Abstract
Leveraging the immunomodulatory effects of radiation therapy (RT) for synergy with immunotherapeutic agents for the treatment of cancer is an area of immense interest. A pressing deficiency in the translation of this strategy into the clinic is the lack of clarity on the impact of radiation dose-fractionation on host anti-cancer immune defences. Using a series of rationally selected radiation schedules in mouse models of solid cancer, we demonstrated that radiation dose per fraction and total dose can independently and differentially shape anti-tumor immune responses and their interplay with immune checkpoint blockade (ICB) therapy. In this commentary, we extend our discussions on the complex and far-reaching impact of radiation dose-fractionation on the tumor immune microenvironment and the therapeutic implications thereof. We also outline the nuances in examining this question and focus on the clinical translatability of our current body of knowledge to re-evaluate RT and ICB combination strategies in the clinic.
Keywords
Radiation therapy, Radiation dose-fractionation, Immune checkpoint blockade therapy, Innate and adaptive anti-cancer immune responses, T regulatory cells (Tregs), Immunosuppression, Preclinical models of solid cancer, Immuno-oncology
Introduction
Radiation therapy (RT) is a highly sophisticated and effective cancer treatment modality that is of central importance in modern oncology. It is estimated that RT contributes to 40% of all cancer cures and at least 1 in 2 cancer patients would benefit from RT during their course of illness [1,2]. An exciting development in radiation oncology is the appreciation that RT can also induce host immune responses that contribute to tumor control, beyond direct radiation-induced cytotoxicity. Underlying mechanisms include the induction of immunogenic cell death, generation of CD8+ T cell responses, increased immune infiltration of tumors, and modulation of tumor cell recognition and killing by immune cells [4-6]. Moreover, there is a growing body of work to suggest that RT and antibody-based immune checkpoint blockade (ICB) can act via non-overlapping and complementary mechanisms to promote anti-tumor immunity [5,6].
The early wave of single-arm studies testing the combination of RT and ICB promisingly reported abscopal responses (radiation-induced immune-mediated regression of tumor lesions distant to the irradiated site) at an increased frequency compared to historical controls of RT alone [7-10]. These data highlighted a beneficial interplay between the two modalities that can restore the ability of the patient’s immune system to control tumors. This was corroborated by the randomized PACIFIC, PEMBRO-RT and CA184-043 trials, which established superior survival in patients receiving RT and ICB compared to either modality alone [11-13]. Nonetheless as more clinical trials mature, an increasing number of studies are emerging that do not demonstrate a survival benefit with RT and ICB [14-16]. The seemingly contradicting data is a reflection of the major gap in our current understanding of the complex interplay between RT, tissue niche and the immune system [17]. Mechanistic insights to help guide the selection of radiation dose-fractionation schedules in combination with ICB remains an area of unmet medical need.
We recently published preclinical findings demonstrating that different radiation dose-fractionation schedules are not equivalent in their capacity to evoke innate and adaptive anti-tumor immune responses and synergize with ICB agents [18]. Radiation-induced T regulatory cell (Treg) responses were identified as a central player in influencing the extent and timing of immune activation by different radiation schedules. Our data also suggested that not all cancer types are conducive to radiation-induced local recruitment and/or expansion of Tregs, highlighting that consideration of both radiation dose-fractionation and cancer type is necessary for the optimal use of ICB with RT. Herein, we elaborate on these points by providing a brief historical overview of the concept of radiation dose-fractionation and the complexities in understanding its impact on immune responses. We also suggest possible approaches to leverage this aspect of RT in the design of RT and ICB combination strategies.
Shifts in Our Understanding and Application of Radiation Dose-Fractionation
When X-rays were first adopted for treatment of tumors in the 1900’s, large single-dose techniques were commonplace, which controlled tumors at the cost of severe radiation toxicity. Pivotal work in the 1920’s discovered that fractionation, or the dividing of a radiation dose into multiple daily fractions, greatly increased the therapeutic window between tumor control and normal tissue toxicity [19]. Subsequent foundational radiobiology work in the 1970’s clarified that fractionation exploits differences between tumor and normal tissues via mechanisms that are often summarized as the “four R’s” of radiobiology: repair, reoxygenation, reassortment, and repopulation [20]. Due to this, conventionally fractionated radiation schedules, which employ a protracted number of low dose fractions over multiple weeks, have dominated clinical practice. More recently, multifront technological advances in image guidance, motion tracking, and dose calculation algorithms have reintroduced the delivery of high radiation doses in one or a few fractions in the form of stereotactic radiosurgery (SRS) and stereotactic ablative body radiotherapy (SABR) for the intra- and extracranial settings, respectively. Although the differential cell kill between tumor and normal tissues is lost with such schedules, this is compensated by geographic and dosimetric precision in radiation delivery. A major advantage of SRS and SABR is the convenience afforded to the patient by reducing the number of treatment sessions without compromising tumor control. This is particularly desirable in the palliative setting, but increasingly, SABR has also taken on roles both as viable surgical alternatives in the curative setting of various cancers [21-24] and as a uniquely suited modality for aggressive metastasis-directed therapy in oligometastatic patients to improve survival [25,26].
Undergirding the effective clinical application of these different radiation schedules is the study of radiobiology, which has primarily focused on radiation-induced DNA damage and resultant cell death mechanisms. Without doubt, classical radiobiology has led to cornerstone clinical gains such as the development of altered fractionation and the use of hypoxic modifiers [27,28]. Several threads of evidence over the last few decades have however converged to now establish that the tumoricidal activity of RT extends beyond the direct cytotoxicity of DNA damage induction in irradiated cells to “non-targeted effects”, of which tumor cell killing by immunological processes is principal [4]. Therefore, a deeper understanding of the impact of radiation dose-fractionation on downstream immune responses has become an important and urgent research need especially in the current immunooncology era.
The Complex Impact of Radiation Dose-Fractionation on the Tumor Microenvironment
Interpretation of published data examining the immunomodulatory effects of radiation dose-fractionation is complicated by several factors. Firstly, most pre-clinical studies in this field have only examined high doses of radiation in a few fractions (SABR-like schedules), most likely because conventionally fractionated schedules present technical and logistic difficulties for in vitro and in vivo investigation. Thus, there is an inherent bias in the body of pre-clinical data that has led to the impression that SABR-like schedules are better inducers of cancer immunity [29]. A review of clinical reports of the abscopal effect suggests that a wide range of radiation schedules can evoke these immune-mediated responses, including both conventionally fractionated and SABR-like schedules [29,30]. Secondly, the non-linear relationship between radiation dose and cell kill means that comparison of biological sequelae between dose-fractionation schedules is not as simple as matching the arithmetic sum of the radiation schedules’ doses per fraction (DPF). To address this issue, the linear-quadratic model is most widely adopted to fit the relationship between radiation dose and cell kill [31]. Its equation can then be rearranged to standardize for the effect of fractionation and derive the biological effective dose (BED) [32]. BED is therefore a surrogate for “total dose” and can be used for comparison between different radiation schedules. This consideration is rarely accounted for when pre-clinical studies have compared radiation schedules.
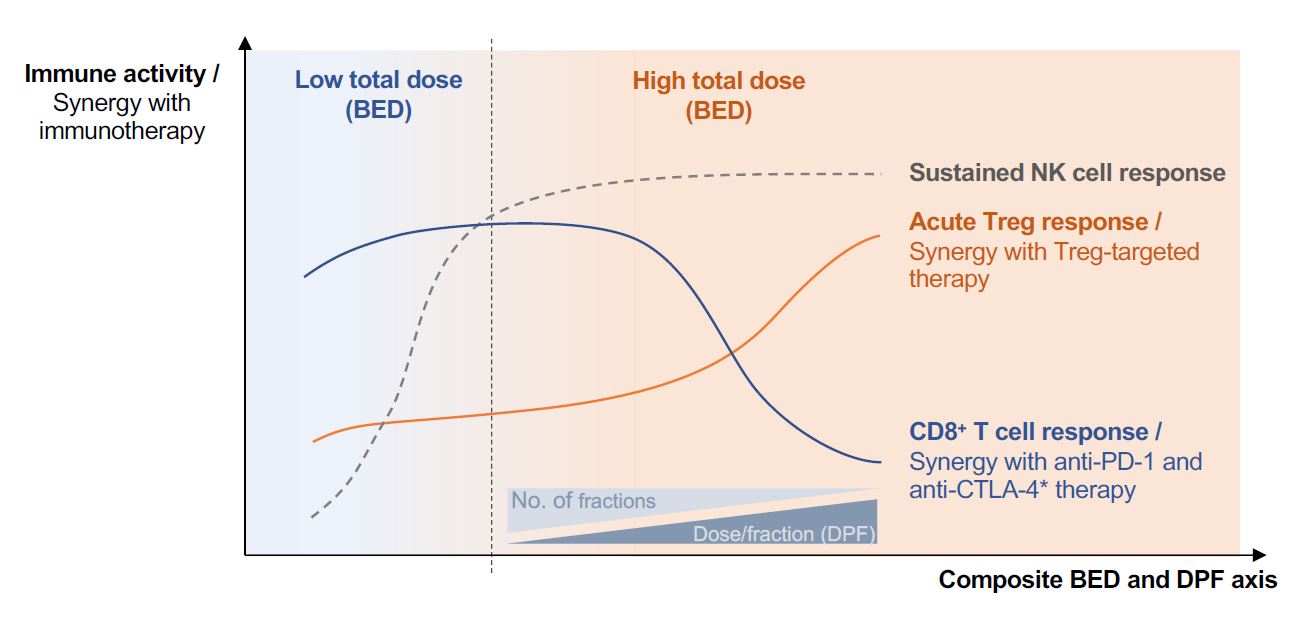
Critically, the tumor microenvironment is an ecosystem of tightly inter-dependent tumor, immune, and non-immune cells, the composition of which can vary significantly between tumor type and metastatic niche [33,34]. In response to irradiation of the tumor, each cellular subset may exhibit distinctive radiation response characteristics that collectively dictate the response of the tumor lesion to therapy [35]. Illustrating this dictum, in an orthotopic murine model of mammary carcinoma we demonstrated that RT, regardless of radiation schedule, transiently enriched tumors for Tregs, CD4+ effector T and NK cells [18]. In contrast, total CD8+ T cell numbers remained constant, but the recovery of activated CD8+ T cells with cytotoxic potential was more rapid in tumors exposed to RT schedules of ≤ 8 Gy per fraction compared to a single fraction of 20 Gy, even when BED was controlled for. A similar pattern of recovery of mature tumor-associated CD86+ type 1 conventional dendritic cells (cDC1s) was also observed in response to the different radiation schedules. These findings pointed to radiation DPF rather than BED as being a key determinant of the ability of RT to prime local anti-tumor CD8+ T cell responses (Figure 1). Central to the induction of these responses was the DPF-dependent effects of RT on the rate and amplitude of Treg accumulation within irradiated tumors. Indeed, we found a direct negative correlation between the abundance of tumor-associated Tregs and that of CD86+ cDC1s. Correspondingly, local depletion of radiationinduced Tregs salvaged the ability of high DPF schedules, such as a single fraction of 20 Gy, to evoke effective antitumor CD8+ T cell responses and synergize with anti-PD-1 therapy. The activity of NK cells within irradiated tumors was also compromised by the increased accumulation of Tregs following RT. However, radiation schedules of sufficiently high BED (> 36 Gy in the context of AT3-OVA tumors) supported the persistence of elevated NK cell numbers with heightened activity beyond contraction of the Treg response, which underpinned their long-term tumor control (Figure 1) [18].Interestingly, in MC38 colon adenocarcinoma tumors, a single fraction of 20 Gy evoked both heightened CD8+ T and NK cell anti-tumor responses, independent of immunotherapy. This was likely a consequence of the inability of these tumors to generate a suppressive Treg response to RT [18].
Ultimately, the impact of radiation dose-fractionation on local immune responses can shape ensuing systemic responses that mediate the abscopal effect, as well as immunological memory responses that can protect against disease recurrence. Work by Demaria et al. demonstrated that activation of tumor cellassociated type I interferon signaling via STING underpinned the ability of RT to prime CD8+ T cell activity and in turn support the induction of abscopal responses [36]. They showed that high DPF schedules such as a single fraction of 20 Gy induced increased levels of cytosolic exonuclease TREX1, resulting in the degradation of cytosolic double-stranded DNA and preclusion of STING-mediated release of interferon-β. We showed that targeted depletion of radiation-induced tumorassociated Tregs was sufficient to rescue the ability of a single fraction of 20 Gy to support abscopal responses. Furthermore, abrogation of Treg responses to RT, regardless of radiation schedule, was critical to the generation of anti-cancer memory responses. Thus, anti-CTLA-4-mediated suppression of radiation-induced Treg activity equalized the ability of both high and low-to-moderate DPF schedules to induce local and systemic anti-tumor immunity. We also generated data suggesting that a single fraction of high dose RT, in the context of anti-CTLA-4-mediated Treg suppression, evoked a broader repertoire of memory T cells compared to low-tomoderate DPF schedules. Corroborating this observation, increases in peripheral and tumor-associated T cell diversity has been associated with the clinical use of SABR [8,26,37]. Taken together, the immunological impact of radiation dosefractionation is highly multifaceted, differentially affecting the amplitude, kinetics as well as quality of innate and adaptive immune responses, both locally and systemically.
Translational Perspectives
Moving forward, it is imperative to re-evaluate how radiation dose-fractionation can be leveraged to exploit the full therapeutic potential of ICB across different cancer indications. In this regard, it is helpful to consider the two angles from which RT is uniquely positioned to be partnered with cancer immunotherapy: priming and debulking. The two approaches are not necessarily mutually exclusive. It is well recognized that a significant barrier to the successful application of ICB is a “cold” tumor microenvironment in which there is little or no pre-existing T cell infiltration. In considering RT as a mechanism to convert the status of such tumors to inflamed and ICB-responsive, a departure from the orthodox radiation oncology dogma of “treat once and treat to tolerance” may be surprisingly helpful. Radiation doses as low as 0.5-2 Gy have been shown pre-clinically to repolarize tumor-associated myeloid cells that support T cell recruitment into the tumor [38,39]. More provocatively, some pre-clinical and clinical data have raised the possibility that the low dose penumbra in partial irradiation of tumors may be adequate to elicit robust immune responses that can control the entire tumor and even distant tumors [9,40]. Promisingly, early exploration of these novel concepts in the clinic, involving sub-tumoricidal radiation doses, metronomic scheduling, and irradiation of all involved lesions, have shown exciting synergy with ICB therapy [41,42].
RT is also well-suited for use as a debulking strategy, both in the metastatic and localized disease settings to reduce the overall tumor load. Underpinning this line of thought is the observation that patients with a more limited burden of disease respond better to ICB [43-45]. Because various high radiation BED schedules can be used to reduce tumor burden, an understanding of how immune response characteristics are shaped by radiation dose-fractionation may help the strategic co-opting of host immune defenses to achieve maximum tumor cell kill. Along these lines, we found that radiation schedules of low-to-moderate DPF, but of high BED, achieved more durable tumor control compared to high DPF schedules of comparable BED, likely because the former evoked both CD8+ T and NK cell responses whereas the latter supported persistent NK cell activity but failed to trigger early priming events necessary to engage host adaptive immune activity (Figure 1) [18]. Notably, the PACIFIC randomized clinical trial reported significant combinatorial efficacy between a conventionally fractionated (low DPF) schedule of high BED (54-66 Gy in 1.8-2.0 Gy per fraction) and anti-PD-1 therapy [11]. It is worth noting that this trial, which achieved one of the most remarkable synergistic clinical outcomes between RT and ICB thus far, was conducted in patients with Stage III nonsmall cell lung cancer with only overt locoregional disease and in whom curative-intent RT was employed to essentially achieve maximal tumor debulking.
Conversely, SABR is often the preferred RT technique for aggressive metastasis-directed therapy in the oligometastatic setting, due to its geographic precision, ablative nature, and convenience for the patient. Here, concomitant targeting of Treg accumulation, often a feature of such radiation schedules, as we and others have demonstrated [38-40], may be a rational strategy to optimize the treatment’s immunoadjuvant potential. Notably, our analysis of publicly available transcriptomic datasets of human breast and prostate cancers treated with SABR-like schedules also identified enriched Treg but not CD8+ T cell responses [18,46]. Significantly elevated expression of CTLA4 observed within these irradiated tumors may provide an opportunity for selective depletion of radiation induced Treg responses. Indeed, in our study, an anti- CTLA-4-targeted depleting antibody selectively eliminated radiation-induced Tregs owing to their heightened expression of CTLA-4 [18]. While the FDA-approved anti-CTLA-4 antibody ipilimumab has the inherent capacity to deplete Tregs via antibody-dependent cell-mediated cytotoxicity (ADCC), evidence to support this mechanism of action in patients is scarce [47]. A version of ipilimumab engineered with a nonfucosylated Fc domain to increase its ADCC capacity is being trialled in patients (NCT03110307). Other antibody-based strategies to deplete Tregs including the targeting of GITR and CD73 are also promising and could similarly be considered in combination with SABR [48,49].
Overall, radiation dose-fractionation is a critical facet of RT that has the potential to be tuned to leverage tumor biology and complement cancer immunotherapy. Unravelling this important but complex subject begins with a foundation of good pre-clinical model systems and relies on the continual and tightly integrated feedback into and from clinical investigations. It will be a tremendous success for oncology if in the not-too-distant future, just as immunotherapeutic agents can be selected to bolster desired immunological pathways, personalized radiation dose-fractionation schedules can be tailored to do the same.
Conflict of Interest Disclosure
The authors have no competing financial interests.
Author Contribution Statement
J.S wrote the manuscript. N.M.H edited the manuscript.
Acknowledgements
Data by Sia J et al., which forms the focus of this commentary was supported by the imCORE Network on behalf of F. Hoffmann-La Roche Ltd (N.M.H.) and the Australian Government Research Training Program Scholarship and the Royal Australian New Zealand College of Radiologists (RANZCR) Withers and Peters Grant (J.S.). We would also like to acknowledge the generous support of The Peter MacCallum Foundation and Australian Cancer Research Foundation.
References
2. Delaney G, Jacob S, Featherstone C, Barton M. The role of radiotherapy in cancer treatment: estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer. 2005;104(6):1129-37.
3. Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359(6382):1350-5.
4. Sia J, Szmyd R, Hau E, Gee HE. Molecular Mechanisms of Radiation- Induced Cancer Cell Death: A Primer. Front Cell Dev Biol. 2020;8:41.
5. Haikerwal SJ, Hagekyriakou J, MacManus M, Martin OA, Haynes NM. Building immunity to cancer with radiation therapy. Cancer Lett. 2015;368(2):198-208.
6. Weichselbaum RR, Liang H, Deng L, Fu YX. Radiotherapy and immunotherapy: a beneficial liaison? Nat Rev Clin Oncol. 2017;14(6):365-79.
7. Golden EB, Chhabra A, Chachoua A, Adams S, Donach M, Fenton- Kerimian M, et al. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumors: a proof-of-principle trial. Lancet Oncol. 2015;16(7):795-803.
8. Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520(7547):373-7.
9. Luke JJ, Lemons JM, Karrison TG, Pitroda SP, Melotek JM, Zha Y, et al. Safety and Clinical Activity of Pembrolizumab and Multisite Stereotactic Body Radiotherapy in Patients With Advanced Solid Tumors. J Clin Oncol. 2018;36(16):1611-8.
10. Formenti SC, Rudqvist NP, Golden E, Cooper B, Wennerberg E, Lhuillier C, et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat Med. 2018;24(12):1845-51.
11. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R,et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med. 2018;379(24):2342-50.
12. Theelen W, Peulen HMU, Lalezari F, van der Noort V, de Vries JF, Aerts J, et al. Effect of Pembrolizumab After Stereotactic Body Radiotherapy vs Pembrolizumab Alone on Tumor Response in Patients With Advanced Non-Small Cell Lung Cancer: Results of the PEMBRO-RT Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019.
13. Fizazi K, Drake CG, Beer TM, Kwon ED, Scher HI, Gerritsen WR, et al. Final Analysis of the Ipilimumab Versus Placebo Following Radiotherapy Phase III Trial in Postdocetaxel Metastatic Castrationresistant Prostate Cancer Identifies an Excess of Long-term Survivors. European Urology. 2020;78(6):822-30.
14. Voorwerk L, Slagter M, Horlings HM, Sikorska K, van de Vijver KK, de Maaker M, et al. Publisher Correction: Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: the TONIC trial. Nat Med. 2019;25(7):1175.
15. McBride S, Sherman E, Tsai CJ, Baxi S, Aghalar J, Eng J, et al. Randomized Phase II Trial of Nivolumab With Stereotactic Body Radiotherapy Versus Nivolumab Alone in Metastatic Head and Neck Squamous Cell Carcinoma. J Clin Oncol. 2020:JCO2000290.
16. Lee NY, Ferris RL, Psyrri A, Haddad RI, Tahara M, Bourhis J, et al. Avelumab plus standard-of-care chemoradiotherapy versus chemoradiotherapy alone in patients with locally advanced squamous cell carcinoma of the head and neck: a randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. The Lancet Oncology. 2021;22(4):450-62.
17. Turchan WT, Pitroda SP, Weichselbaum RR. Radiotherapy and Immunotherapy Combinations in the Treatment of Patients with Metastatic Disease: Current Status and Future Focus. Clinical Cancer Research. 2021.
18. Sia J, Hagekyriakou J, Chindris I, Albarakati H, Leong T, Schlenker R, et al. Regulatory T Cells Shape the Differential Impact of Radiation Dose-Fractionation Schedules on Host Innate and Adaptive Antitumor Immune Defenses. Int J Radiat Oncol Biol Phys. 2021.
19. Bernier J, Hall EJ, Giaccia A. Radiation oncology: a century of achievements. Nat Rev Cancer. 2004;4(9):737-47.
20. Joiner M, Kogel Avd. Basic clinical radiobiology. Fifth edition. ed. Boca Raton, FL: CRC Press/Taylor & Francis Group; 2018. p. p.
21. Loblaw A, Cheung P, D’Alimonte L, Deabreu A, Mamedov A, Zhang L, et al. Prostate stereotactic ablative body radiotherapy using a standard linear accelerator: toxicity, biochemical, and pathological outcomes. Radiother Oncol. 2013;107(2):153-8.
22. Crane CH. Hypofractionated ablative radiotherapy for locally advanced pancreatic cancer. J Radiat Res. 2016;57 Suppl 1:i53-i7.
23. Ball D, Mai GT, Vinod S, Babington S, Ruben J, Kron T, et al. Stereotactic ablative radiotherapy versus standard radiotherapy in stage 1 non-small-cell lung cancer (TROG 09.02 CHISEL): a phase 3, open-label, randomised controlled trial. Lancet Oncol. 2019;20(4):494-503.
24. Siva S, Louie AV, Warner A, Muacevic A, Gandhidasan S, Ponsky L, et al. Pooled analysis of stereotactic ablative radiotherapy for primary renal cell carcinoma: A report from the International Radiosurgery Oncology Consortium for Kidney (IROCK). Cancer. 2018;124(5):934-42.
25. Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet. 2019;393(10185):2051-8.
26. Phillips R, Shi WY, Deek M, Radwan N, Lim SJ, Antonarakis ES, et al. Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer: The ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol. 2020.
27. Marcu LG. Altered fractionation in radiotherapy: from radiobiological rationale to therapeutic gain. Cancer Treat Rev. 2010;36(8):606-14.
28. Overgaard J. Hypoxic modification of radiotherapy in squamous cell carcinoma of the head and neck--a systematic review and metaanalysis. Radiother Oncol. 2011;100(1):22-32.
29. Demaria S, Formenti SC. Radiation as an immunological adjuvant: current evidence on dose and fractionation. Front Oncol. 2012;2:153.
30. Siva S, MacManus MP, Martin RF, Martin OA. Abscopal effects of radiation therapy: a clinical review for the radiobiologist. Cancer Lett. 2015;356(1):82-90.
31. McMahon SJ. The linear quadratic model: usage, interpretation and challenges. Phys Med Biol. 2018;64(1):01TR.
32. Fowler JF. 21 years of biologically effective dose. Br J Radiol. 2010;83(991):554-68.
33. Turley SJ, Cremasco V, Astarita JL. Immunological hallmarks of stromal cells in the tumor microenvironment. Nat Rev Immunol. 2015;15(11):669-82.
34. Jiménez-Sánchez A, Memon D, Pourpe S, Veeraraghavan H, Li Y, Vargas HA, et al. Heterogeneous Tumor-Immune Microenvironments among Differentially Growing Metastases in an Ovarian Cancer Patient. Cell. 2017;170(5):927-38.e20.
35. Barker HE, Paget JT, Khan AA, Harrington KJ. The tumor microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer. 2015;15(7):409-25.
36. Vanpouille-Box C, Alard A, Aryankalayil MJ, Sarfraz Y, Diamond JM, Schneider RJ, et al. DNA exonuclease Trex1 regulates radiotherapyinduced tumor immunogenicity. Nature Communications. 2017;8(1):15618.
37. Rudqvist NP, Pilones KA, Lhuillier C, Wennerberg E, Sidhom JW, Emerson RO, et al. Radiotherapy and CTLA-4 Blockade Shape the TCR Repertoire of Tumor-Infiltrating T Cells. Cancer Immunol Res. 2018;6(2):139-50.
38. Klug F, Prakash H, Huber PE, Seibel T, Bender N, Halama N, et al. Low-dose irradiation programs macrophage differentiation to an iNOS(+)/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. 2013;24(5):589-602.
39. Barsoumian HB, Ramapriyan R, Younes AI, Caetano MS, Menon H, Comeaux NI, et al. Low-dose radiation treatment enhances systemic antitumor immune responses by overcoming the inhibitory stroma. Journal for ImmunoTherapy of Cancer. 2020;8(2):e000537.
40. Markovsky E, Budhu S, Samstein RM, Li H, Russell J, Zhang Z, et al. An Antitumor Immune Response Is Evoked by Partial-Volume Single-Dose Radiation in 2 Murine Models. Int J Radiat Oncol Biol Phys. 2019;103(3):697-708.
41. Menon H, Chen D, Ramapriyan R, Verma V, Barsoumian HB, Cushman TR, et al. Influence of low-dose radiation on abscopal responses in patients receiving high-dose radiation and immunotherapy. Journal for ImmunoTherapy of Cancer. 2019;7(1):237.
42. Herrera FG, Ronet C, Ochoa de Olza M, Barras D, Crespo I, Andreatta M, et al. Low Dose Radiotherapy Reverses Tumor Immune Desertification and Resistance to Immunotherapy. Cancer Discovery. 2021:candisc.0003.2021.
43. Huang AC, Postow MA, Orlowski RJ, Mick R, Bengsch B, Manne S, et al. T-cell invigoration to tumor burden ratio associated with anti- PD-1 response. Nature. 2017;545(7652):60-5.
44. Joseph RW, Elassaiss-Schaap J, Kefford R, Hwu WJ, Wolchok JD, Joshua AM, et al. Baseline Tumor Size Is an Independent Prognostic Factor for Overall Survival in Patients with Melanoma Treated with Pembrolizumab. Clin Cancer Res. 2018;24(20):4960-7.
45. Robert C, Ribas A, Hamid O, Daud A, Wolchok JD, Joshua AM, et al. Durable Complete Response After Discontinuation of Pembrolizumab in Patients With Metastatic Melanoma. J Clin Oncol. 2018;36(17):1668-74.
46. Keam SP, Halse H, Nguyen T, Wang M, Van Kooten Losio N, Mitchell C, et al. High dose-rate brachytherapy of localized prostate cancer converts tumors from cold to hot. J Immunother Cancer. 2020;8(1).
47. Wei SC, Duffy CR, Allison JP. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018;8(9):1069-86.
48. Chen S, Wainwright DA, Wu JD, Wan Y, Matei DE, Zhang Y, et al. CD73: an emerging checkpoint for cancer immunotherapy. Immunotherapy. 2019;11(11):983-97.
49. Zappasodi R, Sirard C, Li Y, Budhu S, Abu-Akeel M, Liu C, et al. Rational design of anti-GITR-based combination immunotherapy. Nat Med. 2019;25(5):759-66.