Abstract
Over the past decade, immune checkpoint inhibitors (ICIs) have transformed the treatment landscape for many types of cancers, leading to significant improvements in patient survival. Treatments like nivolumab, pembrolizumab, durvalumab, and atezolizumab have shown remarkable efficacy. Among the various biomarkers used to guide ICI therapy, PD-L1 is one of the most extensively studied. The PD-L1 SP263 assay is one of the most commonly used assays for identifying PD-L1 status. The Food and Drug Administration has approved the PD-L1 SP263 (Ventana) assay as a companion diagnostic test for atezolizumab in early-stage NSCLC and cemiplimab-rwlc in advanced non-small cell lung cancer (NSCLC), thus offering a standardized method to predict patient responses to immunotherapy based on PD-L1 expression levels. It is the product indicated for use in NSCLC that has received Conformité Européenne marking for in vitro diagnostic (CE-IVD) designation as a companion diagnostic assay for treatment with four immunotherapeutic drugs (durvalumab, pembrolizumab, cemiplimab-rwlc, and atezolizumab) and as a complementary diagnostic assay for nivolumab. In this review, we have consolidated the findings about the PD-L1 SP263 assay, focusing on its use and methodology in NSCLC and other tumors. We discuss why the SP263 assay is important, how it compares with the 22C3 assay—the other widely used PD-L1 assay in NSCLC—and its effectiveness across various types of cancer. The PD-L1 SP263 assay has been used in multiple clinical trials (such as ARCTIC, PACIFIC, and IMpower series) to quantify PD-L1 expression. Concordance studies between the PD-L1 SP263 assay and other PD-L1 assays, especially the 22C3 assay, highlight the SP263 assay’s reliability and the potential for interchangeability when quantifying PD-L1 in NSCLC cases. Beyond NSCLC, the PD-L1 SP263 assay demonstrates clinical utility in gastric, hepatocellular, head and neck squamous cell, urothelial, and renal cell carcinomas. Additionally, this review discusses future directions, including utilizing artificial intelligence to enhance diagnostic accuracy and predict treatment responses, thereby optimizing personalized cancer care.
Keywords
Cancer immunotherapy, PD-L1, Diagnostic assay, Clinical utility, Immune checkpoint inhibitor (ICI), SP263, Non-small cell lung cancer (NSCLC)
Introduction
Immune checkpoint inhibitors (ICIs) of programmed death 1 (PD-1)/programmed death ligand 1 (PD-L1) have revolutionized cancer immunotherapy due to their potential in improving patient outcomes and survival [1-3]. PD-L1, a transmembrane protein expressed on the surface of tumor cells and immune cells, is a co-inhibitory factor of the immune response. PD-1, which is expressed on T cells, binds to its ligand, leading to the inhibition of T cell proliferation, reduction in cytokine secretion, and induction of apoptosis in PD-1 positive cells [4]. The assessment of the PD-L1 biomarker plays a crucial role in predicting responses to immunotherapy, aiding clinical decision-making, and stratifying patients in clinical trials that assess the efficacy of PD-1/PD-L1 inhibitors [5]. PD-L1 acts as a 'brake' on the immune system, helping to control immune responses. Immune checkpoint inhibition can modulate T cell activity, promoting the apoptosis of antigen-specific T cells (brings the T cell function back to life) and preventing the apoptosis of regulatory T cells. This mechanism enhances the immune system's ability to eliminate cancer cells more effectively [4].
Inhibiting the PD-1/PD-L1 pathway consistently demonstrates remarkable antitumor responses in individuals with advanced cancer and is recognized as the gold standard for advancing novel strategies in immune checkpoint blockade and combination treatments [6]. Various studies have shown that high PD-L1 levels are associated with better responses to PD-1/PD-L1 inhibitors. ICIs targeting PD-1/PD-L1 pathway such as nivolumab (Opdivo), pembrolizumab (Keytruda), durvalumab (Imfinzi), atezolizumab (Tecentriq), and cemiplimab-rwlc (Libtayo) exhibit clinical utility in non-small cell lung cancer (NSCLC) management and have been shown to improve patient survival outcomes [7]. For instance, studies have demonstrated that pembrolizumab improves overall survival in patients with previously untreated advanced NSCLC [8]. Similarly, nivolumab has been associated with increased survival rates in previously treated NSCLC patients [9].
Multiple assays exist for the quantification of PD-L1 using immunohistochemical (IHC) staining of both tumor and immune cells, performed with a range of PD-L1 antibodies and following different scoring algorithms. Many of these PD-L1 IHC assays have been studied and approved by the United States Food and Drug Administration (US FDA) [10]. PD-L1 IHC assays currently approved by the USFDA include four distinct PD-L1 antibodies—SP263, 22C3, 28–8, and SP142—on two IHC platforms (Dako and Ventana). Two of the most commonly used assays are SP263 on the Ventana platform and 22C3 on the Dako platform [11]. Additionally, the 28-8 assay is used on the Dako platform, while SP142 is used on the Ventana platform [12,13].
The PD-L1 SP263 assay (Ventana) is in commercial use since September 2016. It has received Conformité Européenne marking for in vitro diagnostic designation in Europe as a companion diagnostic assay for treatment with durvalumab, pembrolizumab, cemiplimab-rwlc, and atezolizumab, and as a complementary predictive assay for treatment with nivolumab [14,15]. It is approved by the US FDA as a companion diagnostic assay for atezolizumab and cemiplimab-rwlc and as a complementary diagnostic assay for pembrolizumab and durvalumab in NSCLC [16]. In addition to its approval for use in NSCLC, the PD-L1 SP263 assay demonstrates clinical utility in various cancers, including gastric, hepatocellular, renal cell, head and neck squamous cell, and urothelial carcinomas [17].
Although multiple PD-L1 assays are available, significant variability exists in their performance and clinical utility. This review consolidates key aspects and findings related to the PD-L1 SP263 assay in non-small cell lung cancer (NSCLC) and other tumors. Despite the widespread use of PD-L1 SP263 assay, there is a notable lack of comprehensive reviews that critically compare the PD-L1 SP263 assay with other PD-L1 assays in terms of diagnostic accuracy, clinical utility, and concordance. By addressing this gap, our review aims to provide insights into how the PD-L1 SP263 assay performs relative to other assays such as 22C3, 28-8, and SP142. This comparison will aid clinicians in selecting the most appropriate PD-L1 assay for their patients, particularly in the context of NSCLC.
This review offers the following unique contributions:
- Clinical utility analysis: An evaluation of the clinical utility of the PD-L1 SP263 assay in guiding immunotherapy decisions across various cancers, focusing on NSCLC.
- Comprehensive comparison: An in-depth comparison of the PD-L1 SP263 assay with other PD-L1 assays (22C3, 28-8, SP142), highlighting concordance and discrepancies.
- PD-L1 SP263 in other tumors: A discussion on the application and performance of the PD-L1 SP263 assay in tumor types beyond NSCLC, broadening its clinical relevance and helping pathologists take an appropriate informed decision on choosing the PD-L1 assay.
- Guidance on laboratory-developed tests (LDTs): Insights into the use of LDTs and their implications for clinical practice.
This review also provides insights into the various scoring systems used for PD-L1 testing, including novel systems such as the Tumor Proportion Score (TPS) and the Tumor Area Proportion (TAP) scoring system. Additionally, the discussion of the assay's application in other tumors expands the narrative and potential impact in oncology.
Review Methodology
Literature search strategy
The systematic search was conducted using primary information resources, including PubMed and Google Scholar databases. The research strategy included the following MeSH terms: “cancer immunotherapy”, “PD-L1”, “diagnostic assay”, “clinical utility”, “immune checkpoint inhibitor (ICI)”, “SP263”, and “non-small cell lung cancer (NSCLC).”
Inclusion criteria
Articles were included in the review if they: (1) investigated the role of PD-L1 SP263 assay in guiding treatment, and (2) provided data on diagnostic accuracy, clinical effectiveness, or outcomes regarding the use of the SP263 assay in NSCLC patients.
Exclusion criteria
Studies not focused on the PD-L1 SP263 assay or not related to cancer immunotherapy, articles with no original data (e.g., editorials, commentaries), studies with minimal methodological quality or incomplete information, and sources that were not peer-reviewed were excluded from the study.
Data extraction and synthesis
Two independent authors screened the titles and abstracts of the initial search results. Full-text evaluation of potentially relevant studies was performed on articles to ascertain their eligibility status. Discrepancies between the two authors were resolved through discussion. Data extracted from the selected studies include study design, population characteristics, details of the implementation of the PD-L1 SP263 assay, clinical outcomes, and conclusions related to the assay's utility in cancer immunotherapy. Extracted data were qualitatively summarized to offer an overall impression of the state of knowledge on the PD-L1 SP263 assay in NSCLC treatment.
Results and Discussion
Overview
NSCLC is the most common histologic type of lung cancer, accounting for around 85% of all lung cancers [18]. The prognosis of NSCLC is poor, especially in the advanced stages. Traditional treatments included surgery, radiation therapy, and chemotherapy but had limited patient outcomes in an advanced stage [19]. However, newly developed targeted therapies and immunotherapies have created great hope with significant improvement in outcomes for NSCLC patients. Immunotherapy has set a milestone in treating several malignancies by harnessing the body's immune system to attack and destroy cancer cells. In this regard, immunotherapy is different from traditional cancer treatments, such as chemotherapy and radiation, in that it does not attack cancer cells directly but instead aims to get the body's immune response to recognize and attack the cancer [20]. Immunotherapy plays a pivotal role in cancer treatment by leveraging the body's immune system to fight tumors effectively. Key biomarkers such as PD-L1 expression levels, microsatellite instability or mismatch repair deficiency status, and tumor mutational burden are essential in identifying patients who are likely to benefit from these therapies. In non-small cell lung cancer (NSCLC), pembrolizumab exemplifies the success of biomarker-guided immunotherapy. For instance, the landmark KEYNOTE-024 trial showcased pembrolizumab's superiority over chemotherapy in patients with high PD-L1 expression, significantly improving both progression-free survival and overall survival outcomes [20-26].
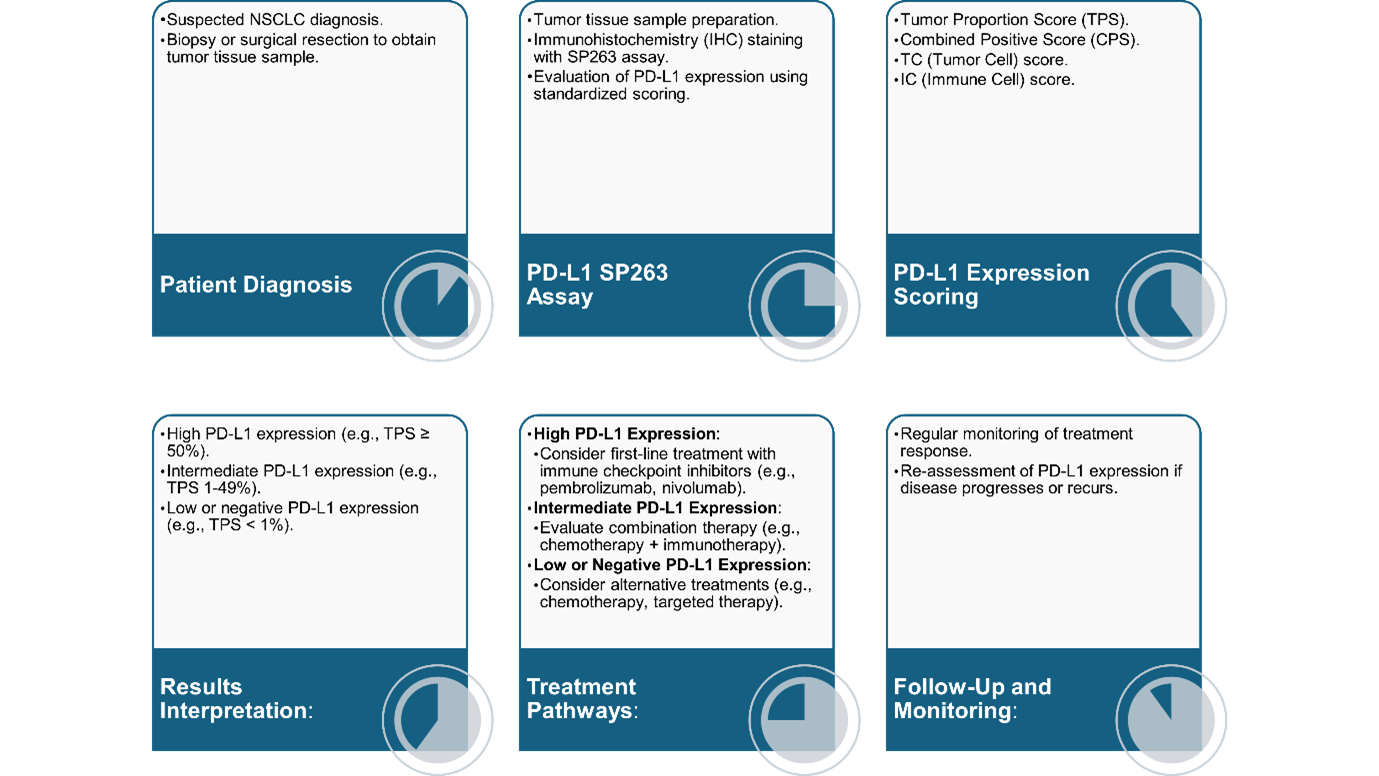
The PD-L1 SP263 assay is a diagnostic tool for predicting patient responses to PD-1/PD-L1 inhibitors by measuring the PD-L1 expression level on tumor cells and immune cells [16]. It has been widely used in clinical practice, providing an approach to therapeutic decision-making for patients with NSCLC. The assay’s ability to accurately stratify patients based on PD-L1 expression levels helps identify those most likely to benefit from PD-L1/PD-1 inhibitor therapies [27]. Clinical utility, diagnostic accuracy, and prognostic value have been extensively explored for SP263 to be an integral part of personalized treatment in NSCLC. An illustration of the procedure to PD-L1 SP263 assay is presented in Figure 1.
Figure 1. Process of PD-L1 testing using the SP263 assay and subsequent treatment pathways based on PD-L1 expression levels.
Clinical utility of PD-L1 SP263 (Ventana) assay in NSCLC
Scoring systems used for assessing PD-L1 expression: Several interpretive scoring algorithms are available for PD-L1 testing, including the TPS (percentage of tumor cells [TCs] that express PD-L1 relative to all viable TCs in the sample), combined positive score (CPS; considers both TCs and immune cells expressing PD-L1), TC score (considers the expression of PD-L1 only in TCs), and immune cell (IC) score (considers the expression of PD-L1 only in immune cells) [17,28,29]. A novel scoring system, the TAP score, combines TC and IC and eliminates the need to perform cell counting [30]. Standardized scoring systems enable comparability across different studies and laboratories, which is essential for researchers and clinicians to interpret and validate results consistently, fostering collaboration and advancing the understanding of PD-L1 as a biomarker [31,32]. The scoring systems for PD-L1 quantification in IHC assays involve various scoring methods that assess PD-L1 expression in tumor tissues (Table 1) [17]. The scoring system for the PD-L1 SP263 assay is based on the percentage of TCs presenting partial or complete membrane staining of any intensity.
|
Scoring system |
Description |
Interpretation |
|
TPS |
Represents the percentage of TCs expressing PD-L1 relative to all viable TCs in the sample |
Positive if TPS exceeds a specific threshold (e.g., 1%, 5%, 10%, or 50%) |
|
CPS |
Considers both TCs and ICs expressing PD-L1 |
Positive if CPS, calculated as (Number of PD-L1-stained cells/Number of viable tumor cells) ×100, exceeds a specific threshold |
|
TC score |
Focuses on the expression of PD-L1 specifically in TCs |
Positive result based on the intensity and distribution of PD-L1 expression in TCs. The TC value is classified into different cutoff subgroups (TC0: < 1%; TC1: 1% – 4%; TC2: 5% – 49%; TC3: ≥ 50%) |
|
IC score |
Assesses the expression of PD-L1 in immune cells (e.g., lymphocytes and macrophages) |
Positive result based on the intensity and distribution of PD-L1 expression in immune cells. The IC value is classified into different cutoff subgroups (IC0: < 1%; IC1: 1% – 4%; IC2: 5% – 9%; IC3: ≥ 10%) |
|
TAP score |
Involves a visual estimation of the tumor area covered by PD-L1-positive TC and IC relative to the total tumor area |
Positive, if TAP score is ≥ 5% (negative borderline, if 2%– 4%, positive borderline, if 5%–9%) |
|
CPS: Combined Positivity Score; IC: Immune Cell; PD-L1: Programmed Death Ligand 1; TAP: Tumor Area Positivity; TC: Tumor Cell; TPS: Tumor Proportion Score |
||
Clinical trials showing the key role of PD-L1 SP263 assay
The PD-L1 SP263 assay is used to quantify PD-L1 in several clinical trials. The Phase III, randomized, open-label, multicenter ARCTIC study assessed the safety and effectiveness of durvalumab with or without tremelimumab as third-line treatment or beyond in comparison to standard-of-care treatment with erlotinib, gemcitabine, or vinorelbine in patients with advanced NSCLC with PD-L1-positive tumors [33]. PD-L1 positivity in this trial is based on a ≥ 25% cutoff for the TC score using the PD-L1 SP263 assay. For patients with PD-L1 expression ≥ 25%, clinically meaningful improvements are noted in the overall survival (OS) and progression-free survival (PFS) with durvalumab; for patients with PD-L1 expression below the cutoff, only numerical improvements are noted, compared to standard of care.
The IMpower110 study evaluated atezolizumab versus platinum-based chemotherapy in treatment-naïve patients with stage IV nonsquamous or squamous NSCLC lacking EGFR or ALK alterations [34]. Initially using the PD-L1 SP142 assay, atezolizumab showed superior OS benefit over chemotherapy in patients with high PD-L1 expression regardless of histology type (≥ 50% TC and ≥ 10% IC) [35]. The US FDA-approved atezolizumab as a first-line treatment option for wild-type NSCLC patients with high PD-L1 expression is based on the results of this interim analysis. The improvement in OS among patients with high or intermediate PD-L1 expression (≥ 5% TC or IC) was significant for the patients treated with atezolizumab. In an updated exploratory OS analysis of the IMpower110 study, wherein various PD-L1 subgroups were defined by the PD-L1 SP263, 22C3, and SP142 assays, the results were comparable across equivalent PD-L1 subgroups i.e., high PD-L1 (SP263: ≥ 50% TC; 22C3: ≥ 50% TPS; SP142: ≥ TC or ≥ 10% IC) and low PD-L1 (SP263: 1% – 49% TC; 22C3: 1% – 49% TPS; SP142: ≥ 1% TC or IC excluding ≥ 50% TC or ≥ 10% IC). However, subsequent analysis integrating PD-L1 subgroups defined by SP263, 22C3, and SP142 assays revealed comparable OS outcomes across equivalent PD-L1 expression categories. This comprehensive assessment highlighted the SP263 assay's clinical utility in confirming the efficacy of atezolizumab in NSCLC patients with high PD-L1 expression, affirming its role as a predictive biomarker in guiding treatment decisions in this setting [34].
The Phase III, randomized IMpower150 study evaluated the effectiveness of atezolizumab (A) and/or bevacizumab (B) with carboplatin/paclitaxel chemotherapy (CP) in patients with NSCLC (N=1202). In this study, improvements in OS differed based on the PD-L1 status, as determined using the PD-L1 SP263 assay. Among patients with Kirsten rat sarcoma viral oncogene homolog mutation-bearing tumors (n=225), the improvement in OS was similar with ABCP and ACP treatments if the patients had high PD-L1 expression (≥ 50% TC). However, if these patients had low or negative PD-L1 status, the OS improvement was greater with ABCP than ACP [36]. These findings suggest that the PD-L1 status influences improvement in OS, emphasizing the importance of accurate PD-L1 testing as it can facilitate informed treatment decision-making by clinicians. A summary of clinical trials in which the SP263 assay was used for PD-L1 quantification is shown in Table 2 [28,33,34,36-38].
|
Author, year |
Clinical trial |
Design/phase |
Patient population |
Intervention |
PD-L1 assessment with SP263 assay |
Main findings |
|
Planchard et al., 2020 [33] |
ARCTIC |
Randomized, open-label, multicenter, Phase III |
Advanced NSCLC with PD-L1-positive tumors |
Durvalumab vs. standard of care |
PD-L1 positivity based on ≥ 25% TC score using PD-L1 SP263 assay |
For patients with PD-L1 expression greater than the cutoff, clinically meaningful improvements were seen in the OS and PFS with durvalumab, but for patients with PD-L1 expression below the cutoff, only numerical improvements were seen compared to standard of care. |
|
Paz-Ares et al., 2020 [38]; Spigel et al., 2022 [37] |
PACIFIC |
Randomized, multicenter, Phase III |
Unresectable stage III NSCLC without disease progression after chemoradiotherapy |
Durvalumab for 12 months |
PD-L1 expression assessed with SP263 assay based on TPS thresholds of 25% and 1% |
Significant improvements in OS and PFS were seen with durvalumab after chemoradiotherapy; OS benefit was seen in all PD-L1 subgroups except the TC < 1% subgroup. |
|
Felip et al., 2021 [28] |
IMpower010 |
Open-label, multicenter, Phase III |
Resected stage II–IIIA NSCLC |
Atezolizumab vs. best supportive care |
SP263 assay used to identify patients benefiting from atezolizumab (TPS ≥ 1%) |
Significant disease-free survival benefit was observed with atezolizumab in patients with early-stage NSCLC, especially in TPS ≥ 1%. |
|
Jassem et al., 2021 [34] |
IMpower110 |
Open-label, multicenter, Phase III |
Chemotherapy-naive stage IV nonsquamous or squamous NSCLC with EGFR- and ALK-negative status |
Atezolizumab monotherapy vs. platinum-based chemotherapy |
SP263 (TC ≥ 50%), 22C3 (TPS ≥ 50%), and SP142 (TC ≥ 50% or IC ≥ 10%) assays used to identify patient subgroups based on PD-L1 expression |
A significant improvement in OS was noted in patients with high PD-L1 expression who received atezolizumab compared to chemotherapy |
|
West et al., 2022 [36] |
IMpower150 |
Randomized, Phase III |
NSCLC patients (n=1202) |
Atezolizumab (A) and/or bevacizumab (B) with carboplatin/paclitaxel chemotherapy (CP) |
SP263 assay detected PD-L1 expression; mutations in KRAS tumors with TP53 co-mutations enriched for high PD-L1 expression; PD-L1 cutoff was TC ≥ 50% |
Improvements in OS differed based on the PD-L1 status; if patients exhibited high PD-L1 expression, both ABCP and ACP showed comparable improvements in OS, but for those with low or negative PD-L1 status, ABCP demonstrates greater OS benefits than ACP. |
|
ABCP: Atezolizumab + Bevacizumab + Carboplatin/Paclitaxel Chemotherapy; ACP: Atezolizumab + Bevacizumab + Carboplatin/Paclitaxel Chemotherapy; ALK: Anaplastic Lymphoma Kinase; KRAS: Kirsten Rat Sarcoma Viral Oncogene Homolog; EGFR: Epidermal Growth Factor Receptor; IC: Immune Cell; NSCLC: Non-Small Cell Lung Cancer; OS: Overall Survival; PD-L1: Programmed Death Ligand 1; PFS: Progression-Free Survival; TC: Tumor Cell; TPS: Tumor Proportional Score. |
||||||
In the Phase III PACIFIC trial conducted on patients with unresectable, stage III NSCLC with no disease progression after treatment with platinum-based concurrent chemoradiotherapy, administering durvalumab for 12 months resulted in significant improvements in OS and PFS [37]. The SP263 assay was used in this trial to evaluate PD-L1 expression in pre-chemoradiotherapy tumor tissue based on cutoff TPS thresholds of 25% and 1% [37,38]. The IMpower010 study, an open-label, multicenter, Phase III study assessing the survival benefit achieved with atezolizumab treatment compared to best supportive care after platinum-based adjuvant chemotherapy in patients with resected stage II – IIIA NSCLC, demonstrates that apart from metastatic settings, the SP263 assay demonstrates clinical utility in identifying patients with early-stage NSCLC, who would likely benefit from treatment with atezolizumab in terms of significantly longer disease-free survival [28]. The survival benefit was especially pronounced in patients with stage II – IIIA NSCLC in whom the TPS was ≥ 1%.
Concordance of the PD-L1 SP263 assay with other PD-L1 assays
Concordance studies are essential for validating the interchangeability of PD-L1 assays, as it has implications for patient treatment decisions. A summary of studies assessing the concordance of the SP263 assay with other PD-L1 assays is presented in Table 3 [39-49].
|
Author, year |
Study |
PD-L1 assays |
Key findings |
|
Hirsch et al., 2017 [39] |
Blueprint PD-L1; IHC comparability project |
SP263, 22C3, 28-8, and SP142 |
Analytical and clinical comparability of PD-L1 IHC assays (SP263, 28-8, and 22C3) reveals poor concordance between the assays for ICs. SP142 detected fewer stained TCs. |
|
Tsao et al., 2018 [40] |
Blueprint project; Phase II |
SP263, 22C3, 28-8, and SP142 |
Validation of Phase I results using real-world data showed interchangeability among the PD-L1 SP263, 22C3, and 28-8 assays, although the SP142 assay had lower sensitivity and the 73-10 assay had higher sensitivity than the rest. The reliability among pathologists was poor when using IC PD-L1 scoring. |
|
Yu et al., 2023 [42] |
RING study |
SP263 and 22C3 |
The study reveals moderate to almost perfect agreement for SP263 in TC staining at 25% cutoff, indicating interchangeability between SP263 and 22C3 assays in NSCLC, especially at the 50% cutoff. |
|
Sughayer et al., 2019 [43] |
|
SP263 and 22C3 |
The study evaluated interchangeability between 22C3 and SP263 assays using NSCLC samples, which showed high concordance in TPS (Pearson correlation coefficient: 0.95). |
|
Zhou et al., 2023 [44] |
IMpower010 trial |
2 SP263 and 2C3 |
The study compared SP263 and 22C3 PD-L1 tests for atezolizumab in NSCLC patients and reported high concordance, suggesting both assays may be used in the identification of patients with early-stage NSCLC who are likely to benefit from atezolizumab treatment. |
|
Nambirajan et al., 2019 [49] |
|
SP263 and SP142 |
The results of the investigation indicated that the LDT employing the SP142 clone and the PD-L1 SP263 assay had only modest concordance and were not comparable. |
|
Kim et al., 2022 [45] |
|
SP263 and 22C3 |
The study compared 22C3 and SP263 PD-L1 expression in patients with NSCLC (n=431), indicating strong concordance with 92.3% positivity for SP263 in 22C3-positive cases, and 89.9% vice versa. |
|
Marchetti et al., 2017 [46] |
|
SP263 and 22C3 |
The study compared SP263 and 22C3 assays in lung adenocarcinoma samples, demonstrating > 90% analytical correlation and high overall concordance (Light’s kappa) values of 0.99 and 0.80 at TPS cutoffs of ≥ 50% and ≥ 1%, respectively. |
|
Savic et al., 2019 [48] |
Swiss RING study |
SP263 and 22C3 |
The study assessed the interchangeability between the SP263 (Ventana) and 22C3 (Dako) assays in Switzerland, and it suggested that the SP263 assay is another valid option for laboratories with the Ventana platform. |
|
Tsimafeyeu et al., 2020 [47] |
CLOVER study |
SP263, 22C3, and SP142 |
The study compared SP263, SP142, and 22C3 assays for PD-L1, which showed excellent positive and negative agreement ( > 91%) between SP263 and 22C3 assays. Caution is needed when replacing 22?3 with SP263, especially in the first-line setting, as the positive agreement was 57%. |
|
Ratcliffe et al., 2017 [41] |
|
SP263, 22C3, and 28-8 |
The study validated the concordance between SP263, 28-8, and 22C3 assays for quantifying PD-L1 in NSCLC samples, which indicated harmonization between the three assays. |
|
IC: Immune Cell; IHC: Immunohistochemical; LDT: Laboratory-Developed Test; NSCLC: Non-Small Cell Lung Cancer; PD-L1: Programmed Death Ligand 1; TC: Tumor Cell; TPS: Tumor Proportion Score. |
|||
Studies evaluating the concordance between SP263, 22C3, and 28-8 assays
The Blueprint PD-L1 IHC Comparability Project Phase I evaluated the clinical and analytical comparability of PD-L1 IHC assays. The study showed that the percentage of PD-L1-positive TCs stained was similar when the PD-L1 SP263, 22C3, and 28-8 assays were used, but the SP142 assay detected fewer stained TCs than the other three assays [39]. Poor concordance between the assays was seen for immune cells. Although the PD-L1 SP263, 22C3, and 28-8 assays exhibited similar analytical performance, there was a possibility of misclassification of the PD-L1 status if the assays and cutoffs were interchanged. The study emphasized the need for real-world data to comment on the interchangeability of these assays since it could misclassify the PD-L1 status.
Building on the findings from the previous study, Phase II of the Blueprint project validated the Phase I results using real-world data and showed that SP263, 22C3, and 28-8 were interchangeable, although SP142 had lower sensitivity and the 73-10 (Dako) assay (potential assay for avelumab) had higher sensitivity than the rest [40]. This study addressed some of the shortcomings of the Phase I study. For example, the Phase I study used resected NSCLC samples, but Phase II was conducted using a larger number (81 in Phase II versus 39 in Phase I) of routinely encountered samples such as core needle/bronchial and lymph node biopsy samples, resected NSCLC samples, and needle aspirate biopsy samples for cytological analysis, as well as a larger number of pathologists (18 in Phase II versus 3 in Phase I). The wide variety of samples used in this study allowed analysis of the full range of PD-L1 expression levels. The assessment also allowed the accurate identification of PD-L1-negative cases, especially among lower-stage, surgically resected cases. More than half of the patients with advanced NSCLC will have only cytological samples available for diagnosis [40]. The Phase II Blueprint study showed very strong reliability among the included pathologists in terms of assessing the PD-L1 expression in TC in cytological samples [40]. However, poor reliability was seen in IC PD-L1 scoring.
Another study validating the concordance among the SP263, 28-8, and 22C3 assays for NSCLC samples showed that harmonization between the three assays is possible [41]. In this study, the SP263 assay had good and similar overall agreements with the 22C3 and 28-8 assays at TC cutoffs of ≥ 1% (88.7% and 89.3%), ≥ 10% (90.5% and 90.7%), ≥ 25% (92.3% and 93.0%), and ≥ 50% (91.4% and 94.2%).
Studies evaluating the concordance between SP263 and 22C3 assays
The RING study, which was conducted in six countries (Australia, Brazil, Korea, Mexico, Russia, and Taiwan) to compare the performance of SP263 and 22C3 assays, exhibits moderate to almost perfect agreement for SP263 in TC staining at the 25% cutoff, and interchangeability between SP263 and 22C3 assays in NSCLC, especially at the 50% cutoff [42]. Another study evaluating the interchangeability between the 22C3 and SP263 assays using NSCLC samples showed high concordance at the 50% TPS cutoff (Pearson correlation coefficient: 0.95) [43].
A comparison of the two assays (SP263 and 22C3) for quantifying PD-L1 in tumor samples from the Phase III IMpower010 trial for determining the effectiveness of atezolizumab in patients with NSCLC showed high concordance between the two assays and demonstrates similar clinical predictive efficacy for atezolizumab compared to best supportive care, validated at PD-L1 thresholds, in individuals with early-stage NSCLC [44].
A comprehensive study comparing the two PD-L1 assays in patients with NSCLC (n=431) showed that, at ≥ 1% TPS cutoff, the SP263 assay was positive in 92.3% of 22C3-positive cases and the 22C3 assay was positive in 89.9% of SP263-positive cases, indicating a strong concordance between the two assays [45]. Formalin-fixed paraffin-embedded blocks greater than 3-years-old and sections stored at room temperature for more than 1 week might underestimate the PD-L1 status, specifically with the 22C3 PD-L1 assay. Nevertheless, the SP263 assay demonstrates increased sensitivity compared to the 22C3 assay under these circumstances [45].
A multicenter study in Italy comparing the SP263 assay with the 22C3 assay for quantifying PD-L1 in lung adenocarcinoma samples demonstrates high analytical correlation ( > 90%) between the two assays, with high overall concordance (Light’s kappa) values of 0.99 and 0.80 at the TPS cutoffs of ≥ 50% and ≥ 1%, respectively [46].
The CLOVER study demonstrates excellent (> 91%) positive and negative agreement between the PD-L1 SP263 and 22C3 assays. In this study, the conditional probability of one test indicating a positive or negative PD-L1 staining result was estimated given the outcome of another test. When the PD-L1 SP263 and 22C3 assays were compared, it was found that among all samples that were tested positive by the PD-L1 SP263 assay, only 57% of the samples also tested positive using the 22C3 assay in the first-line setting [47]. This suggests that the PD-L1 22C3 assay missed identifying positive cases in about half of the samples that the PD-L1 SP263 assay could identify. However, if a sample tests negative using the SP263 assay, retesting using any other assay may not be needed.
As the Dako Autostainer ASL48 for the 22C3 assay is not broadly available in Europe, a study was conducted across different laboratories in Switzerland to determine if the 22C3 assay can be performed using the widely available Ventana Benchmark Ultra Immunostainer [48]. The results showed that the PD-L1 SP263 assay was interchangeable with the 22C3 (Dako) assay and suggested that the SP263 assay is another valid option for PD-L1 testing for laboratories having only the Ventana platform.
Studies evaluating the concordance between the PD-L1 SP263 and SP142 assays
The PD-L1 SP142 assay demonstrates modest (76%) concordance with the PD-L1 SP263 assay in a validation study aimed at identifying a single harmonized PD-L1 assay that would allow for tumor tissue conservation and be cost-effective for patients with NSCLC (N=80) [49]. Although the PD-L1 SP263 assay detected more TCs than the SP142 assay in 16% of the cases, the SP142 assay detected more immune cells than the SP263 assay in 24% of the cases [49].
Comparisons of the PD-L1 SP263 assay with other assays such as the 22C3 and 28-8 assays have revealed similar TC staining but poor agreement in IC staining. The PD-L1 SP263 assay has demonstrated interchangeability with the 22C3 assay, particularly at higher cutoffs, and on different staining platforms, but it may not be feasible in all cases to use the 22?3 assay in place of the SP263 assay.
Laboratory-developed tests (LDTs)
There are multiple PD-L1 inhibitors, each with different clinically validated IHC assays, and these, in turn, utilize antibody clones against different epitopes of PD-L1 and different amplification systems. This wide variety makes it challenging to achieve uniformity in assay performance and to choose the most appropriate and cost-effective PD-L1 detection methodology although considering the availability of instruments (testing platform i.e., Dako versus Ventana) and reagents [50]. US-FDA approved or CE-IVD-marked PD-L1 assays are considered as validated tools in clinical trials. Any test or assay which is not clinically validated is categorized as an LDT [51]. LDTs are tests that involve the cross-platform use of PD-L1 antibodies. For instance, the SP263 assay may be performed on the Dako platform or the 22C3 assay may be performed on the Ventana platform. Although LDTs may provide some advantages over commercially available assays, it is crucial to ensure that the results obtained from the SP263 assay align with those from different PD-L1 assays across different laboratories for the standardization and reliability of PD-L1 testing.
A tissue microarray analysis of 21 pulmonary carcinoma specimens stained using SP263, 22C3, 28-8, and SP142 assays along with eleven in-house LDTs at ten different sites in Germany demonstrates that the assays were reproducible at the different sites with moderate interlaboratory concordance with a TPS cutoff of ≥ 50% [52]. In a study comparing the performance of the Ventana SP263 assay, the Dako 22C3 assay, and an LDT using the 22C3 clone on the Ventana platform for quantifying PD-L1 expression in NSCLC samples, the greatest agreement (93%; Light’s kappa = 0.86) was seen between the Dako 22C3 assay and the LDT when the TPS cutoff was 1%, although there was slightly less agreement (84%; Light’s kappa = 0.66) between the Dako 22C3 assay and the Ventana SP263 assay [50]. However, caution is needed when referring to results reported for LDTs. When LDTs are reported to perform well, it reflects the potential capabilities of such tests within the specific laboratory context rather than implying that these results can be universally replicated across all laboratories. In other words, just because a test performs well in one laboratory setting does not guarantee that it will perform equally well in other laboratories. Each laboratory may have different equipment, methodologies, and expertise levels, which can impact the test performance and reproducibility. Therefore, although positive results in one laboratory are promising, they do not automatically guarantee similar outcomes elsewhere [53].
PD-L1 SP263 assay in other tumors
Although regulatory approvals currently focus on NSCLC, extensive literature exists supporting the clinical usefulness of the SP263 assay in various other tumor types. A study comparing the expression of different anti PD-L1 antibody clones on the cells of various cancer types (melanoma, colorectal cancer, NSCLC, breast cancer, head and neck squamous cell carcinoma (HNSCC), pancreatic cancer, prostate cancer, stomach cancer, urinary bladder cancer, clear cell renal carcinoma, and mesothelioma) showed that the SP263 clone had a generally higher mean value of expression in TCs of most tumor groups, with 69.3% expression in the adenocarcinoma subtype of NSCLC [54]. A multicenter evaluation of the precision and reliability of the SP263 rabbit monoclonal primary antibody in seven cancer types showed that although the inter-pathologist overall percent agreement (OPA) for NSCLC was 93.5%, it was also high for other cancer types such as urothelial cancer (93.0%), HNSCC (98.0%), melanoma (96.0%), renal cell carcinoma (100%), gastric or gastroesophageal junction adenocarcinoma (99.3%), and hepatocellular carcinoma (100%) [27]. These OPA values compare favorably to the OPA values reported previously using the 22C3 assay for NSCLC samples [55]. In this study, at the TPS cutoff of 1%, the intra- and inter-pathologist agreement was 84.2% and 89.7%, respectively. Moreover, batch-to–batch variability and variability associated with the use of different instruments had no negative effect on the OPA. The reproducibility of the SP263 assay supports broader clinical use, aiding PD-1/PD-L1 therapy decisions across various cancers.
The 22C3 assay and the SP263 assays are known to be interchangeable in HNSCC, with the inter-observer reliability for CPS reported to be 0.83 for the 22C3 assay and 0.87 for the SP263 assay and the correlation between the two assays to be 0.901[56]. Substantial agreement (80% – 90%) among the SP263, 22C3, 28-8, and SP142 assays for PD-L1 analysis exists in urothelial carcinoma [57]. A high correlation (Spearman’s correlation: 0.914) was reported between the SP263 and 22C3 assays for detecting PD-L1 expression in gastric cancer samples at CPS cutoffs ≥ 1 (60.9% and 57.8% positive samples) and ≥ 10 (9.5% and 9.8% positive samples) [58]. A similarly high correlation (Pearson correlation: 0.932) was reported between the SP263 and 22C3 assays for gastric cancer samples, with excellent sensitivity and specificity at CPS cutoffs of ≥ 1 (100% and 95.67% positive samples) and ≥ 10 (96.30% and 95.52% positive samples) [59].
In ESCC, the SP263 assay had the highest sensitivity in a comparison against the 22C3, E1L3N, and SP142 assays [60]. Excellent inter-observer agreement was reported when the SP263 (Fleiss's kappa = 0.972) and 22C3 (0.939) assays were used for assessing PD-L1 in metastatic triple-negative breast cancer [61]. In bladder urothelial cancer, there was a significant difference in the number of PD-L1-positive cases identified using the SP263 and SP142 assays at the CPS ≥ 1 cutoff but the SP263 and 22C3 assays were found to be relatively similar in performance [62]. A TC cutoff of 10% is suggested for predicting the efficacy of ICI therapy for upper tract urothelial carcinoma [63]. In bladder cancer samples, the 28-8 assay provided a higher PD-L1 positivity rate than the SP263 and 22C3 assays (42.3% with 28-8 versus 22.1% with SP263 and 22C3), and PD-L1 detection with the SP263 assay was more consistent and had a higher prognostic value [64].
Cutoff values for a positive result with the SP263 assay are shown in Table 4 [10,15,65,66]. However, caution must be exercised when switching between PD-L1 assays and analyzing efficacy data in some cancers. For example, although the 22C3 and SP263 assays detected a greater number of PD-L1-positive patients (IC ≥ 1%) than the SP142 assay in a study assessing the comparative performance of the PD-L1 SP263, 22C3, and SP142 assays in patients with triple-negative breast cancer, there was no observed analytical equivalence between these different assays and has subpar concordance for harmonized cutoff values for 22C3 (CPS ≥ 10) and SP263 (IC ≥ 4%) to SP142 (IC ≥ 1%) [67]. Low concordance values (72% – 75%) between the SP263 assay and the 22C3, SP142, and 28-8 assays have been reported in a study evaluating the performance of these PD-L1 assays in patients with malignant pleural mesothelioma (N=32), suggesting that the SP263 assay may not be the best choice in this patient population [68].
|
PD-L1 SP263-positive cutoff |
Indication for use |
US FDA status |
CE-IVD status |
Therapy |
|
TC ≥ 25% or ICP > 1% and IC+ ≥ 25% or ICP = 1% and IC + = 100% |
Second-line metastatic urothelial cancer |
Complementary diagnostic |
-- |
Durvalumab |
|
TC ≥ 1% |
Post CRT stage III NSCLC |
-- |
Companion diagnostic |
Durvalumab |
|
TC ≥ 1% |
Second-line non-squamous NSCLC |
-- |
Complementary diagnostic |
Nivolumab |
|
TC ≥ 1% second line; TC ≥ 50% first line |
First-line, second-line NSCLC monotherapy |
Complementary diagnostic |
Companion diagnostic |
Pembrolizumab |
|
TC ≥ 25% |
First-line NSCLC |
Complementary diagnostic |
-- |
Durvalumab |
|
TC ≥ 25% |
HNSCC |
Complementary diagnostic |
-- |
Durvalumab |
|
TC ≥ 50% |
NSCLC |
Companion diagnostic |
Companion diagnostic |
Cemiplimab-rwlc |
|
TC ≥ 50% |
NSCLC |
Companion diagnostic |
Companion diagnostic |
Atezolizumab |
|
CE-IVD: Conformité Européenne In vitro Diagnostic; CRT: Chemoradiotherapy; HNSCC: Head and Neck Squamous Cell Carcinoma; IC: Immune Cell; ICP: Immune Cell Present; NSCLC: Non-Small Cell Lung Cancer; PD-L1: Programmed Death Ligand 1; TC: Tumor Cell; US FDA: United States Food and Drug Administration |
||||
Factors influencing PD-L1 assay reliability and concordance between different PD-L1 assays
Challenges in concordance may arise due to differences in assay methodologies, antibodies used, scoring systems, and other technical factors. These include inter-observer variability, intra-tumor heterogeneity, issues with tissue preparation, antibody specificity, scoring system variations, tumor microenvironment complexities, dynamic nature of PD-L1 expression, analytical variability, establishing clinical cutoffs, and obtaining adequate tissue samples [69]. The various factors affecting the reliability of a PD-L1 assay may be classified as preanalytical (handling of cytology specimens, tissue fixation and decalcification, and selection of the most representative tissue specimen), analytical (choice of validated assays), and postanalytical (interpretation and reporting of PD-L1 results) [70]. Although differences in PD-L1 expression in the tissue are independent of the PD-L1 antibody clone used in the assay, tumor heterogeneity and variables related to the assay or platform might affect concordance results [71].
The poor concordance between different PD-L1 assays reported in the literature may also be due to the variable IC PD-L1 expression and variable effects of inter- and intra-tumoral heterogeneity on PD-L1 expression [72]. The differences in PD-L1 expression between primary and metastatic sites, as well as dynamic changes at different time points, are additional challenges to be considered when assessing the feasibility of PD-L1 assessment and deciding the number of sites to be biopsied to make an accurate treatment decision. Evaluating PD-L1 expression on circulating TCs is suggested as a potential method to address the dynamic nature of PD-L1 and monitor therapeutic efficiency [73]. Liquid biopsy may be considered in such cases [74].
Pathologists’ familiarity with the cell types and tissue structures present in a sample is also important for the assessment of PD-L1 expression, which emphasizes the need for tumor type-specific training for pathologists [10]. There is a need for pathologists to focus on accurately identifying IC (lymphocytes and macrophages) and attempting to standardize the interpretation of PD-L1 expression based on staining intensity and sites [60]. Interpretation of results is mainly a challenge for cases near the threshold [60]. Therefore, it is important to enhance the knowledge of pathologists in dealing with specific cases, likely complex or challenging ones near the threshold. Incorporating artificial intelligence into routine practice may enhance diagnostic accuracy and efficiency, ultimately leading to improved patient outcomes. Moreover, collaboration between oncologists and pathologists is vital to decide the most appropriate PD-L1 assay [51].
Application of artificial intelligence (AI) and machine learning/ deep learning (ML)/ (DL) for PD-L1 detection/estimation in cancer immunotherapy
Predicting response to ICI therapy remains a significant challenge. One major obstacle is the labor-intensive process of manually counting PD-L1 positive cells and distinguishing between immune cells and tumor cells. Various scoring systems, such as the TC, IC, TAP, TPS, and CPS, have been developed to standardize PD-L1 evaluation, yet they require meticulous assessment by pathologists [39,75]. AI models can analyze complex image data, including radiology and histopathology images. These methods aim to predict therapy response either directly from images or through surrogate markers. Despite current limitations in clinical use, ongoing academic and commercial advancements suggest that AI-based approaches may soon find clinical application in predicting immunotherapy outcomes [76]. It is difficult to quantitatively assess PD-L1 expression based on immunohistochemistry staining. A study presented DL-based AI models which automatically assessed the immunohistochemical expression of PD-L1 in lung cancer patients. Using 84 slides stained by PD-L1 SP263, the AI models demonstrate excellent accuracy (>96%) of detection at a TPS cutoff of 1%. One model divided the tumor patches based on the presence or absence of PD-L1-positive immune cells, then used the YOLO (You Only Look Once) detection model to detect PD-L1-positive or PD-L1-negative tumor cells within those patches. Another model classified patches into four categories: tumor-positive, tumor-negative, immune positive, and others, and then detected PD-L1-positive and PD-L1-negative cells using the YOLO model [77]. These results suggest that AI-developed diagnostic models for PD-L1 expression could be valuable tools for clinical pathologists. Similarly, an AI system using whole slide images from the 22C3 assay automatically assesses the TPS of PD-L1 expression based on a DL model of tumor detection. Comparison tests in the 22C3 assay show strong consistency between the TPS calculated by the AI system and trained pathologists (R = 0.9429–0.9458). AI-assisted diagnosis confirms that the AI system improves the repeatability and efficiency of untrained pathologists. The Ventana PD-L1 (SP263) assay demonstrates high consistency in TPS calculations between the AI system and pathologists (R = 0.9787). This underscores the benefits of using an AI-assisted system to enhance diagnostic repeatability and efficiency for pathologists [78].
A study on PD-L1 as a predictive biomarker for immunotherapy in breast cancer patients utilized DL models to predict PD-L1 expression from H&E-stained images. Using a dataset of 26,763 tissue microarray images from independent clinical trial cohorts, the study consistently achieved high predictive performance, with an area under the curve of 0.91 to 0.93 for PD-L1 status prediction in a cohort of 3,376 patients. This system helps identify cases prone to misinterpretation by pathologists, potentially serving as a decision support and quality assurance tool in clinical practice [79]. In 11 cases of head and neck squamous cell carcinoma, a computational approach assessed correlations between stain features and pathologists’ assessments. A lab-developed test (LDT) using 22C3 and SP263 clones on Dako or Ventana platforms compared with Dako 22C3 pharmDx (a companion diagnostic), revealed technical discordances (45% of cases) and interpretative discordances (54% of cases) between the tests. Differences in diaminobenzidine stain intensity/distribution, identified by computational pathology, partly explained discrepancies in two cases (18%) between LDT and CDx. This study lays the groundwork for digital pathology as a second opinion in PD-L1 scoring for challenging cases. Hence, computational and artificial intelligence tools are expected to enhance clinical decision-making and patient outcomes [80].
Limitations of This Review
This review has several limitations, including subjectivity in data extraction due to the nature of the narrative review, and the lack of quantitative synthesis, as the qualitative summary approach may lack the rigor and objectivity provided by meta-analyses or other quantitative synthesis methods. Additionally, the included studies exhibit variability in methodological quality, leading to heterogeneity in clinical outcomes and potentially affecting the reliability of the conclusions. There is also a potential for publication bias, as studies with positive results are more likely to be published. While this review does not delve deeply into the use of AI and ML, it acknowledges the significant potential these technologies hold for the future of PD-L1 testing and immunotherapy response prediction.
Conclusion
The PD-L1 SP263 assay continues to be an essential tool in predicting the likelihood of a patient benefiting from PD-1/PD-L1 immunotherapeutic drugs. The assay has remarkable reproducibility across different pathologists, timeframes, materials, and laboratories, indicating its adaptability for training and its performance consistency across various tumor types. The prominent concordance between the PD-L1 SP263 assay and 22C3 assay in NSCLC suggests that the assay can be used interchangeably. Given that prior studies have highlighted the consistency and superior diagnostic sensitivity of the PD-L1 SP263 assay in comparison to other US FDA-approved assays for NSCLC and the higher overall performance of this assay for other cancer types, the PD-L1 SP263 assay may be a good choice for meaningful PD-L1 detection in various clinical indications. The reproducibility of SP263 supports broader clinical use, aiding PD-1/PD-L1 therapy decisions across different cancer types. However, the limited data on concordance reported between the PD-L1 SP263 assay and other PD-L1 assays highlight the need for caution when attempting to apply the PD-L1 SP263 assay to cancers other than NSCLC. With the approval of an expanding array of PD-1/PD-L1–targeted therapies for multiple tumor types, the PD-L1 SP263 assay is expected to continue to play a substantial role in quantifying PD-L1 expression and identifying the right patients for receiving the right immunotherapies at the right time.
Conflicts of Interest
All authors declare no conflict of interest.
Funding
This review did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgement
We would like to thank BioQuest Solutions, Pvt Ltd. for their editorial support.
Author Contribution Statement
Conceptualization: Dr Sandeep Sewlikar, Dr Jayesh Deshmukh; Methodology: Dr Trupti Pai, Dr Hema Malini Aiyer, Dr Bijal Kulkarni, Dr Vidya MN, Dr Shashikant C.U. Patne, Dr Sandeep Sewlikar, Dr Jayesh Deshmukh; Project administration: Dr Jayesh Deshmukh; Resources: Dr Jayesh Deshmukh; Supervision: Dr Jayesh Deshmukh; Validation: Dr Trupti Pai, Dr Hema Malini Aiyer, Dr Bijal Kulkarni, Dr Vidya MN, Dr Shashikant C.U. Patne, Dr Sandeep Sewlikar, Dr Jayesh Deshmukh; Writing- original draft: Dr Trupti Pai, Dr Sandeep Sewlikar, Dr Jayesh Deshmukh; Writing- review & editing: Dr Trupti Pai, Dr Hema Malini Aiyer, Dr Bijal Kulkarni, Dr Vidya MN, Dr Shashikant C.U. Patne, Dr Sandeep Sewlikar, Dr Jayesh Deshmukh.
References
2. Shen X, Zhao B. Efficacy of PD-1 or PD-L1 inhibitors and PD-L1 expression status in cancer: meta-analysis. BMJ. 2018 Sep 10; 362:k3529.
3. Li Z, Sun G, Sun G, Cheng Y, Wu L, Wang Q, et al. Various Uses of PD1/PD-L1 Inhibitor in Oncology: Opportunities and Challenges. Front Oncol. 2021 Nov 17;11:771335.
4. Han Y, Liu D, Li L. PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res. 2020 Mar 1;10(3):727-742.
5. Liu D, Wang S, Bindeman W. Clinical applications of PD-L1 bioassays for cancer immunotherapy. J Hematol Oncol. 2017 May 17;10(1):110.
6. Cha JH, Chan LC, Li CW, Hsu JL, Hung MC. Mechanisms Controlling PD-L1 Expression in Cancer. Mol Cell. 2019 Nov 7;76(3):359-70.
7. Breimer LH, Nousios P, Olsson L, Brunnström H. Immune checkpoint inhibitors of the PD-1/PD-L1-axis in non-small cell lung cancer: promise, controversies and ambiguities in the novel treatment paradigm. Scand J Clin Lab Invest. 2020 Sep;80(5):360-9.
8. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2016 Nov 10;375(19):1823-33.
9. Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015 Jul 9;373(2):123-35.
10. Prince EA, Sanzari JK, Pandya D, Huron D, Edwards R. Analytical Concordance of PD-L1 Assays Utilizing Antibodies From FDA-Approved Diagnostics in Advanced Cancers: A Systematic Literature Review. JCO Precis Oncol. 2021 Jun 8;5:953-73.
11. Akhtar M, Rashid S, Al-Bozom IA. PD-L1 immunostaining: what pathologists need to know. Diagn Pathol. 2021 Oct 25;16(1):94.
12. Oyan B, Sonmez O, Yazar A, Teomete M. Comparison of SP142 (Ventana) and SP263 (Ventana) assays to test PD-L1 expression in metastatic cancer patients to be treated with immune checkpoint inhibitors. J Clin Oncol. 2018 May 20;36(15_suppl):e24306-e24306.
13. Maule JG, Clinton LK, Graf RP, Xiao J, Oxnard GR, Ross JS, et al. Comparison of PD-L1 tumor cell expression with 22C3, 28-8, and SP142 IHC assays across multiple tumor types. J Immunother Cancer. 2022 Oct;10(10):e005573.
14. Lantuejoul S, Sound-Tsao M, Cooper WA, Girard N, Hirsch FR, Roden AC, et al. PD-L1 Testing for Lung Cancer in 2019: Perspective From the IASLC Pathology Committee. J Thorac Oncol. 2020 Apr;15(4):499-519.
15. Roche Diagnostics. VENTANA PD-L1 (SP263) Assay (CE IVD) [Internet]. 2023 [cited 2023 Oct 19]. Available from: https://diagnostics.roche.com/global/en/products/tests/ventana-pd-l1-sp263-assay.html
16. FDA. VENTANA PD-L1 (SP263) Assay – P160046/S010. FDA [Internet]. 2022 Jan 3 [cited 2023 Oct 18]; Available from: https://www.fda.gov/medical-devices/recently-approved-devices/ventana-pd-l1-sp263-assay-p160046s010.
17. Marletta S, Fusco N, Munari E, Luchini C, Cimadamore A, Brunelli M, et al. Atlas of PD-L1 for Pathologists: Indications, Scores, Diagnostic Platforms and Reporting Systems. J Pers Med. 2022 Jun 29;12(7):1073.
18. Khajuria O, Sharma N. Epigenetic targeting for lung cancer treatment via CRISPR/Cas9 technology. Adv Cancer Biol - Metastasis. 2021 Dec;3:100012.
19. Ganti AK, Klein AB, Cotarla I, Seal B, Chou E. Update of Incidence, Prevalence, Survival, and Initial Treatment in Patients With Non-Small Cell Lung Cancer in the US. JAMA Oncol. 2021 Dec 1;7(12):1824-32.
20. Lahiri A, Maji A, Potdar PD, Singh N, Parikh P, Bisht B, et al. Lung cancer immunotherapy: progress, pitfalls, and promises. Mol Cancer. 2023 Feb 21;22(1):40.
21. Sellars MC, Wu CJ, Fritsch EF. Cancer vaccines: Building a bridge over troubled waters. Cell. 2022 Jul 21;185(15):2770-88.
22. Houot R, Schultz LM, Marabelle A, Kohrt H. T-cell-based Immunotherapy: Adoptive Cell Transfer and Checkpoint Inhibition. Cancer Immunol Res. 2015 Oct;3(10):1115-22.
23. Scagliotti GV, Hirsh V, Siena S, Henry DH, Woll PJ, Manegold C, et al. Overall survival improvement in patients with lung cancer and bone metastases treated with denosumab versus zoledronic acid: subgroup analysis from a randomized phase 3 study. J Thorac Oncol. 2012 Dec;7(12):1823-29.
24. Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. Journal of Clinical Oncology. 2018 Jun 10;36(17):1714-68.
25. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015 Jul 2;373(1):23-34.
26. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012 Jun 28;366(26):2443-54.
27. Manriquez L, Hayden D, Lian F, Liu C, Feng J, Nielsen A, et al. Precision and Repeatability of the Analytical VENTANA PD-L1 (SP263) Rabbit Monoclonal Primary Antibody Across Seven Different Tumor Types [Internet]. Roche Tissue Diagnostics; 2022 [cited 2023 Oct 19]. Available from: https://diagnostics.roche.com/content/dam/acadia/whitepaper/918/25/PD-L1%20SP263%20White%20Paper_US%20Veeva%20Copy_MC-US-07988.pdf
28. Felip E, Altorki N, Zhou C, Csőszi T, Vynnychenko I, Goloborodko O, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet. 2021 Oct 9;398(10308):1344-57.
29. Vranic S, Gatalica Z. PD-L1 testing by immunohistochemistry in immuno-oncology. Biomol Biomed. 2023 Feb 1;23(1):15-25.
30. Liu C, Fang F, Kong Y, ElGabry EA. Tumor Area Positivity (TAP) score of programmed death-ligand 1 (PD-L1): a novel visual estimation method for combined tumor cell and immune cell scoring. Diagn Pathol. 2023 Apr 19;18(1):48.
31. Udall M, Rizzo M, Kenny J, Doherty J, Dahm S, Robbins P, et al. PD-L1 diagnostic tests: a systematic literature review of scoring algorithms and test-validation metrics. Diagn Pathol. 2018 Feb 9;13(1):12.
32. Igarashi T, Teramoto K, Ishida M, Hanaoka J, Daigo Y. Scoring of PD-L1 expression intensity on pulmonary adenocarcinomas and the correlations with clinicopathological factors. ESMO Open. 2016 Aug 26;1(4):e000083.
33. Planchard D, Reinmuth N, Orlov S, Fischer JR, Sugawara S, Mandziuk S, et al. ARCTIC: durvalumab with or without tremelimumab as third-line or later treatment of metastatic non-small-cell lung cancer. Ann Oncol. 2020 May;31(5):609-18.
34. Jassem J, de Marinis F, Giaccone G, Vergnenegre A, Barrios CH, Morise M, et al. Updated Overall Survival Analysis From IMpower110: Atezolizumab Versus Platinum-Based Chemotherapy in Treatment-Naive Programmed Death-Ligand 1-Selected NSCLC. J Thorac Oncol. 2021 Nov;16(11):1872-82.
35. Herbst RS, Giaccone G, de Marinis F, Reinmuth N, Vergnenegre A, Barrios CH, et al. Atezolizumab for First-Line Treatment of PD-L1-Selected Patients with NSCLC. N Engl J Med. 2020 Oct 1;383(14):1328-39.
36. West HJ, McCleland M, Cappuzzo F, Reck M, Mok TS, Jotte RM, et al. Clinical efficacy of atezolizumab plus bevacizumab and chemotherapy in KRAS-mutated non-small cell lung cancer with STK11, KEAP1, or TP53 comutations: subgroup results from the phase III IMpower150 trial. J Immunother Cancer. 2022 Feb;10(2):e003027.
37. Spigel DR, Faivre-Finn C, Gray JE, Vicente D, Planchard D, Paz-Ares L, et al. Five-Year Survival Outcomes From the PACIFIC Trial: Durvalumab After Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. J Clin Oncol. 2022 Apr 20;40(12):1301-11.
38. Paz-Ares L, Spira A, Raben D, Planchard D, Cho BC, Özgüroğlu M, et al. Outcomes with durvalumab by tumour PD-L1 expression in unresectable, stage III non-small-cell lung cancer in the PACIFIC trial. Ann Oncol. 2020 Jun;31(6):798-806.
39. Hirsch FR, McElhinny A, Stanforth D, Ranger-Moore J, Jansson M, Kulangara K, et al. PD-L1 Immunohistochemistry Assays for Lung Cancer: Results from Phase 1 of the Blueprint PD-L1 IHC Assay Comparison Project. J Thorac Oncol. 2017 Feb;12(2):208-22.
40. Tsao MS, Kerr KM, Kockx M, Beasley MB, Borczuk AC, Botling J, et al. PD-L1 Immunohistochemistry Comparability Study in Real-Life Clinical Samples: Results of Blueprint Phase 2 Project. J Thorac Oncol. 2018 Sep;13(9):1302-11.
41. Ratcliffe MJ, Sharpe A, Midha A, Barker C, Scott M, Scorer P, et al. Agreement between Programmed Cell Death Ligand-1 Diagnostic Assays across Multiple Protein Expression Cutoffs in Non-Small Cell Lung Cancer. Clin Cancer Res. 2017 Jul 15;23(14):3585-91.
42. Yu SL, Hsiao YJ, Cooper WA, Choi YL, Avilés-Salas A, Chou TY, et al. The Ring Study: an international comparison of PD-L1 diagnostic assays and their interpretation in non-small cell lung cancer, head and neck squamous cell cancer and urothelial cancer. Pathology. 2023 Feb;55(1):19-30.
43. Sughayer MA, Alnaimy F, Alsughayer AM, Qamhia N. Comparison of 22C3 PharmDx and SP263 Assays to Test PD-L1 Expression in NSCLC. Appl Immunohistochem Mol Morphol. 2019 Oct;27(9):663-6.
44. Zhou C, Srivastava MK, Xu H, Felip E, Wakelee H, Altorki N, et al. Comparison of SP263 and 22C3 immunohistochemistry PD-L1 assays for clinical efficacy of adjuvant atezolizumab in non-small cell lung cancer: results from the randomized phase III IMpower010 trial. J Immunother Cancer. 2023 Oct;11(10):e007047.
45. Kim SY, Kim TE, Park CK, Yoon HK, Sa YJ, Kim HR, et al. Comprehensive Comparison of 22C3 and SP263 PD-L1 Expression in Non-Small-Cell Lung Cancer Using Routine Clinical and Conditioned Archives. Cancers (Basel). 2022 Jun 27;14(13):3138.
46. Marchetti A, Barberis M, Franco R, De Luca G, Pace MV, Staibano S, et al. Multicenter Comparison of 22C3 PharmDx (Agilent) and SP263 (Ventana) Assays to Test PD-L1 Expression for NSCLC Patients to Be Treated with Immune Checkpoint Inhibitors. J Thorac Oncol. 2017 Nov;12(11):1654-63.
47. Tsimafeyeu I, Imyanitov E, Zavalishina L, Raskin G, Povilaitite P, Savelov N, et al. Agreement between PDL1 immunohistochemistry assays and polymerase chain reaction in non-small cell lung cancer: CLOVER comparison study. Sci Rep. 2020 Mar 3;10(1):3928.
48. Savic S, Berezowska S, Eppenberger-Castori S, Cathomas G, Diebold J, Fleischmann A, et al. PD-L1 testing of non-small cell lung cancer using different antibodies and platforms: a Swiss cross-validation study. Virchows Arch. 2019 Jul;475(1):67-76.
49. Nambirajan A, Husain N, Shukla S, Kumar S, Jain D. Comparison of laboratory-developed test & validated assay of programmed death ligand-1 immunohistochemistry in non-small-cell lung carcinoma. Indian J Med Res. 2019 Oct;150(4):376-84.
50. Naso JR, Wang G, Banyi N, Derakhshan F, Shokoohi A, Ho C, et al. Comparability of laboratory-developed and commercial PD-L1 assays in non-small cell lung carcinoma. Ann Diagn Pathol. 2021 Feb;50:151590.
51. Pasricha S, Durga G, Koyyala VP, Jajodia A, Gupta G, Mehta A. PD-L1 Testing and Assessment: Practical Considerations for Oncologist and Pathologist. Indian J Med Paediatr Oncol. 2022 Nov 28: s-0042-1748797.
52. Scheel AH, Baenfer G, Baretton G, Dietel M, Diezko R, Henkel T, et al. Interlaboratory concordance of PD-L1 immunohistochemistry for non-small-cell lung cancer. Histopathology. 2018 Feb;72(3):449-59.
53. Torlakovic E, Lim HJ, Adam J, Barnes P, Bigras G, Chan AWH, et al. "Interchangeability" of PD-L1 immunohistochemistry assays: a meta-analysis of diagnostic accuracy. Mod Pathol. 2020 Jan;33(1):4-17.
54. Kintsler S, Cassataro MA, Drosch M, Holenya P, Knuechel R, Braunschweig T. Expression of programmed death ligand (PD-L1) in different tumors. Comparison of several current available antibody clones and antibody profiling. Ann Diagn Pathol. 2019 Aug;41:24-37.
55. Cooper WA, Russell PA, Cherian M, Duhig EE, Godbolt D, Jessup PJ, et al. Intra- and Interobserver Reproducibility Assessment of PD-L1 Biomarker in Non-Small Cell Lung Cancer. Clin Cancer Res. 2017 Aug 15;23(16):4569-77.
56. Cerbelli B, Girolami I, Eccher A, Costarelli L, Taccogna S, Scialpi R, et al. Evaluating programmed death-ligand 1 (PD-L1) in head and neck squamous cell carcinoma: concordance between the 22C3 PharmDx assay and the SP263 assay on whole sections from a multicentre study. Histopathology. 2022 Jan;80(2):397-406.
57. Rijnders M, van der Veldt AAM, Zuiverloon TCM, Grünberg K, Thunnissen E, de Wit R, et al. PD-L1 Antibody Comparison in Urothelial Carcinoma. Eur Urol. 2019 Mar;75(3):538-40.
58. Park Y, Koh J, Na HY, Kwak Y, Lee KW, Ahn SH, et al. PD-L1 Testing in Gastric Cancer by the Combined Positive Score of the 22C3 PharmDx and SP263 Assay with Clinically Relevant Cut-offs. Cancer Res Treat. 2020 Jul;52(3):661-70.
59. Dabbagh TZ, Sughayer MA. PD-L1 Expression Harmonization in Gastric Cancer Using 22C3 PharmDx and SP263 Assays. Appl Immunohistochem Mol Morphol. 2021 Jul 1;29(6):462-6.
60. Wang X, He J, Li J, Wu C, Yue M, Niu S, et al. Concordance of assessments of four PD-L1 immunohistochemical assays in esophageal squamous cell carcinoma (ESCC). J Cancer Res Clin Oncol. 2024 Jan 28;150(2):43.
61. Ivanova M, Frascarelli C, Cerbelli B, Pignataro MG, Pernazza A, Venetis K, et al. PD-L1 testing in metastatic triple-negative breast cancer: Interobserver and interplatform reproducibility of CE-IVD assays for CPS and IC scores. Hum Pathol. 2024 Feb;144:22-7.
62. Paliogiannis P, Lobrano R, Bella MA, Fara A, Uras MG, Pinna MA, et al. PD-L1 immunohistochemical expression in bladder urothelial cancer with SP263, SP142 and 22C3 antibodies: A comparative study. Ann Diagn Pathol. 2024 Apr;69:152267.
63. Chen Y, Fu J, Li Z, Chen Q, Zhang J, Yang Y, et al. Cutoff values of PD-L1 expression in urinary cytology samples for predicting response to immune checkpoint inhibitor therapy in upper urinary tract urothelial carcinoma. Cancer Cytopathol. 2023 Mar;131(3):179-87.
64. Weng M, Bai Y, Xu L, Chang C, Teng X. Comparison of PD-L1 detection methods, platforms and reagents in bladder cancer. Ann Diagn Pathol. 2022 Oct;60:151986.
65. Badve SS, Penault-Llorca F, Reis-Filho JS, Deurloo R, Siziopikou KP, D'Arrigo C, et al. Determining PD-L1 Status in Patients With Triple-Negative Breast Cancer: Lessons Learned From IMpassion130. J Natl Cancer Inst. 2022 May 9;114(5):664-75.
66. Scorer P, Scott M, Lawson N, Ratcliffe MJ, Barker C, Rebelatto MC, et al. Consistency of tumor and immune cell programmed cell death ligand-1 expression within and between tumor blocks using the VENTANA SP263 assay. Diagn Pathol. 2018 Jul 24;13(1):47.
67. Rugo HS, Loi S, Adams S, Schmid P, Schneeweiss A, Barrios CH, et al. PD-L1 Immunohistochemistry Assay Comparison in Atezolizumab Plus nab-Paclitaxel-Treated Advanced Triple-Negative Breast Cancer. J Natl Cancer Inst. 2021 Nov 29;113(12):1733-43.
68. Watanabe T, Okuda K, Murase T, Moriyama S, Haneda H, Kawano O, et al. Four immunohistochemical assays to measure the PD-L1 expression in malignant pleural mesothelioma. Oncotarget. 2018 Apr 17;9(29):20769-80.
69. Wang M, Wang S, Trapani JA, Neeson PJ. Challenges of PD-L1 testing in non-small cell lung cancer and beyond. J Thorac Dis. 2020 Aug;12(8):4541-8.
70. Sanguedolce F, Zanelli M. Assessing PD-L1 Expression in Different Tumor Types. In: Handbook of Cancer and Immunology 2023 Jan 31: 1-21.
71. Gaule P, Smithy JW, Toki M, Rehman J, Patell-Socha F, Cougot D, et al. A Quantitative Comparison of Antibodies to PDL-1. JAMA Oncol. 2017 Feb 1;3(2):256-9.
72. Büttner R, Gosney JR, Skov BG, Adam J, Motoi N, Bloom KJ, et al. Programmed Death-Ligand 1 Immunohistochemistry Testing: A Review of Analytical Assays and Clinical Implementation in Non-Small-Cell Lung Cancer. J Clin Oncol. 2017 Dec 1;35(34):3867-76.
73. Yue C, Jiang Y, Li P, Wang Y, Xue J, Li N, et al. Dynamic change of PD-L1 expression on circulating tumor cells in advanced solid tumor patients undergoing PD-1 blockade therapy. Oncoimmunology. 2018 Mar 6;7(7):e1438111.
74. Zhao X, Bao Y, Meng B, Xu Z, Li S, Wang X, et al. From rough to precise: PD-L1 evaluation for predicting the efficacy of PD-1/PD-L1 blockades. Front Immunol. 2022 Aug 3;13:920021.
75. Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015 May 21;372(21):2018-28.
76. Ghaffari Laleh N, Ligero M, Perez-Lopez R, Kather JN. Facts and Hopes on the Use of Artificial Intelligence for Predictive Immunotherapy Biomarkers in Cancer. Clin Cancer Res. 2023 Jan 17;29(2):316-323.
77. Cheng G, Zhang F, Xing Y, Hu X, Zhang H, Chen S, et al. Artificial Intelligence-Assisted Score Analysis for Predicting the Expression of the Immunotherapy Biomarker PD-L1 in Lung Cancer. Front Immunol. 2022 Jul 1;13:893198.
78. Wu J, Liu C, Liu X, Sun W, Li L, Gao N, et al. Artificial intelligence-assisted system for precision diagnosis of PD-L1 expression in non-small cell lung cancer. Mod Pathol. 2022 Mar;35(3):403-11.
79. Shamai G, Livne A, Polónia A, Sabo E, Cretu A, Bar-Sela G, et al. Deep learning-based image analysis predicts PD-L1 status from H&E-stained histopathology images in breast cancer. Nat Commun. 2022 Nov 8;13(1):6753.
80. Canini V, Eccher A, d'Amati G, Fusco N, Maffini F, Lepanto D, et al. Digital Pathology Applications for PD-L1 Scoring in Head and Neck Squamous Cell Carcinoma: A Challenging Series. J Clin Med. 2024 Feb 22;13(5):1240.