Abstract
Severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) infection is a serious threat to lung cancer patients. Hereby, we hypothesize that Coronavirus disease 2019 (COVID-19) may contribute to lung cancer progression by increasing extracellular adenosine triphosphate (ATP) levels and hyperactivating the purinergic P2X purinoceptor 7 receptor (P2X7R). Hyperactivation of P2X7R by increased extracellular ATP may stimulate multiple signaling pathways and factors such as NLRP3 inflammasome; as a result, interleukin (IL)-1β, and IL-18 pro-inflammatory cytokines are released, JNK, Rho kinase, HMGB1-RAGE, PI3K/AKT, hypoxia-inducible factor-1 alpha (HIF-1α), and ERK. NLRP3 activation may play a pivotal role in fatal cytokine storm in critically ill patients with COVID-19 and tumor progression in patients with lung cancer. Consequently, inhibiting these signaling pathways may deviate immune responses toward anti-tumoral responses, and suppress lung cancer progression and cytokine storms. Therefore, targeting P2X7R by means of oxidized ATP and anti-P2X7 monoclonal antibodies may provide promising therapeutic approaches to prevent lung cancer progression in COVID-19 patients; however, no clinical trials have yet been conducted, and their clinical efficacy remains to be elucidated.
Keywords
COVID-19, SARS-CoV-2, Lung cancer, P2X7 receptor, Cancer progression
Introduction
COVID-19, which emerged in Dec 2019, has caused a concerning epidemic worldwide by SARS-CoV-2 [1-5]. Glycoprotein S or spike protein is the main contributor to virus entry into the host cells and its infection through binding to angiotensin-converting enzyme 2 (ACE2) receptor [6]. This interaction leads to activation of the transmembrane protease/serine subfamily member 2 (TMPRSS2), a human protease primarily located in the airways and alveolar cell membranes, thereby facilitating the virus entry via supporting the protein S and ACE2 cleavage [1]. At the moment, several researchers/scientists are investigating all possible pathways affected by SARS-CoV-2 infection, particularly in susceptible individuals, such as cancer patients, to develop a rapid treatment and decrease their mortality [7-11]. It has been suggested that COVID-19 can lead to multiple cancer progression and chemo-resistance [1,8-13]. Recently we discussed the P2X7 receptor, as a cation channel highly expressed in the central nervous system (CNS) and lungs that is activated at high concentrations of adenosine triphosphate (ATP) [1,14]. Long-term activation of the P2X7 receptor leads to cell death and increased ATP release into the extracellular environment [1]. On the other hand, a study by Kong et al. showed that COVID-19 patients with lung cancer are more susceptible to severe complications of COVID-19 than patients without cancer [15]. Due to the fact that lung cancer is one of the leading causes of cancer-related mortality [16,17], lung cancer patients must be considered a vulnerable group to COVID-19-related complications and death. Hereby, we hypothesize that hyperactivation of the P2X7 receptor (P2X7R) by COVID-19 not only may predispose lung cancer patients to experience a severe course of COVID-19 but also may contribute to cancer progression in these patients.
COVID-19 and P2X7 Receptor Hyperactivation and Downstream Signaling Pathways
Purinergic P2X7R is a gated ion channel with an ATP ligand. Multiple tissues (e.g., lung, CNS, etc.) and immune cells (e.g., tumor-associated macrophage (TAM), etc.) express P2X7R, which activated P2X7R has been demonstrated to be involved in physiological and pathological processes such as tumor cell growth, inducing apoptosis, or stimulating cell proliferation [1,18,19]. According to recent investigations, P2X7R is overexpressed in lung cancer and may strongly underlie lung cancer progression [18]. The bronchoalveolar lavage fluid (BALF) of patients with acute respiratory distress syndrome (ARDS) also contains elevated ATP levels. Elevated levels of extracellular ATP induced by SARS-CoV-2 infection may induce P2X7 receptor hyperactivity [1,20-24]. A central role of this receptor in inflammation is the stimulation of NACHT, LRR, and PYD domain-containing protein 3 (NLRP3) inflammasome, resulting in caspase-1 activation and IL-1β and IL-18 release. ROS production is triggered by the P2X7 receptor [25]. In severe inflammation, its increased activation may decrease lymphocyte function by impairing mitochondrial function, as has been seen in monocytes in sepsis [26]. In response to the activation of P2X7 receptors, MHC-I expression is down-regulated and IL-6 is released simultaneously [25]. It can also activate JNK and Rho kinase as well as the high mobility group box 1- receptor for advanced glycation end products (HMGB1-RAGE) pathway [27-29]. Researchers found that serum HMGB1 levels were related to the severity of pathogen-induced tissue damage as well as excessive cytokine storms [30,31]. In addition to ACE2, HMGB1 was recently shown to bind to the RNA of SARS-CoV-2, carrying it into the cytoplasm when attached to the RAGE-lysosomal pathway [32].
Lung Cancer and P2X7 Receptor Hyperactivation
As SARS-CoV-2 primarily affects lung tissue and leads to severe acute respiratory syndrome, individuals with respiratory disorders, such as lung cancer, may be more susceptible to COVID-19 and should be given extra attention [33,34]. In this line, previous studies indicated that P2X7R could cause carcinogenesis, progression, survival, and metastasis of lung cancer cells through its up-regulation and activation of numerous intracellular signaling pathways such as JNK, Rho, HMGB1, and Epithelial-Mesenchymal Transition (EMT) pathways in bronchoalveolar cells [35,36]. In normal cells, the P2X7 receptor has moderate activity because of its smooth affinity for low concentrations of ATP. Increased aerobic glycolysis in cancerous cells will result in hyper-activated mitochondria leading to Ca2+ release from the endoplasmic reticulum and subsequently, high levels of ATP. A high concentration of ATP can promote lung cancer cells' progression and survival through the activation of P2X7 receptor [35]. Also, the P2X7R activation itself can increase the cytosolic Ca2+ and ratio of Bcl-2/Bax ratio to promote cancer cell survival [36]. Moreover, it induces several intracellular growth-promoting pathways, such as NFATc1, JNK, Phosphoinositide 3-kinase (PI3K)/AKT, Hypoxia-inducible factor 1-alpha (HIF-1α), and ERK in cancerous cells [14,37]. Besides that, high levels of vascular endothelial growth factor (VEGF) and matrix metalloproteinases (MMP) mediated by activated P2X7R elevate the proliferation and metastasis rate and decrease the apoptosis of cancerous lung cells [15,38]. This observation can be proved by the study of Takai et al. who demonstrated that transforming growth factor-β1 (TGF-β1) activates the P2X7Rs through ATP release and subsequently, accelerates lung cancer cell metastasis via actin remodeling. Then, it was indicated that cell metastasis and actin remodeling are related to mitogen-activated protein kinases, such as JNK, and Rho kinase, respectively [39].
Conclusion
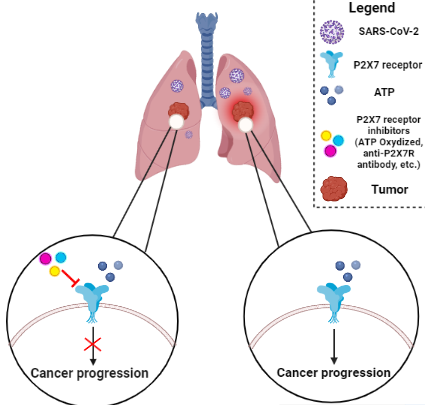
Hyperactivation of P2X7R is involved in either inducing apoptosis or stimulating cell proliferation in many tissues, especially lung cancer cells. SARS-CoV-2 may hyperactivate P2X7R by increasing extracellular ATP levels. It can activate multiple signaling pathways, including NLRP3 (inflammasome), JNK, Rho kinase, HMGB1-RAGE, PI3K/AKT, HIF-1α, and ERK [1,20,22-24,35]. In this regard, IL-1β and IL-18 pro-inflammatory cytokines are released after NLRP3 inflammasome activation. Therefore, NLRP3 activation may play a pivotal role in fatal cytokine storm in critically ill patients with COVID-19 and tumor progression in patients with lung cancer. Consequently, inhibition of this signaling pathway may deviate immune responses toward anti-tumoral responses and suppress cytokine storm. In light of this hypothesis that purinergic P2X7R may facilitate the progression of lung cancer, P2X7R antagonists and inhibitors (e.g. ATP Oxidized, JNJ47965567, AZ11645373, GW791343, Brilliant Blue G (BBG), KN-62 and KN-04, A740003 and A438079, CE-224535, anti-P2X7 monoclonal antibodies, and natural compounds such as Rhein, Emodin, Teniposide, and Baicalein) may inhibit cancer progression in lung cancer patients who are afflicted with COVID-19 (Figure 1) [40]. This is because of several pre-clinical trials of them. For instance, oxidized ATP was utilized to target P2X7R in an in vivo mouse model of islet allograft dismissal and was shown to safeguard islet unites [40,41]. Also, A438079 has been shown to reduce inflammation in an in vivo mouse model of salivary gland exocrinopathy [42]. However, no clinical trials have yet been conducted and their clinical efficacy remains unknown.
Abbreviations
P2X7R: P2X7 Receptor; CNS: Central Nervous System; NLRP3: PYD domain-containing protein 3; EMT: Epithelial-Mesenchymal Transition; ATP: adenosine triphosphate; HIF-1α: Hypoxia-Inducible Factor 1-alpha
Acknowledgment
Not applicable.
Authors’ Contributions
H.Z conceived the study and designed the headings. H.Z, A.A, M.N-A, A.N, and F.S wrote the manuscript text. H.Z created the figure. H.Z, F.S, and M.N-A, edited the manuscript for important intellectual content. M.N-A, H.Z, and A.N revised the manuscript. F.S and M.P supervised the study. All authors read and approved the final manuscript.
Funding
The authors received neither funding/support nor grants for the publication of this work.
Availability of Data and Materials
Data derived from public domain resources.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflicts of interest.
References
2. Samidoust P, Delshad ME, Talemi RN, Mojtahedi K, Samidoust A, Jahangiri S, et al. Incidence, characteristics, and outcome of COVID-19 in patients on liver transplant program: a retrospective study in the north of Iran. New Microbes and New Infections. 2021;44:100935.
3. Aghajanzadeh M, Haghighi M, Rimaz S, Fomani AA, Tangestaninejad A, Ashoobi MT, et al. Pneumomediastinum, pneumopericardium pneumothorax and subcutaneous emphysema in Iranian COVID-19 patients. Journal of Current Biomedical Reports. 2021;2(4):201-5.
4. Dehghanbanadaki H, Aazami H, Shabani M, Amighi D, Seif F, Dehnavi AZ, et al. A systematic review and meta-analysis on the association between lymphocyte subsets and the severity of COVID-19. immunopathologia persa. 2021;8(2):e29303-e.
5. Sedighimehr N, Fathi J, Hadi N, Rezaeian ZS. Rehabilitation, a necessity in hospitalized and discharged people infected with COVID-19: a narrative review. Physical Therapy Reviews. 2021;26(3):202-10.
6. Nabi-Afjadi M, Heydari M, Zalpoor H, Arman I, Sadoughi A, Sahami P, et al. Lectins and lectibodies: potential promising antiviral agents. Cellular & Molecular Biology Letters. 2022;27(1):1-25.
7. Zalpoor H, Akbari A, Nabi-Afjadi M. Ephrin (Eph) receptor and downstream signaling pathways: a promising potential targeted therapy for COVID-19 and associated cancers and diseases. Human Cell. 2022:1-3.
8. Zalpoor H, Bakhtiyari M, Liaghat M, Nabi‐Afjadi M, Ganjalikhani‐Hakemi M. Quercetin potential effects against SARS‐CoV‐2 infection and COVID‐19‐associated cancer progression by inhibiting mTOR and hypoxia‐inducible factor‐1α (HIF‐1α). Phytotherapy Research. 2022.
9. Zalpoor H, Rezaei M, Yahyazadeh S, Ganjalikhani-Hakemi M. Flt3-ITD mutated acute myeloid leukemia patients and COVID-19: potential roles of autophagy and HIF-1α in leukemia progression and mortality. Human Cell. 2022:1-2.
10. Zalpoor H, Shapourian H, Akbari A, Shahveh S, Haghshenas L. Increased neuropilin-1 expression by COVID-19: a possible cause of long-term neurological complications and progression of primary brain tumors. Human Cell. 2022:1-3.
11. Zalpoor H, Akbari A, Nayerain Jazi N, Liaghat M, Bakhtiyari M. Possible role of autophagy induced by COVID-19 in cancer progression, chemo-resistance, and tumor recurrence. Infectious Agents and Cancer. 2022;17(1):1-4.
12. Zalpoor H, Akbari A, Nabi-Afjadi M. Ephrin (Eph) receptor and downstream signaling pathways: a promising potential targeted therapy for COVID-19 and associated cancers and diseases. Human Cell. 2022;35(3):952-4.
13. Zalpoor H, Bakhtiyari M, Shapourian H, Rostampour P, Tavakol C, Nabi-Afjadi M. Hesperetin as an anti-SARS-CoV-2 agent can inhibit COVID-19-associated cancer progression by suppressing intracellular signaling pathways. Inflammopharmacology. 2022:1-7.
14. Zalpoor H, Akbari A, Nabi-Afjadi M, Forghaniesfidvajani R, Tavakol C, Barzegar Z, et al. Hypoxia‐inducible factor 1 alpha (HIF‐1α) stimulated and P2X7 receptor activated by COVID-19, as a potential therapeutic target and risk factor for epilepsy. Human Cell. 2022:1-8.
15. Kong Q, Xiang Z, Wu Y, Gu Y, Guo J, Geng F. Analysis of the susceptibility of lung cancer patients to SARS-CoV-2 infection. Molecular Cancer. 2020;19(1):1-5.
16. Karami Fath M, Babakhaniyan K, Zokaei M, Yaghoubian A, Akbari S, Khorsandi M, et al. Anti-cancer peptide-based therapeutic strategies in solid tumors. Cellular & Molecular Biology Letters. 2022;27(1):1-26.
17. Zalpoor H, Nabi-Afjadi M, Forghaniesfidvajani R, Tavakol C, Farahighasreaboonasr F, Pakizeh F, et al. Quercetin as a JAK–STAT inhibitor: a potential role in solid tumors and neurodegenerative diseases. Cellular & Molecular Biology Letters. 2022;27(1):1-17.
18. Li Q, Zhu X, Song W, Peng X, Zhao R. The P2X7 purinergic receptor: a potential therapeutic target for lung cancer. Journal of Cancer Research and Clinical Oncology. 2020:1-11.
19. Qin J, Zhang X, Tan B, Zhang S, Yin C, Xue Q, et al. Blocking P2X7-mediated macrophage polarization overcomes treatment resistance in lung cancer. Cancer Immunology Research. 2020.
20. van Kempen TA, Deixler E. SARS-CoV-2: influence of phosphate and magnesium, moderated by vitamin D, on energy (ATP) metabolism and on severity of COVID-19. American Journal of Physiology-Endocrinology and Metabolism. 2021;320(1):E2-E6.
21. da Silva GB, Manica D, da Silva AP, Kosvoski GC, Hanauer M, Assmann CE, et al. High levels of extracellular ATP lead to different inflammatory responses in COVID-19 patients according to the severity. Journal of Molecular Medicine. 2022;100(4):645-63.
22. Zhang Y, Cheng H, Li W, Wu H, Yang Y. Highly‐expressed P2X7 receptor promotes growth and metastasis of human HOS/MNNG osteosarcoma cells via PI3K/Akt/GSK3β/β‐catenin and mTOR/HIF1α/VEGF signaling. International Journal of Cancer. 2019;145(4):1068-82.
23. Hirayama Y, Anzai N, Kinouchi H, Koizumi S. P2X7 Receptors in Astrocytes: A Switch for Ischemic Tolerance. Molecules. 2022;27(12):3655.
24. Lara R, Adinolfi E, Harwood CA, Philpott M, Barden JA, Di Virgilio F, et al. P2X7 in cancer: from molecular mechanisms to therapeutics. Frontiers in Pharmacology. 2020;11:793.
25. Di Virgilio F, Tang Y, Sarti AC, Rossato M. A rationale for targeting the P2X7 receptor in Coronavirus disease 19. British Journal of Pharmacology. 2020;177(21):4990-4.
26. Martínez-García JJ, Martínez-Banaclocha H, Angosto-Bazarra D, de Torre-Minguela C, Baroja-Mazo A, Alarcón-Vila C, et al. P2X7 receptor induces mitochondrial failure in monocytes and compromises NLRP3 inflammasome activation during sepsis. Nature Communications. 2019;10(1):1-14.
27. Yue Q, Song Y, Liu Z, Zhang L, Yang L, Li J. Receptor for Advanced Glycation End Products (RAGE): A Pivotal Hub in Immune Diseases. Molecules. 2022;27(15):4922.
28. Egaña-Gorroño L, López-Díez R, Yepuri G, Ramirez LS, Reverdatto S, Gugger PF, et al. Receptor for advanced glycation end products (RAGE) and mechanisms and therapeutic opportunities in diabetes and cardiovascular disease: insights from human subjects and animal models. Frontiers in Cardiovascular Medicine. 2020;7:37.
29. Teissier T, Boulanger É. The receptor for advanced glycation end-products (RAGE) is an important pattern recognition receptor (PRR) for inflammaging. Biogerontology. 2019;20(3):279-301.
30. Chen L, Long X, Xu Q, Tan J, Wang G, Cao Y, et al. Elevated serum levels of S100A8/A9 and HMGB1 at hospital admission are correlated with inferior clinical outcomes in COVID-19 patients. Cellular & Molecular Immunology. 2020;17(9):992-4.
31. Salehi M, Amiri S, Ilghari D, Hasham LFA, Piri H. The Remarkable Roles of the Receptor for Advanced Glycation End Products (RAGE) and Its Soluble Isoforms in COVID-19: The Importance of RAGE Pathway in the Lung Injuries. Indian Journal of Clinical Biochemistry. 2022:1-13.
32. Islam MT, Hossen M, Kamaz Z, Zali A, Kumar M, Docea AO, et al. The role of HMGB1 in the immune response to SARS-COV-2 infection: From pathogenesis towards a new potential therapeutic target. Farmacia. 2021;69:621-34.
33. Malkani N, Rashid MU. SARS-COV-2 infection and lung tumor microenvironment. Molecular Biology Reports. 2021;48(2):1925-34.
34. Khomari F, Nabi-Afjadi M, Yarahmadi S, Eskandari H, Bahreini E. Effects of cell proteostasis network on the survival of SARS-CoV-2. Biological Procedures Online. 2021;23(1):1-10.
35. Matyśniak D, Oslislok M, Pomorski P. P2X7 receptor in normal and cancer cells in the perspective of nucleotide signaling. Acta Biochimica Polonica. 2020.
36. Li Q, Zhu X, Song W, Peng X, Zhao R. The P2X7 purinergic receptor: a potential therapeutic target for lung cancer. Journal of Cancer Research and Clinical Oncology. 2020;146(11):2731-41.
37. Wang Z, Zhu S, Tan S, Zeng Y, Zeng H. The P2 purinoceptors in prostate cancer. Purinergic Signalling. 2022:1-9.
38. Zalpoor H, Aziziyan F, Liaghat M, Bakhtiyari M, Akbari A, Nabi-Afjadi M, et al. The roles of metabolic profiles and intracellular signaling pathways of tumor microenvironment cells in angiogenesis of solid tumors. Cell Communication and Signaling. 2022;20(1):1-25.
39. Takai E, Tsukimoto M, Harada H, Sawada K, Moriyama Y, Kojima S. Autocrine regulation of TGF-β1-induced cell migration by exocytosis of ATP and activation of P2 receptors in human lung cancer cells. Journal of cell science. 2012;125(21):5051-60.
40. Drill M, Jones NC, Hunn M, O’Brien TJ, Monif M. Antagonism of the ATP-gated P2X7 receptor: A potential therapeutic strategy for cancer. Purinergic Signalling. 2021;17(2):215-27.
41. Fan X, Zhang J, Dai Y, Shan K, Xu J. Blockage of P2X7R suppresses Th1/Th17-mediated immune responses and corneal allograft rejection via inhibiting NLRP3 inflammasome activation. Experimental Eye Research. 2021;212:108792.
42. Khalafalla MG, Woods LT, Jasmer KJ, Forti KM, Camden JM, Jensen JL, et al. P2 receptors as therapeutic targets in the salivary gland: from physiology to dysfunction. Frontiers in Pharmacology. 2020;11:222.