Abstract
In this literature review, we will introduce pharmacology in addition to most of the up-to-date reported methods that have been developed for determination of important oral hypoglycemic drugs which are metformin and glimepiride in their pure forms, combined forms with other drugs, combined forms with degradation products, and in biological samples.
Keywords
Literature review, Metformin, Glimepiride; Degradation products, Biological samples
Introduction
Diabetes mellitus is characterized by abnormally high levels of sugar (glucose) in the blood, When the amount of glucose in the blood increases, e.g., after a meal, it triggers the release of the hormone insulin from the pancreas. Insulin stimulates muscle and fat cells to remove glucose from the blood and stimulates the liver to metabolize glucose, causing the blood sugar level to decrease to normal level. In people with diabetes, blood sugar levels remain high. This may be because insulin is not being produced at all, or is not made at sufficient levels, or is not as effective as it should be. The most common forms of diabetes are type-1 diabetes (5%), which is an autoimmune disorder, and type 2 diabetes (95%), which is associated with obesity. Gestational diabetes is a form of diabetes that occurs in pregnancy, and other forms of diabetes are very rare and are caused by a single gene mutation [1].
Metformin (MTF, as seen in Figure 1), sold under the brand name Glucophage, among others, is the first-line medication for the treatment of type 2 diabetes. MTF is an antihyperglycemic agent that improves glucose tolerance in patients with type 2 diabetes, lowering both basal and postprandial plasma glucose. Its pharmacologic mechanisms of action are different from other classes of oral antihyperglycemic agents. MTF decreases hepatic glucose production, decreases intestinal absorption of glucose, and improves insulin sensitivity by increasing peripheral glucose uptake and utilization. MTF does not produce hypoglycemia in either patients with type 2 diabetes or normal subjects and does not cause hyperinsulinemia. With MTF therapy, insulin secretion remains unchanged while fasting insulin levels and daylong plasma insulin response may decrease [2-4].
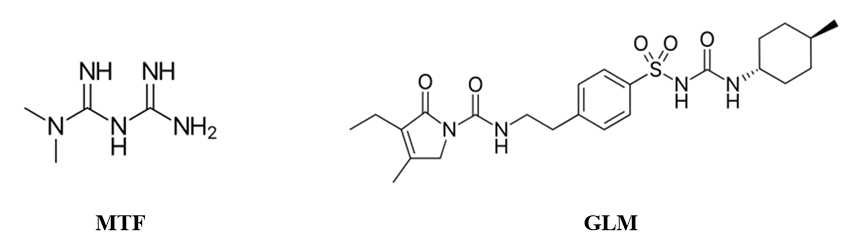
Figure 1: Chemical structures of Metformin (MTF) and Glimepiride (GLM).
Glimepiride (GLM, as depicted in Figure 1) is indicated for the management of type 2 diabetes in adults as an adjunct to diet and exercise to improve glycemic control as monotherapy. It may also be indicated for use in combination with metformin or insulin to lower blood glucose in patients with type 2 diabetes whose high blood sugar levels cannot be controlled by diet and exercise in conjunction with an oral hypoglycemic (a drug used to lower blood sugar levels) agent alone [5].
Its mechanism of action is based on ATP-sensitive potassium channels on pancreatic beta cells that are gated by intracellular ATP and ADP. The hetero-octomeric complex of the channel is composed of four pore-forming Kir6.2 subunits and 4 regulatory sulfonylurea receptor (SUR) subunits. Alternative splicing allows the formation of channels composed of varying subunit isoforms expressed at different concentrations in different tissues [7]. In pancreatic beta cells, ATP-sensitive K channels play a role as essential metabolic sensors and regulators that couple membrane excitability with glucose-stimulated insulin secretion (GSIS). When there is a decrease in the ATP:ADP ratio, the channels are activated and open, leading to K+ efflux from the cell, membrane hyperpolarization, and suppression of insulin secretion. In contrast, increased uptake of glucose into the cell leads to elevated intracellular ATP:ADP ratio, leading to the closure of channels and membrane depolarization. Depolarization leads to activation and opening of the voltage-dependent Ca2+ channels and consequently an influx of calcium ions into the cell. Elevated intracellular calcium levels cause the contraction of the filaments of actomyosin responsible for the exocytosis of insulin granules stored in vesicles [3]. GLM blocks the ATP-sensitive potassium channel by binding non-specifically to the B sites of both sulfonylurea receptor-1 (SUR1) and sulfonylurea receptor-2A (SUR2A) subunits as well as the A site of SUR1 subunit of the channel to promote insulin secretion from the beta cell [6].
Review of Analytical Methods
Various techniques were used for the analysis of MTF and GLM in its pure forms, in pharmaceutical formulations and in biological fluids. The available reported methods in the literature can be summarized as follows:
Spectroscopic Methods
|
Spectrophotometric methods |
||||||
|
Ref. |
LOD |
Linearity range |
λmax (nm) |
Method or reagent |
Matrix |
Drugs |
|
[7] |
------------ |
8-18 μg/mL |
570 |
Ninhydrin in alkaline medium |
Tablets |
MTF |
|
[8] |
0.167 & 0.320 μg/mL |
5–30 μg/mL.0 |
233 & 238 |
Q – Absorption ratio method |
Tablets |
MTF, Anagliptin |
|
[9] |
------------- |
2-12 μg/mL |
295 |
Charge-transfer complex with iodine |
Tablets |
MTF |
|
[10] |
0.083 µg/mL |
0.5-4 µg/mL |
385 |
Oxidation using sodium hypochlorite in alkaline medium |
Tablets and industrial waste water |
MTF |
|
[11] |
------------ |
0.5-2 mg/mL |
540 |
Cu2+ in basic medium |
Tablets |
MTF |
|
[12] |
0.4 μg/mL |
5-30 μg/mL |
249 |
UV spectrophotometry. |
Tablets |
GLM |
|
[13] |
1.311 mg/L |
2-40 mg/L |
279.0, 257.5 & 256.3 |
Derivative UV spectrophotometry |
Tablets |
GLM |
|
[14] |
0.4 μg/mL |
1- 500 μg/mL |
263.3–268.2 |
Derivative UV spectrophotometry |
Tablets |
GLM |
|
[15] |
------------ |
40–160 μg/mL |
413.5 |
2,3,5-Triphenyl-2H-tetrazolium chloride in basic media |
Tablets |
GLM |
|
[16] |
2.6 μg/mL 2.8 μg/mL
|
10–80 μg/mL 20–120 μg/mL |
843 415 |
1-Charge-transfer complex using TCNQ 2-Ion-pair complex using bromo thymol blue |
Tablets |
GLM |
|
[17] |
0.088 μg/mL |
0.981-9.812 μg/mL |
416 |
Ion-pair complex formation using bromocresol green |
Tablets |
GLM |
|
[18] |
0.35 μg/mL |
0.5-22 μg/mL |
231 |
UV spectrophotometry |
Tablets |
GLM |
|
Spectrofluorometric methods |
|||||||
|
Ref. |
LOD |
Linearity range |
λem |
λmax |
Fluorogenic method |
Matrix |
Drug |
|
[11] |
------------ |
20-200 μg/mL |
520 |
450 |
Chrysenequinone in alkaline medium |
Tablets |
MTF |
|
[19] |
0.01 μg/mL
|
0.04-1.2 μg/mL |
416 |
240 |
9,10-phenanthraquinone in alkaline media |
Tablets |
MTF, Glibenclamide |
Chromatographic methods
HPLC-UV methods
|
Drugs |
Matrix |
Column |
Mobile phase |
UV-Detector (nm) |
Linearity range |
LOD |
Ref. |
|
MTF |
Tablets & formulated microspheres |
phenomenex C18 ODS (5 μ, 250 × 4.60 mm) |
Acetonitrile:phosphate buffer (65:35) pH adjusted to 5.75 with o-phosphoric acid |
233 |
0-25 μg/mL |
-------- |
[20] |
|
MTF |
Human plasma |
RP C18 (250 × 4.6 mm, 5 μm) |
34% acetonitrile & 66% aqueous phase, containing 10 mM KH2PO4 and 10 mM SLS. |
233 |
0.125-2.5 μg/mL |
62 ng/mL |
[21] |
|
MTF, nateglinide |
Tablets |
Inertsil C18-ODS 3V (250 × 4.6 mm, 5 μm) |
Phosphate buffer (pH4.0): Acetonitrile: methanol (30:60:10) |
221 |
60-140 μg/mL |
2.18 μg/mL |
[22] |
|
MTF, Gliclazide & GLM |
Tablets |
Thermo Scientific® BDS Hypersil C8 (5µm, 2.50 x 4.60 mm) |
MeOH : 0.025M KH2PO4 adjusted to pH 3.20 using ortho - phosphoric acid (70 : 30, v/v) |
235 |
5-100 µg/mL |
0.05 (MET), 0.11 μg/mL (GLM) |
[23] |
|
MTF, ertugliflozin |
Tablets |
Kromasil C18 (150 mm × 4.6 mm, 5 μm) |
0.1% ortho-phosphoric acid buffer (pH 2.7):acetonitrile (65:35% v/v) |
224 |
62.5-375 μg/mL |
0.87 μg/mL |
[24] |
|
MTF, Repaglinide |
Tablets |
XBridge C18 column (4.6 x 150 mm, 3.5 μm) |
Potassium dihyrogen ortho phosphate (2.2 pH): Acetonitrile (35:65%v/v) |
240 |
5-50 μg/mL |
0.018 μg/mL |
[25] |
|
MTF, pioglitazone & GLM |
Tablets |
Inertsil-ODS-3 C18 (250 × 4.60 mm, 5 µm) |
Methanol–phosphate buffer (pH 4.3) in the ratio of 75:25 v/v |
258 |
10-5000 (MET), 1-10 μg/mL (GLM) |
-------- |
[26] |
|
MTF, atorvastatin & GLM |
Tablets |
Grace Smart Altima C8 (250 × 4.6 mm, 5 μm) |
Acetonitrile : phosphate buffer (60 : 40 (v/v), pH 3.0) |
235 |
20- 200 µg/mL |
-------- |
[27] |
|
MTF, Sitagliptin |
Tablets |
Li-chrosphere-100 C18 ODS (250 × 4.6 mm, 5 μm) |
Methanol: potassium di-hydrogen phosphate buffer at a ratio of 70:30 v/v |
266 |
20-100 μg/mL
|
0.14 μg/mL |
[28] |
|
MTF |
Tablets |
RP C18 (250 mm x 4.6 mm, 5.0 µm) |
34% acetonitrile and 66% 10 mM KH2PO4 and 10 mM sodium lauryl sulfate (pH 5.2) |
233 |
2.5-20 µg/mL |
0.1 µg/mL |
[29] |
|
MTF, gliclazide |
Tablets |
Alltima CN (250 mm × 4.6 mm x5μm) |
20 mM ammonium formate buffer (pH 3.5) and acetonitrile (45:55, v/v) |
227 |
2.5-150 μg/mL |
0.8 μg/mL
|
[30] |
|
GLM |
Self-nanoemulsifying powder (SNEP) formulation |
octadesyl silane (ODS) (250 x 4.6 mm, 5μm) |
Acetonitrile: 0.2 M phosphate buffer (pH= 7.4) 40:60 v/v |
228 |
0.2-2 μg/mL |
0.38 μg/mL |
[31] |
|
GLM |
Rat serum samples |
LiChrosphere 100 RP 18 e (125 × 4.0 mm, 5 µm) |
MeOH: 10mM Phosphate buffer (80:20 v/v) adjusted to pH 3.0 with orthophosphoric acid |
230 |
0.5-500 μg/mL |
0.15 μg/mL |
[32] |
|
GLM |
Tablets |
Hypersil C18 (15 cm x 3.9 mm) |
Acetonitrile 0.05 M monobasic potassium phosphate (pH 6.0) 40:60 v/v |
210 |
10–40 μg/mL |
0.8 μg/mL |
[33] |
|
GLM |
Tablets |
Lichrosorb RP-18 (125 x 4 mm, 5µm) |
Acetonitrile-water-glacial acetic acid (550:450:0.6 v/v) |
230 |
15-120 μg/mL |
4 ng |
[34] |
|
GLM |
Tablets |
spherisorb S5NH2 (250 mm x 4.6 mm, 5 μm) |
40% acetonitrile and 60% aqueous acetate buffer (5.0 mM at pH 6.3) |
228 |
50.0 μg/L - 6.0 mg/L |
15.0 μg/L |
[35] |
|
MTF, pioglitazone & GLM |
Tablets |
Phenomenex-ODS-3 C18 (250 × 4.60 mm, 5 μm) |
MeOH:acetonitrile:15 mM KHPO4 (pH 4), 40:35:25 |
240 |
0.2-50 (MET), 0.2-30 μg/mL (GLM) |
0.04 (MTF), 0.08 μg/mL (GLM) |
[36] |
|
MTF, pioglitazone & GLM |
Human plasma |
MAGELLEN 5U C18 (5 μm, 150 mm × 4.60 mm) |
MeOH-0.025 M KH2PO4 adjusted to PH 3.20 using O-phosphoric acid (85:15, v/v) |
235 |
2.50-100 μg/mL |
0.05 (MET), 0.10 μg/mL (GLM) |
[37] |
2.2: HPLC-MS methods
|
Drug |
Matrix |
Column |
Mobile phase |
Detector |
Linearity range |
LOD |
Ref. |
|
MTF, canagliflozin |
Human plasma |
C18 column (50 mm × 4.6 mm, 5 µm) |
0.1% formic acid and acetonitrile (60:40, v/v) |
MS |
50–5000 ng/mL |
15 ng/mL |
[38] |
|
MTF, Empagliflozin |
Tablets |
Bridged Ethylene Hybrid C18 (50 mm × 2.1 mm, 1.7 μm) |
0.1% aqueous formic acid: acetonitrile (75:25, v/v) |
MS |
50–25,000 ng/mL |
7.3– 21.9 ng/mL |
[39] |
|
MTF |
Human plasma |
a Zorbax SB C8 (150 mm × 4.6 mm, 5 μm) |
Acetonitrile−water− formic acid (70:30:1, v/v) |
MS |
2.0–2000 ng/mL |
0.7 ng/mL |
[40] |
|
MTF |
Human plasma |
Nucleosil C18 (5 μm, 50 mm × 4.6 mm) |
Acetonitrile: methanol:10 mM ammonium acetate pH 7.0 (20:20:60, v/v/v) |
MS |
1–2000 ng/mL |
250 pg/mL |
[41] |
|
MTF, gliclazide |
Human plasma |
Hypersil BDS C18 (50 mm × 2.1 mm, 3 µm) |
Methanol–water (containing 1% formic acid)– acetonitrile (30: 31: 39, v/v/v) |
MS |
7.8–4678.9 ng/mL |
2.6 ng/mL |
[42] |
|
MTF, vildagliptin |
Human plasma |
Atlantis HILIC Silica (150 × 2.1 mm, 3μm) |
20% water and 80% acetonitrile/water solution 95:5 (v/v), containing both 0.1% formic acid and 3mM ammonium formate. |
MS |
5-500 ng /mL |
1.5 ng/mL |
[43] |
|
GLM |
Human plasma |
5 µm (50 mm × 2.1 mm, i.d.) C18 XTerra column |
Ammonium acetate buffer (0.02 M, pH = 3.5): acetonitrile: methanol in the ratio of 40:35:25 (v/v) |
MS |
5.0– 500.0 ng/mL |
1.5 ng/ml. |
[44] |
|
GLM |
Human plasma |
ACE 5C18, 50×4 mm, 5μm column |
200mL water, 450mL acetonitrile, 350mL methanol, 0.6mL glacial acetic acid |
MS |
5–1,000 ng/mL |
1.5 ng/mL |
[45] |
|
GLM |
Human plasma |
Zorbax Eclipse Plus C18 column (3.5 µm, 4.6 × 100 mm) |
Acetonitrile: 0.1% formic in water (70: 30, v/v) |
MS |
2.0–650 ng/mL |
60 pg/mL |
[46] |
|
GLM |
Human Plasma |
BEH C18 1.7µm (2.1x50 mm). |
A: 30% (Ammonium formate 0.1 %) B:70% Acetonitrile |
MS |
1– 100 ng/mL |
0.33 ng/mL |
[47] |
HPTLC methods
|
Drug |
Matrix |
Stationary phase |
Mobile phase |
Detector |
Linearity range |
LOD |
Ref. |
|
GLM, Empagliflozin & Linagliptin |
Tablets |
Aluminum plates precoated with silica gel 60 F254 |
Toluene: methanol: ethyl acetate (4: 3: 2 v/v/v) |
Reflectance/fluorescence mode at λmax 228 nm and λem 320 nm |
2.61–60 ng/band |
1.84 ng/band |
[48] |
|
MTF, Atorvastatin & GLM |
Tablets |
Silica gel 60 F254 |
Water: methanol: ammonium sulphate (1: 1: 4 v/v/v) |
Densitometric detection at 237 nm |
200-700 (MET), 600-2100 ng/spot (GLM) |
100 (MET), 500 ng/spot (GLM) |
[49] |
|
MTF, GLM
|
Tablets |
TLC aluminium plates precoated with silica gel 60 F254 |
0.5% Ammonium Sulfate: Methanol (7.5:2.5 v/v) |
Densitometric detection at 228 nm |
200-700 (MET), 600-2100 ng/band (GLM) |
0.32 (MET), 0.05 ng/band (GLM) |
[50] |
|
MTF, Nateglinide,
|
Tablets |
TLC aluminium plates precoated with silica gel 60 F254 |
Chloroform:ethyl acetate:acetic acid (4:6:0.1 v/v/v) |
Reflectance/absorbance mode at 216 nm |
500–3000 ng/band
|
0.022 ng/band |
[51] |
|
GLM, Rosiglitazone |
Tablets |
Silica gel 60 F254
|
Methanol: toluene: ethyl acetate (1:8:1, v/v/v) |
Densitometric detection at 228 nm |
100 - 1500 ng/spot |
30 ng/spot |
[52] |
Electrochemical methods
|
Drug |
Matrix |
Electrode |
Linearity range |
LOD |
Ref. |
|
MTF |
Tablets |
Glassy carbon |
1.0 nmol L−1 - 1.0 μmol L−1 |
0.3 nmol L−1. |
[53] |
|
MTF |
Tablets |
Carbon paste |
0.1 - 80 μM |
0.014 μM |
[54] |
|
MTF |
Tablets |
Glassy carbon |
10 - 70 μM |
0.7 μM |
[55] |
|
MTF |
Tablets |
Glassy carbon |
0.5 - 25 μM |
0.12 μM |
[56] |
|
MTF |
sol–gel matrix |
Citrate-capped gold nanoparticle |
0.02 - 80 ng mL−1 |
0.005 ng mL−1 |
[57] |
|
MTF |
Tablets |
Carbon paste |
0.1–65 μM |
30 nM |
[58] |
|
MTF |
Tablets |
Pencil graphite |
0.1–1000 µM |
6.8 nM |
[59] |
|
MTF |
Urine |
Mercury drop |
5×10−8 - 4×10−6 mol l−1 |
1.8 × 10−8 mol l−1 |
[60] |
|
MTF |
Tablets & human serum |
Carbon paste |
10.4-1125.0 µM |
3.4 µM |
[61] |
|
MTF |
Tablets & urine |
Carbon paste |
50 nM - 60 μM |
9 nM |
[62] |
|
MTF |
Tablets |
CB[6]-modified gold electrode |
10 pmol/L - 20 nmol/L |
1.35 pmol/L |
[63] |
|
MTF |
Biological samples |
Copper hydroxide - carbon ionic liquid electrode |
1 µM–4 mM |
0.5 µM |
[64] |
|
GLM |
Tablets |
Glassy carbon Carbon paste |
1.0×10–5 - 3.2×10–5 mol l–1 2.0×10–6 - 1.5×10–5 mol l–1 |
2.0 × 10–6 mol l–1 7.5 × 10–7 mol l–1 |
[65] |
|
GLM, Valsartan |
-------- |
Hanging mercury drop electrode (HMDE) |
0.25x10-7 - 3.25x10-7 M |
3.48x10-8 M |
[66] |
By the end of this literature review, we would like to emphasize that we continue in our current project to provide an updated review on diseases and drugs chemistry that help the humanity all over the world [67-102].
Conclusion
In this literature review, we shed the light on pharmacology of metformin and glimepiride in addition to most of up-to-date reported methods related to their determination in their pure forms, combined forms with other drugs, combined forms with degradation products, and in biological samples.
References
2. Scarpello JH, Howlett HC. Metformin therapy and clinical uses. Diabetes and Vascular Disease Research. 2008 Sep;5(3):157-67.
3. Viollet B, Guigas B, Garcia NS, Leclerc J, Foretz M, et al. Cellular and molecular mechanisms of metformin: an overview. Clinical science. 2012 Mar 1;122(6):253-70.
4. DeFronzo RA, Goodman AM, Multicenter Metformin Study Group. Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. New England Journal of Medicine. 1995 Aug 31;333(9):541-9.
5. Hardie DG. AMP-activated protein kinase as a drug target. Annu. Rev. Pharmacol. Toxicol.. 2007 Feb 10;47:185-210.
6. Davis SN. Insulin, oral hypoglycemic agents and the pharmacology of the endocrine pancreas. Goodman and Gilmans the Pharmacological Basis of Therapeutics. Brunton, LL (Ed.). McGraw-Hill, New York. 2006:1613-45.
7. Mubeen G, Noor K. Spectrophotometric method for analysis of metformin hydrochloride. Indian journal of Pharmaceutical Sciences. 2009 Jan;71(1):100.
8. Majithia RH, Khodadiya A, Patel VB. Spectrophotometric method development and validation for simultaneous estimation of Anagliptin and Metformin HCl BY Q-Absorption ratio method in synthetic mixture. Heliyon. 2020 May 1;6(5):e03855.
9. El-bardicy MG, El-khateeb SZ, Ahmad AS, Assaad HN. Spectrophotometric determination of metformin via charge-transfer complex with iodine. Spectroscopy Letters. 1989 Nov 1;22(9):1173-81.
10. Ahmad NR. Facial visible spectrophotometric determination of metformin hydrochloride in glucosam tablets and industrial waste water: Application to content uniformity testing. Iraq J Pharm Vol. 2012;12(1).
11. Hassan SS, Mahmoud WH, Elmosallamy MA, Othman AH. Determination of metformin in pharmaceutical preparations using potentiometry, spectrofluorimetry and UV-visible spectrophotometry. Analytica Chimica Acta. 1999 Jan 4;378(1-3):299-311.
12. Bhargavi S, Suryasagar G, Sowmya DK, Ashok K, Nama S. UV spectrophotometric method for determination of glimepiride in pharmaceutical dosage forms. Int J Pharm Sci Rev Res. 2013 Jul;21(2):131-4.
13. Bonfilio R, Araujo MB, Salgado H. Development and validation of an UV-derivative spectrophotometric method for determination of glimepiride in tablets. Journal of the Brazilian Chemical Society. 2011;22:292-9.
14. Altinoz S, Tekeli D. Analysis of glimepiride by using derivative UV spectrophotometric method. Journal of Pharmaceutical and Biomedical Analysis. 2001 Jan 1;24(3):507-15.
15. Khan IU, Aslam F, Ashfaq M, Asghar MN. Determination of glimepiride in pharmaceutical formulations using HPLC and first-derivative spectrophotometric methods. Journal of Analytical Chemistry. 2009 Feb;64:171-5.
16. Ulu ST. Spectrophotometric determination of glimepiride in pharmaceutical preparations based on the formation of charge-transfer and ion-pair complexes. Journal of Analytical Chemistry. 2013 Jul;68:606-10.
17. Ramadan AA, Mandil H, Zeino S. Spectrophotometric determination and validation of glimepiride in pure and tablet dosage forms through ion-pair complex formation using bromocresol green. International Journal of Pharmacy and Pharmaceutical Sciences. 2016 Jun 1:216-21.
18. Afieroho OE, Okorie O, Okonkwo TJ. An ultraviolet-spectrophotometric method for the determination of glimepiride in solid dosage forms. Diabetes Technology & Therapeutics. 2011 Jun 1;13(6):671-4.
19. Abdelrahman MM, Emam RA, Ali NW, Abdelaleem EA. Validated spectrofluorometric determination of hypoglycemic combination, in pure form and pharmaceutical formulation using 9, 10-phenanthraquinone reagent. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2021 Feb 15;247:119078.
20. Kar M, Choudhury PK. HPLC method for estimation of metformin hydrochloride in formulated microspheres and tablet dosage form. Indian Journal of Pharmaceutical Sciences. 2009 May;71(3):318.
21. Dhulikhel N. of the Research Article: Simple HPLC-UV Method For The Quantification Of Metformin In Human Plasma With One Step Protein Precipitation.
22. Chengalva P, Parameswari AS, Aruna G. Development and validation of RP-HPLC method for metformin hydrochloride and nateglinide in bulk and combined dosage form. Int. J. Pharm. Pharm. Sci. 2016;8:267-71.
23. Sebaiy MM, El-Adl SM, Baraka MM, Hassan AA. Rapid RP-HPLC method for simultaneous estimation of some antidiabetics; Metformin, Gliclazide and Glimepiride in Tablets. Egyptian Journal of Chemistry. 2019 Mar 1;62(3):429-40.
24. Kumari KS, Bandhakavi S. Development and validation of stability-indicating RP-HPLC method for the simultaneous determination of ertugliflozin pidolate and metformin hydrochloride in bulk and tablets. Future Journal of Pharmaceutical Sciences. 2020 Dec;6:1-0.
25. Patan A, Basha SR, Ketha RK, Cheriyan BV, Muthukumar VA. Development and validation of new RP-HPLC method for the simultaneous estimation of metformin hydrochloride and repaglinide in pure and pharmaceutical formulations. Research Journal of Pharmacy and Technology. 2021;14(3):1323-8.
26. Jain D, Jain S, Jain D, Amin M. Simultaneous estimation of metformin hydrochloride, pioglitazone hydrochloride, and glimepiride by RP-HPLC in tablet formulation. Journal of Chromatographic Science. 2008 Jul 1;46(6):501-4.
27. Ramesh D, Habibuddin M. Stability indicating RP-HPLC method for the simultaneous determination of atorvastatin calcium, metformin hydrochloride, and glimepiride in bulk and combined tablet dosage form. International Scholarly Research Notices. 2014;2014.
28. Prasad PB, Satyanarayana K, Krishnamohan G. Development and validation of a method for simultaneous determination of metformin hydrochloride and sitagliptin phosphate in a formulation by RP-HPLC. American Journal of Analytical Chemistry. 2014 Aug 8;2014.
29. Chhetri HP, Thapa P, Van Schepdael A. HPLC method for the quantification of metformin hydrochloride in bulk and dosage forms. International Journal of Pharmaceutical Sciences and Research. 2013 Jul 1;4(7):2600.
30. Gedawy A, Al-Salami H, Dass CR. Development and validation of a new analytical HPLC method for simultaneous determination of the antidiabetic drugs, metformin and gliclazide. Journal of Food and Drug Analysis. 2019 Jan 1;27(1):315-22.
31. Mohd AB, Sanka K, Gullapelly R, Diwan PV, Shastri N. Development and validation of RP-HPLC method for glimepiride and its application for a novel self-nanoemulsifying powder (SNEP) formulation analysis and dissolution study. Journal of Analytical Science and Technology. 2014 Dec;5:1-8.
32. Samala S, Tatipamula SR, Veeresham C. Determination of glimepiride in rat serum by RP-HPLC method. American Journal of Analytical Chemistry. 2011 May 1;2(2):152.`
33. Wanjari DB, Gaikwad NJ. Reversed phase HPLC method for determination of glimepiride in tablet dosage form. Indian Journal of Pharmaceutical Sciences. 2005;67(2):253.
34. Maslarska V. Development, estimation and validation of glimepiride in pharmaceutical formulation by HPLC method. International Journal of Pharmaceutical Sciences and Research. 2014 Aug 1;5(8):3195.
35. Meng F, Xu P, Wang X, Huang Y, Wu L, Chen Y, Teng L, Wang D. Investigation on the immunomodulatory activities of Sarcodon imbricatus extracts in a cyclophosphamide (CTX)-induced immunosuppressanted mouse model. Saudi Pharmaceutical Journal. 2017 May 1;25(4):460-3.
36. Pandit V, Pai RS, Singh G, Devi K, Narayana S, Suresh S. Development and validation of the liquid chromatographic method for simultaneous estimation of metformin, pioglitazone, and glimepiride in pharmaceutical dosage forms. Pharmaceutical Methods. 2012 Jan 1;3(1):9-13.
37. Sebaiy MM, El-Adl SM, Baraka MM, Hassan AA. Rapid RP-HPLC method for simultaneous estimation of metformin, pioglitazone, and glimepiride in human plasma. Acta Chromatographica. 2020 Mar;32(1):16-21.
38. Mohamed D, Elshahed MS, Nasr T, Aboutaleb N, Zakaria O. Novel LC-MS/MS method for analysis of metformin and canagliflozin in human plasma: application to a pharmacokinetic study. BMC Chemistry. 2019 Dec;13(1):1-1.
39. Ayoub BM, Mowaka S. LC-MS/MS determination of empagliflozin and metformin. Journal of Chromatographic Science. 2017 Aug 1;55(7):742-7.
40. Chen X, Gu Q, Qiu F, Zhong D. Rapid determination of metformin in human plasma by liquid chromatography? tandem mass spectrometry method. Journal of Chromatography B. 2004 Apr 5;802(2):377-81.
41. Wang Y, Tang Y, Gu J, Fawcett JP, Bai X. Rapid and sensitive liquid chromatography-tandem mass spectrometric method for the quantitation of metformin in human plasma. Journal of Chromatography B. 2004 Sep 5;808(2):215-9.
42. Zhong GP, Bi HC, Zhou S, Chen X, Huang M. Simultaneous determination of metformin and gliclazide in human plasma by liquid chromatography-tandem mass spectrometry: application to a bioequivalence study of two formulations in healthy volunteers. Journal of Mass Spectrometry. 2005 Nov;40(11):1462-71.
43. Pontarolo R, Gimenez AC, de Francisco TM, Ribeiro RP, Pontes FL, et al.Simultaneous determination of metformin and vildagliptin in human plasma by a HILIC-MS/MS method. Journal of Chromatography B. 2014 Aug 15;965:133-41.
44. Salem II, Idrees J, Al Tamimi JI. Determination of glimepiride in human plasma by liquid chromatography-electrospray ionization tandem mass spectrometry. Journal of Chromatography B. 2004 Jan 5;799(1):103-9.
45. Yüzüak N, Özden T, Eren S, Özilhan S. Determination of glimepiride in human plasma by LC–MS–MS. Chromatographia. 2007 Sep;66:165-8.
46. Kammoun AK, Awan ZA, Elawady T, Khedr A, El-Awady MI. LC-MS/MS determination of glimepiride in human plasma with a high recovery at picogram scale: Pharmacokinetic study after oral administration. Acta Chromatographica. 2022;34(1).
47. Ayad MM, Hosny MM, El-Sayed HM, Elwan MG. A Validated Bioanalytical Method for Determination of Glimepiride in Human Plasma Using UPLC-MS/MS.
48. Abbas NS, Derayea SM, Omar MA, Saleh GA. Innovative TLC-densitometric method with fluorescent detection for simultaneous determination of ternary anti-diabetic mixture in pharmaceutical formulations and human plasma. Microchemical Journal. 2021 Jun 1;165:106131.
49. Dhaneshwar SR, Salunkhe JV, Bhusari VK. Validated HPTLC method for simultaneous estimation of metformin hydrochloride, atorvastatin and glimepiride in bulk drug and formulation. Journal of Analytical and Aioanalytical Techniques. 2010;1(3):1-5.
50. Patel KK, Karkhanis VV, Gajjar SS. Development and validation of stability indicating HPTLC method for estimation of glimepiride and metformin hydrochloride. International Journal of Pharmaceutical Sciences and Research. 2015 Mar 1;6(3):1222.
51. Thomas AB, Patil SD, Nanda RK, Kothapalli LP, Bhosle SS, Deshpande AD. Stability-indicating HPTLC method for simultaneous determination of nateglinide and metformin hydrochloride in pharmaceutical dosage form. Saudi Pharmaceutical Journal. 2011 Oct 1;19(4):221-31.
52. Dhole SM, Khedekar PB, Amnerkar ND. Development and validation of hptlc densitometry method for simultaneous estimation of rosiglitazone and glimepiride in fixed tablet dosage form. Journal of the Chilean Chemical Society. 2013;58(2):1663-6.
53. Ghanbari MH, Sharafi P, Nayebossadr S, Norouzi Z. Utilizing a nanocomposite consisting of zinc ferrite, copper oxide, and gold nanoparticles in the fabrication of a metformin electrochemical sensor supported on a glassy carbon electrode. Microchimica Acta. 2020 Oct;187:1-1.
54. Mirzajani R, Karimi S. Preparation of ?-Fe2O3/hydroxyapatite/Cu (II) magnetic nanocomposite and its application for electrochemical detection of metformin in urine and pharmaceutical samples. Sensors and Actuators B: Chemical. 2018 Oct 1;270:405-16.
55. Gorska A, Paczosa?Bator B, Wyrwa J, Piech R. New Electrochemical Sensor of Prolonged Application for Metformin Determination Based on Hydrated Ruthenium Dioxide?Carbon Black?Nafion Modified Glassy Carbon Electrode. Electroanalysis. 2020 Aug;32(8):1875-84.
56. Hadi M, Poorgholi H, Mostaanzadeh H. Determination oF metformin at metal-organic framework (Cu-BTC) Nanocrystals/multi-walled carbon nanotubes modified glassy carbon electrode. South African Journal of Chemistry. 2016 Jan 1;69(1):132-9.
57. Roy E, Patra S, Madhuri R, Sharma PK. Gold nanoparticle mediated designing of non-hydrolytic sol-gel cross-linked metformin imprinted polymer network: A theoretical and experimental study. Talanta. 2014 Mar 1;120:198-207.
58. Dehdashtian S, Gholivand MB, Shamsipur M, Karimi Z. A nano sized functionalized mesoporous silica modified carbon paste electrode as a novel, simple, robust and selective anti-diabetic metformin sensor. Sensors and Actuators B: Chemical. 2015 Dec 31;221:807-15.
59. Nezhadali A, Khalili Z. Computer-aided study and multivariate optimization of nanomolar metformin hydrochloride analysis using molecularly imprinted polymer electrochemical sensor based on silver nanoparticles. Journal of Materials Science: Materials in Electronics. 2021 Dec;32:27171-83.
60. Skrzypek S, Mir?eski V, Ciesielski W, Soko?owski A, Zakrzewski R. Direct determination of metformin in urine by adsorptive catalytic square-wave voltammetry. Journal of Pharmaceutical and Biomedical Analysis. 2007 Oct 18;45(2):275-81.
61. Hassasi S, Hassaninejad-Darzi SK, Vahid A. Production of copper-graphene nanocomposite as a voltammetric sensor for determination of anti-diabetic metformin using response surface methodology. Microchemical Journal. 2022 Jan 1;172:106877.
62. Gholivand MB, Mohammadi-Behzad L. Differential pulse voltammetric determination of metformin using copper-loaded activated charcoal modified electrode. Analytical Biochemistry. 2013 Jul 1;438(1):53-60.
63. Wang Y, Ding L, Yu H, Liang F. Cucurbit [6] uril functionalized gold nanoparticles and electrode for the detection of metformin drug. Chinese Chemical Letters. 2022 Jan 1;33(1):283-7.
64. Momeni S, Farrokhnia M, Karimi S, Nabipour I. Copper hydroxide nanostructure-modified carbon ionic liquid electrode as an efficient voltammetric sensor for detection of metformin: a theoretical and experimental study. Journal of the Iranian Chemical Society. 2016 Jun;13:1027-35.
65. Radi AE, Eissa SH. Electrochemical study of glimepiride and its complexation with ?-cyclodextrin. Collection of Czechoslovak Chemical Communications. 2010 Dec 1;76(1):13-25.
66. AL-TAEE AT, AL-HAFIDH AZ. Electrochemical Behavior of Valsartan, Glimepiride and Their Interaction with Each Other Using Square Wave Voltammetry. The Eurasia Proceedings of Science Technology Engineering and Mathematics. 2019 Nov 24;7:236-48.
67. Sebaiy M. M., Abdelazeem A. I., Aboulfotouh A., Rouk A. A., Mohamed A. A., Mahny A. G. (2022) Instrumental Analysis of Chloroquine and Hydroxychloroquine in Different Matrices. Curr. Res: Integr. Med. 7(2):1-8. DOI:10.37532.2022.7.2
68. Batakoushy H. A., Omar M. A., Ahmed H. M., Abdel Hamid M. A., Sebaiy M. M. (2022) Review article: Pharmacology and Analytical Chemistry Profile of Dapagliflozin, Empagliflozin andSaxagliptin. Modern App. Pharm. Pharmacol., 2(5): MAPP.000548 DOI: 10.31031/MAPP.2022.02.000548
69. Ali O. T., Elgendy K. M., Saad M. Z., Hassan W. S., Sebaiy M. M. (2021) Analytical Techniques for Determination of Albendazole, Fenbendazole, Omeprazole and Fluconazole in Pharmaceutical and Biological Samples. Int. J. Pathol. Immunol., 2(1): 1-24.
70. Lashine E. M., El-Sayed A. S., Elshahat A. K., Zaki A. R., El-Halaby A. S., Mostafa A. S., Shabaan A. K., Sobhy A. S., Abd Alsamed A. S., El-attar A. S., Farrag A. S., Sebaiy M. M. (2021) Spinal Muscle Atrophy (Types I & II & III & IV): Literature Review. Clin. Pharmacol. Toxicol. Res., 4(6): 1-6.
71. Ramadan A., Abd-Elaziz A., Ismail E. M., Maher A., Hegazy K. M., Sebaiy M. M. (2021) Review article: Pharmacological and Analytical Profile of Celecoxib. Pharm. Sci. Biomed. Anal. J., 4(1):128 https://scientificliterature.org/Pharmaceutics/Pharmaceutics-21-128.pdf
72. Elsabbagh O. I., Soror A. W., Moselhy A. Y., Elayat A. E., Hafez A. M., Saleh A. M., Nagib O. A., Khorkhash E. I., Abdelgalil E. I., Abdelmaksod E. I., Elsayed E. A., Sebaiy M. M. (2021) Literature Review on Obesity: Causes, Treatment and Correlation with Pandemic COVID-19. Pharm. Drug Regul. Affair J. (PDRAJ)., 4(1): 000124. DOI: 10.23880/pdraj-16000124
73. Ibrahim SM, Elshafiey EH, Abdulrahim ER, Azazy ER, Abd-Elghany EZ, et al. Steroids in Medicinal Chemistry: Literature Review. Academic Journal of Chemistry. 2021;6(3):69-78.
74. Ibrahim AE, Elhenawee M, Saleh H, Sebaiy MM. Overview on liquid chromatography and its greener chemistry application. Annals of Advances in Chemistry. 2021 Apr 7;5(1):004-12.
75. Abdel-Aziz L. M., Soror A. A., Hassan A. A., Ali A. S., Hafez A. A., Hemdan A. A., Sebaiy M. M. (2021) Review article: Instrumental Analysis of Chlordiazepoxide in Different Matrices. Int. Res. J. Multidiscipl. Technovat. (IRJMT)., 3(5): 1-10. https://doi.org/10.34256/irjmt2151
76. Sebaiy MM. Mini-review on Glaucoma Drugs, Timolol and Latanoprost: Mode of Action and Analytical Methods. Open J Pharma Sci. 2021;1:1-3.
77. Sebaiy MM. Analytical Review: Methods of Determination for Ledipasvir and Velpatasvir in Pharmaceutical and Biological samples. BR Nahata Smriti Sansthan International Journal of Phramaceutical Sciences & Clinical Research. 2021 Aug 12;1(2).
78. Ibrahim AE, Elhenawee M, Saleh H, Sebaiy MM. Sci Forschen.
79. Sebaiy M. M., Shanab A. G., Nasr A. K., Hosney A. E., Elsaid A. G., Ramadan A. H. (2021) Literature Review on Spectrophotometric, Chromatographic and Voltammetric Analysis of Ivermectin. Med. Anal. Chem. Int. J. (MACIJ)., 5(1): 000170. https://doi.org/10.23880/macij-16000170
80. Kumaraswamy G, Pranay K, Rajkumar M, Lalitha R. Novel stabilty indicating rp-hplc method simultaneous determination of sofosbuvir and velpatasvir in bulk and combined tablet dosage forms. Innov Int J Med Pharm Sci. 2017;2(7).
81. Abdel-Aziz LM, Sapah AA, Naser A, Abd-Elaziz A, El-Emary A, et al.. Spectroscopic, Chromatographic and Electrochemical Determination of Indomethacin in Different Matrices. European Journal of Science, Innovation and Technology. 2021 Jun 29;1(2):32-40.
82. Sebaiy MM, Farouk EM, Lotfy EM, Mokhtar EM, Abd-Elgwad EN, et al. (2021) Review article: Spectroscopic, Chromatographic and Electrochemical Analysis of Azithromycin in Different Matrices. J Drug Des Res 8(2):1084.
83. Adel E. Ibrahim, Magda Elhenawee, Hanaa Saleh, Mahmoud M. Sebaiy. “Overview on Hepatitis C, Treatment Strategy, Instrumental Analysis of Anti-HCV drugs’’. J Pharmacy and Drug Innovations, 2(2); DOI: http;//doi.org/03.2020/1.1014.
84. SARAYA RE, ELHENAWEE M, SALEH H, SEBAIY MM. Mini Review: Insights on Instrumental Analysis of Ombitasvir, Paritaprevir and Ritonavir. International Journal of Chemistry Research. 2021 Apr 1:1-4.
85. Elrefay H, Ismaiel OA, Hassan WS, Shalaby A, Fouad A, et al. Mini-Review on Various Analytical Methods for Determination of Certain Preservatives in Different Matrices. Int. J. Res. Stud. Sci., Eng. Technol.(IJRSSET). 2021;8:1-8.
86. Ibrahim A. E., Elhenawee M., Saleh H., Sebaiy M. M. (2021) Mini-review on Chromatography of Proteomics. Glob. J Chem. Sci. 1(1): 1-4.
87. Ali OT, Elgendy KM, Saad MZ, Hassan WS, Sebaiy MM. Review Article: Instrumental Analysis of Certain Azoles with Variant Anti-Infective Activity.
88. Saraya R. E., Elhenawee M., Saleh H., Sebaiy M. M. (2021) Review article on Analytical Techniques of Lamivudine Determination in Different Matrices. J. Adv. Pharm. Sci. Tech (JAPST)., 2(3): 37-46. DOI: 10.14302/issn.2328-0182.japst-20-3664
89. Scheme I. Analytical Methods for Determination of Certain Antihypertensive Drugs.
90. Sebaiy MM, Saraya RE, Elhenawee M, Saleh H. Analytical Methods for Determination of Ondansetron hydrochloride and Pantoprazole. Journal of Medical Research and Health Sciences. 2021 Feb 24;4(2):1175-81.
91. Elbaramawi SS, El-Sadek ME, Baraka MM, Abdel-Aziz LM, Sebaiy MM. Instrumental analysis of some anti-ulcer drugs in different matrices. Chemical Reports. 2020 Nov 17;2(1):156-72.
92. Elkady Y, El-Adl SM, Baraka M, Sebaiy MM. Literature Review of Analytical Methods for Determination of Triamcinolone Acetonide and Benzyl Alcohol. Novel Approaches in Drug Designing & Development. 2020;5(3):49-54.
93. Yara Elkady, Sobhy M. El-Adl, Mohamed Baraka and Mahmoud M. Sebaiy (2020) Analytical Methods for Determination of Salbutamol, Ambroxol and Fexofenadine J, Biotechnology and Bioprocessing 1(1); DOI: 10.31579/2766-2314/004
94. Ibrahim F, Sobhy M, Baraka MM, Ibrahim SM, Sebaiy MM. Analytical methods for the determination of certain antibiotics used in critically ill patients. Journal of Pharmaceutical and Biopharmaceutical Research. 2020 Jun 9;2(1):99-117.
95. Sebaiy MM, Abdellatef HE, Elhenawee MM, Elmosallamy MA, Alshuwaili MK. Review Article: Instrumental Analysis of Olopatadine Hydrochloride, Oxeladine Citrate, Amlodipine Besylate and Xipamide. Int J Analyt Bioanalyt Methods. 2020;2(010).
96. Sebaiy MM, Sm EA, Mm B, Aa H. Analytical Methods for Determination of Certain Sartans and Diuretics. J. Chem. Sci. Chem. Eng. 2020;1:11-8.
97. Sebaiy MM, Hegazy KM, Ebrahim AM, Essam FM, Amin FA, et al. Captopril and Hydrochlorothiazide: Insights on Pharmacology and Analytical Chemistry Profile. Journal of Chemistry & its Applications. 2022;1(2):1-2.
98. Elrefay H, Ismaiel OA, Hassan WS, Shalaby A, Fouad A, Sebaiy MM. Literature Review on Instrumental Analysis of Metformin Hydrochloride, Glibenclamide, Glimepiride and Pioglitazone Hydrochloride in Different Matrices. Pharmaceutical Sciences And Biomedical Analysis Journal. 2022; 4(1):130
99. Sebaiy MM, Elrefay H, Ismaiel OA, HassanWS, Shalaby A , et al. (2022) Insights on Analytical Methods for Determination of Risperidone, Levetiracetam, Sodium Valproate and Oxcarbazepine. Der Pharmacia Sinica Vol:13 No:3
100. Mahmoud M. Sebaiy, Mohamed Y . Hassaballah and Noha I. Ziedan. Topoisomerase II Inhibitor as a Potential Therapy for Severe COVID-19: Antiviral Activity and Molecular Docking Studies. Pharmaceutical Sciences And Biomedical Analysis Journal. 2022; 4(2):132
101. Sebaiy MM, Abdelmonem A, Reda A, Fathy A, Gamal A. Insights on COVID-19 Pathophysiology and Treatment. Journal of Pharmaceutical Research and Drug Safety. 2022;1:103.
102. Sebaiy MM, Hegazy KM, Ebrahim AM, Essam FM, Amin FA, et al. Captopril and Hydrochlorothiazide: Insights on Pharmacology and Analytical Chemistry Profile. Journal of Chemistry & its Applications. 2022;1(2):1-2.
