Abstract
HCC is one of the most common malignant tumors. The life and health of humans are gravely threatened by HCC because of its hidden onset, high recurrence rate, poor therapeutic effect, and high mortality. It is essential to explore the particular pathological mechanisms of HCC in order to increase the rate of early diagnosis and enhance patient therapy outcomes. Recent research has demonstrated that SCUBE3 can influence HCC cell proliferation by regulating the TGFβ/PI3K/AKT/GSK3β pathway. The molecular regulatory network of HCC proliferation is improved by this research, which also offers a solid theoretical and experimental foundation for SCUBE3 as a potential new therapeutic target for HCC.
Keywords
Hepatocellular carcinoma, SCUBE3, Cell proliferation, Targeted therapy, TβRII, PI3K/AKT, CCNE1
Commentary
The most prevalent kind of primary liver cancer is hepatocellular carcinoma (HCC). Primary liver cancer has risen to the sixth most prevalent cancer worldwide and the third most common cause of cancer mortality as of 2020 [1]. There have been more therapeutic choices for HCC in recent years. Sorafenib shifts the paradigm of systemic treatment to molecular targeted therapy. Targeted therapy can induce cancer cell death by blocking specific proteins through monoclonal antibodies or small molecule inhibitors, changing cell signaling pathways, thereby affecting apoptosis, and stimulating the immune system. The exploration of HCC immunotherapy targets is essential given the high mortality and poor prognosis of HCC. With the detailed study of the carcinogenic process and signaling pathways of HCC cell proliferation, differentiation, invasion, and metastasis, biomarkers and therapeutic targets related to the pathological mechanism of HCC have been applied to clinical diagnosis and treatment, including AFP [2], Des-γ-Carboxy Prothrombin (DCP) [3], VEGF/VEGF receptor [4], etc. Nonetheless, there is a need to increase the specificity and accuracy of these biomarkers and treatment targets. Therefore, it is crucial to discover biomarkers or novel treatment targets linked to the progression and development of HCC. Surprisingly, an article entitled "SCUBE3 downregulation modulates hepatocellular carcinoma by inhibiting CCNE1 via TGFβ/PI3K/AKT/GSK3β pathway" was published by Cancer Cell International. The authors of this article explored the signal peptide-CUB-EGF-like domain-containing protein 3 (SCUBE3), which is closely related to HCC development [5].
SCUBE3, along with SCUBE1 [6] and SCUBE2 [7], is a member of the signal peptide-CUB-EGF-like domain-containing protein (SCUBE) family. SCUBE3 and other members of the family have similar domain structures and share an overall protein identity of 60%. An N-terminal signal peptide, an amino acid copy of the EGF (epidermal growth factor)-like repeat sequence, a spacer region, three cysteine-rich domains, and the C-terminal domains of the complement proteins C1r/C1s, Uegf, and Bmp1 (CUB) make up the SCUBE3 domain structure [8]. The authors first analyze the expression of SCUBE3 using The Cancer Genome Atlas (TCGA) liver HCC data on UALCAN. SCUBE3 expression was upregulated in tumor tissues as compared to nearby normal tissues. Gender, age, and weight are associated with SCUBE3 expression in liver cancer tissues [5]. The authors further found that the expression of SCUBE3 in HCC cells was higher than that in normal hepatocytes [5]. Subsequently, the authors verified that knockdown of SCUBE3 inhibited HCC cell proliferation, promoted apoptosis, and induced cell cycle arrest [5]. Notably, our in vivo experiments demonstrated that knockdown of SCUBE3 inhibited tumor growth in naked mice [5]. Both in vivo and in vitro experiments showed that down-regulation of SCUBE3 expression reduced the proliferation of HCC cells and significantly reduced the occurrence and development of tumors [5]. Together, our results show that SCUBE3 has the potential to be a therapeutic target for HCC. The SCUBE3 protein has attracted more and more attention in the field of cancer. In many tumors, SCUBE3 is upregulated, which further promotes the development of tumors such as lung cancer [9], breast cancer [10], and glioma [11]. Importantly, CN-3, a new asterosaponin, can reduce SCUBE3, thereby preventing the proliferation of glioma cells [11]. However, our study is the first to explore the association between SCUBE3 and HCC [5].
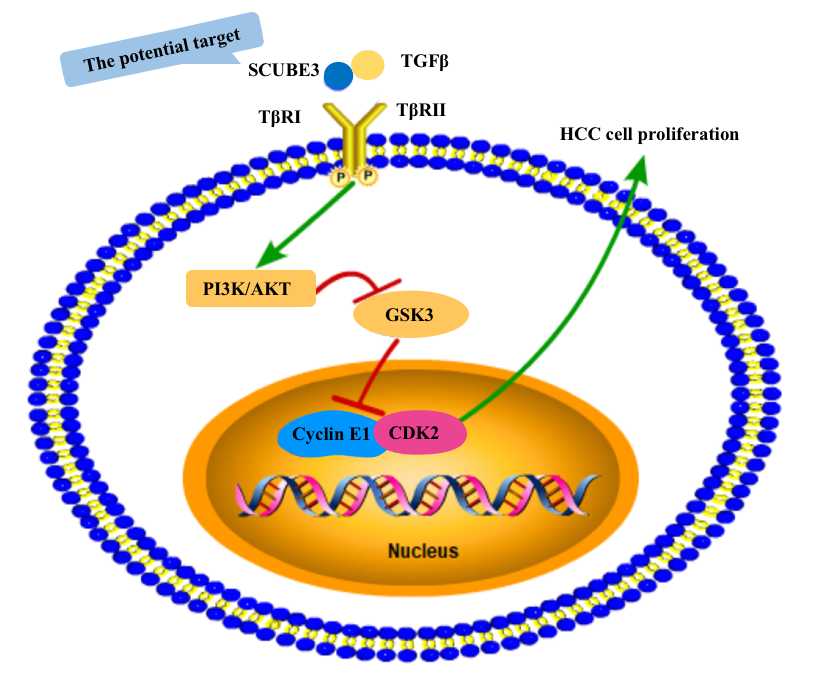
Figure 1. The schematic diagram of SCUBE3 regulating HCC cell proliferation.
The authors further explored the molecular mechanism of SCUBE3 regulating HCC proliferation. The ability of the signal transduction network to transfer cell-perceived signals to targets farther down the signaling chain in order to coordinate cell growth, migration, survival, and differentiation is well known. A bioinformatics analysis confirmed that the function of SCUBE3 in promoting proliferation may be related to cyclin E1 (CCNE1) and PI3K-AKT signaling pathways [5]. According to our in vitro experiments, SCUBE3 binds to the TGFβ type II receptor (TβRII) and activates the non-Smad signaling pathway, the PI3K/AKT signaling pathway [5]. TGF-β activates non-Smad signaling pathways, also known as "non-canonical signaling pathways," even though the Smad pathway is the canonical signaling pathway of TGF-β family proteins. The ligands can activate non-Smad signaling pathways by binding with varying affinity to the three TGFβ- receptor subtypes (TβRI, II, and III). The TGF-β receptors can activate the non-Smad signaling pathway’ the PI3K/AKT signaling pathway by activating intermediates PI3K [12].
The PI3K/AKT signaling pathway is one of the most crucial pathways for regulating cell division, autophagy, survival, and differentiation. Malignant tumors are associated with aberrant PI3K/AKT signaling pathway activation, and this dysfunction will catalyze the phosphorylation of a few proteins, promoting cancer cell survival, proliferation, invasion, and migration. Phosphatidylinositol-3,4,5-triphosphate (PI3,4,5-P3), a second messenger that is produced when PI3K is activated, aggregates a subset of signaling proteins (such as Akt). GSK3β and other downstream effector molecules can receive signals from a variety of upstream regulatory proteins through Akt, which controls cell cycle progression and survival [13,14]. Our study showed that knockdown of SCUBE3 significantly reduced GSK3 phosphorylation [5]. Additionally, there is evidence that the PI3K/AKT signaling network, which is regarded as a crucial signaling pathway in cell cycle regulation, positively influences G1/S cell cycle progression by inactivating GSK3β [14]. Studying the cell cycle proteins involved in the molecular mechanism of SCUBE3 promoting HCC proliferation has become our focus.
SCUBE3 regulates CCNE1 to modulate HCC cell proliferation. Cyclin E-CDK2 has long been considered a significant and critical regulator in the G1 phase of the cell cycle. The C (PSTAIRE) helix and activation segment of CDK2 serve as the primary points of contact between CCNE1 and CDK2. A positive feedback loop is created by CCNE1 activating CDK2 and phosphorylating cellular proteins to drive mitosis from the G1 phase to the S phase. Cancer cells have the ability to divide indefinitely, and one of their primary characteristics is disruption of the cell cycle. In certain forms of human tumors, an abnormal increase of cyclin E-CDK2 activity has been observed [15]. Similarly, our study demonstrated that CCNE1 is a downstream effector molecule of SCUBE3 promoting HCC proliferation through rescue experiments [5]. The findings revealed that CCNE1 expression was elevated in HCC cells and that SCUBE3 induced HCC cell proliferation through controlling the CCNE1 downstream molecule [5].
In conclusion, the authors first identified the function, mechanism, and significance of SCUBE3 in HCC cancer in the focus article. According to research, SCUBE3 inhibited GSK3 activity by attaching to TβRII and activating the PI3K/AKT signaling pathway, which expanded the accumulation of CCNE1 and resulted the transformation of HCC cells from the G1 phase to the S phase and promoted their proliferation [5]. Our findings support SCUBE3 as a therapeutic target for HCC, but further tissue specimens and clinical data would round out the perspective. SCUBE3 promotes the invasion and metastasis of lung cancer [9] and breast cancer [10]. In the future, the authors will explore whether SCUBE3 is involved in HCC invasion and migration.
Acknowledgement
Not applicable.
Author Contributions Statement
Teng Liu wrote the entire manuscript and edited it. Qiang Luo is the corresponding author who edited the manuscript and provided comments on it. Xia Yang and Ke Wang also provided comments.
Conflicts of Interest
The authors declare no conflict of interest.
Funding
This work is supported by the Science and Health joint Medical Research Project of Nan’an District, Chongqing (No. 2019-01), General Program of Chongqing Natural Science Foundation (No. cstc2019jcyj-msxmX084), the Chongqing Postgraduate Research and Innovation Project (Grant No: CYS18194) and the National Science and Technology Major Project of China (2017ZX10202203-007).
References
2. Lin B, Dong X, Wang Q, Li W, Zhu M, Li M. AFP-Inhibiting Fragments for Drug Delivery: The Promise and Challenges of Targeting Therapeutics to Cancers. Front Cell Dev Biol. 2021;9:635476.
3. Ji J, Wang H, Li Y, Zheng L, Yin Y, Zou Z, et al. Diagnostic Evaluation of Des-Gamma-Carboxy Prothrombin versus ?-Fetoprotein for Hepatitis B Virus-Related Hepatocellular Carcinoma in China: A Large-Scale, Multicentre Study. PloS One. 2016;11(4):e0153227.
4. Zhu AX, Duda DG, Sahani DV, Jain RK. HCC and angiogenesis: possible targets and future directions. Nat Rev Clin Oncol. 2011 May;8(5):292-301.
5. Xu P, Luo A, Xiong C, Ren H, Yan L, Luo Q. SCUBE3 downregulation modulates hepatocellular carcinoma by inhibiting CCNE1 via TGF?/PI3K/AKT/GSK3β pathway. Cancer Cell Int. 2022 Jan 3;22:1.
6. Yang RB, Ng CKD, Wasserman SM, Colman SD, Shenoy S, Mehraban F, et al. Identification of a Novel Family of Cell-surface Proteins Expressed in Human Vascular Endothelium*. J Biol Chem. 2002 Nov 29;277(48):46364-73.
7. Tsai MT, Cheng CJ, Lin YC, Chen CC, Wu AR, Wu MT, et al. Isolation and characterization of a secreted, cell-surface glycoprotein SCUBE2 from humans. Biochem J. 2009 Jul 29;422(1):119-28.
8. Huo Q, He X, Li Z, Yang F, He S, Shao L, et al. SCUBE3 serves as an independent poor prognostic factor in breast cancer. Cancer Cell Int. 2021 May 18;21:268.
9. Wu YY, Peck K, Chang YL, Pan SH, Cheng YF, Lin JC, et al. SCUBE3 is an endogenous TGF-? receptor ligand and regulates the epithelial-mesenchymal transition in lung cancer. Oncogene. 2011 Aug 25;30(34):3682-93.
10. Yang X, Hu J, Shi C, Dai J. Activation of TGF-?1 Pathway by SCUBE3 Regulates TWIST1 Expression and Promotes Breast Cancer Progression. Cancer Biother Radiopharm. 2020 Mar 1;35(2):120-8.
11. Qiu PC, Lu YY, Zhang S, Li H, Bao H, Ji YQ, et al. Reduction of SCUBE3 by a new marine-derived asterosaponin leads to arrest of glioma cells in G1/S. Oncogenesis. 2020 Aug 6;9(8):71.
12. Finnson KW, Almadani Y, Philip A. Non-canonical (non-SMAD2/3) TGF-? signaling in fibrosis: Mechanisms and targets. Semin Cell Dev Biol. 2020 May;101:115-22.
13. Revathidevi S, Munirajan AK. Akt in cancer: Mediator and more. Semin Cancer Biol. 2019 Dec 1;59:80-91.
14. Porta C, Paglino C, Mosca A. Targeting PI3K/Akt/mTOR Signaling in Cancer. Front Oncol. 2014;4.
15. Chu C, Geng Y, Zhou Y, Sicinski P. Cyclin E in normal physiology and disease states. Trends Cell Biol. 2021 Sep;31(9):732-46.
