health. 2013 Nov;10(1):1-4.
30. Lawn JE, Cousens S, Zupan J, Lancet Neonatal Survival Steering Team. 4 million neonatal deaths: when? Where? Why?. The lancet. 2005 Mar 5;365(9462):891- 900.
31. Beck S, Wojdyla D, Say L, Betran AP, Merialdi M, Requejo JH, et al. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bulletin of the World Health Organization. 2010;88:31-8.
32. Blencowe H, Vos T, Lee AC, Philips R, Lozano R, Alvarado MR, et al. Estimates of neonatal morbidities and disabilities at regional and global levels for 2010: introduction, methods overview, and relevant findings from the Global Burden of Disease study. Pediatric Research. 2013 Dec;74(1):4-16.