Abstract
Study on ‘‘cytokine storms’’ has been braced up in infectious diseases. The pertinence of this research begins to be evident in tuberculosis as it was observed that increased levels of Interleukin-6 (IL-6) were connected with disease severity. The IL-6 blockers therapeutics approaches for tuberculosis are currently a key line of research, with many in progress clinical trials, as IL-6 has become an important factor of the immune response to tuberculosis. Here, we focus on the role of IL-6 in tuberculosis.
Keywords
Tuberculosis, IL-6, Cytokine storms, Immune response, Disease severity
Introduction
Over 10 million lives developed tuberculosis (TB) in the world and one out of four people are carrier of latent TB [1]. TB remains a worldwide disease, causing an estimated of 9.8 million deaths per year [2]. A characteristic of TB infection is a ‘‘cytokine storm’’. The term “cytokine storm” is more and more used by both popular media and Authors of scientific articles. The expression “cytokine storm” was first used to describe the response of uncontrolled systemic inflammation in graft versus host disease. Cytokine storms represent a wide spectrum of neurodegenerative and neoplastic diseases, infectious and non-infectious diseases. The status of systemic hyperactivated immunity in ‘‘cytokine storm’’ are the results of organ dysfunction, vascular leakage, transaminitis, coagulopathy and death. The abundance of numerous cytokines is implicated in this uncontrolled systemic inflammatory response, IL-6 plays an important role in its signaling pathways and pathophysiology. IL-6 signaling is studied extensively elsewhere [3].
IL-6 accumulates with other cytokines (IL-6, TNF-α and IL-1), which are crucial inducers of the acute phase response. The signalization of IL-6 is done via soluble and membrane bound IL-6R, specifically through gp130 [4]. A phosphorylation cascade implicating janus kinase/signal transducer (JAK/STAT) pathway mediates downstream signaling [5]. Afterwards, IL-6- STAT3-NF-κB pathway activation increases IL-6-regulated gene expression by provoking a pro-inflammatory arsenal including IL-18 and MCP-1. Mycobacterium tuberculosis provokes the release of IL-6 through mitogen-activated protein kinases (P38 MAPK) activation. IL-6 is generated by several types of immune cells, stromal cells, and tumor cells. In this study, we focus on TB and discuss about the involvement of IL-6 in the inflammatory storm. We also summarize the new IL-6 blocker treatments for TB.
TB and Inflammatory Storm
TB causes a fibrosis and chronic inflammation which might drive to genetic changes and mutations. TB diseases implicate the parenchyma tissue of the lung, continuous cough, vascular morphological variations, lymphocytosis processes, and the generation of immune system mediators such as interleukins, are all among the components driving to the assumption regarding the implication of TB in lung cancer [6-8]. Study in patients with immune deficiency, reported that induction of necrosis and apoptosis appear to drive to an augmentation in TNF-α and IL-17, which will increase the Bcl-2 expression or lower P53 activity, diminish Bax-T and contribute to the impediment of caspase-3 expression because of the low expression of cytochrome oxidase in mitochondria [7,9].
TB is characterized by x-ray images and clinical symptoms (loss of appetite, fever, weight loss, continuous cough, hemoptysis, chest pain, and dyspnea) [10-12]. Because of nontuberculous mycobacteria (NTM), correct diagnosis is necessary for TB and respiratory infections. Actually, lung NTM or lung cancers infections are some of the cases of TB drugresistance. Briefly, TB is believed to be one of the risk factors for certain diseases such as diabetes, cancer and HIV. Among the most substantial solutions for the respiratory diseases and TB diagnosis are acid-fast staining and histological study of phlegm and bronchial lavage, culture and polymerase chain reaction [13,14].
In these instances, a chronic inflammatory environment contributes to the neoplastic change. In order to comprehend how to block this process, it is substantial to know what component or components lead this transition and how this can be impeded. Many studies indicate IL-6 as a principal conductor responsible for TB. The role of IL-6 as a key mediator of TB has been extensively investigated; thus, the following section will focus on TB to illustrate the major role of IL-6 in TB risk and prognosis.
In lung of TB patients, IL-6 acts directly on lung epithelial cells via the NF-κB signaling pathway under conditions of inflammation and TB infection. IL-6 in turn promotes TB production and migration via STAT3 signaling [15]. A complementary source of IL-6 in TB is CD4+ T lymphocytes [16,17]. Increased levels of circulating IL-6 in patients infected by M. tuberculosis predicts TB risk. Moreover, elevated circulating levels of IL-6 are a biomarker of unfavorable TB patients [18].
Except its role in M. tuberculosis, ‘‘cytokine storm’’ is a toxic side effect of T cell engaged therapy. Once more, IL-6 has become a principal driver of inflammatory storm associated with TB. Furthermore, IL-6 from monocytes and macrophages induces ‘‘cytokine storm’’ in response to T-cell engaged therapy or other conditions.
IL-6 in TB
The pertinence of this study begins to be evident in TB as it was observed that increased levels of IL-6 were connected with disease severity and contributed to complications such as acute respiratory distress syndrome (ARDS). IL-6 is also correlated with increased bacterial load, and elevated levels are found in severe disease [19]. Monocyte derived and recruited macrophages produces IL-6 [20]. IL-6 cumulates with other cytokines, including IL-1, TNF-α and IL-6 which are important provokers of the acute phase response. IL-6 is pivotal in the protection against murine M. tuberculosis infection, because of the impact of the response of CD4+ T cells, [16]. Increased bacterial loads and a changed type 1 T helper response was observed in IL-6 deficient animals infected with M. tuberculosis [21]. IL-6 secreted by macrophages infected with M. tuberculosis represses the responses of uninfected macrophages to IFN-γ [22].
In the field of lung cancer, studies imply that early on in infection alveolar type II pneumocytes produces IL-6, later macrophage produce IL-6 which participate to severe disease phenotypes [23]. Moreover, finding stipulate that increased levels of IL-6 is a modification from alveolar resident macrophages to macrophages derived and recruited from IL- 6-producing monocytes, which are found in bronchoalveolar lavage samples from people with severe disease [24]. A key participator to TB pathogenesis is IL-6-induced immune dysregulation characterized by lymphocytic dysregulation with CD4+ lymphopenia [25] and also by production of proinflammatory cytokines downstream of IL-6 by monocytes [26].
IL-6 Blocker Therapeutics Approaches for TB
The blockade of IL-6 has been displayed in the therapeutical plan successfully in numerous rheumatological diseases, malignancies and rheumatoid arthritis [3,27]. For the success of TB treatment, IL-6 was shown to be an important biomarker [28] (Figure 1 and Table 1). In order to discover common mechanisms that might also repress TB, a growing number of repurposed drugs are going through clinical trials.
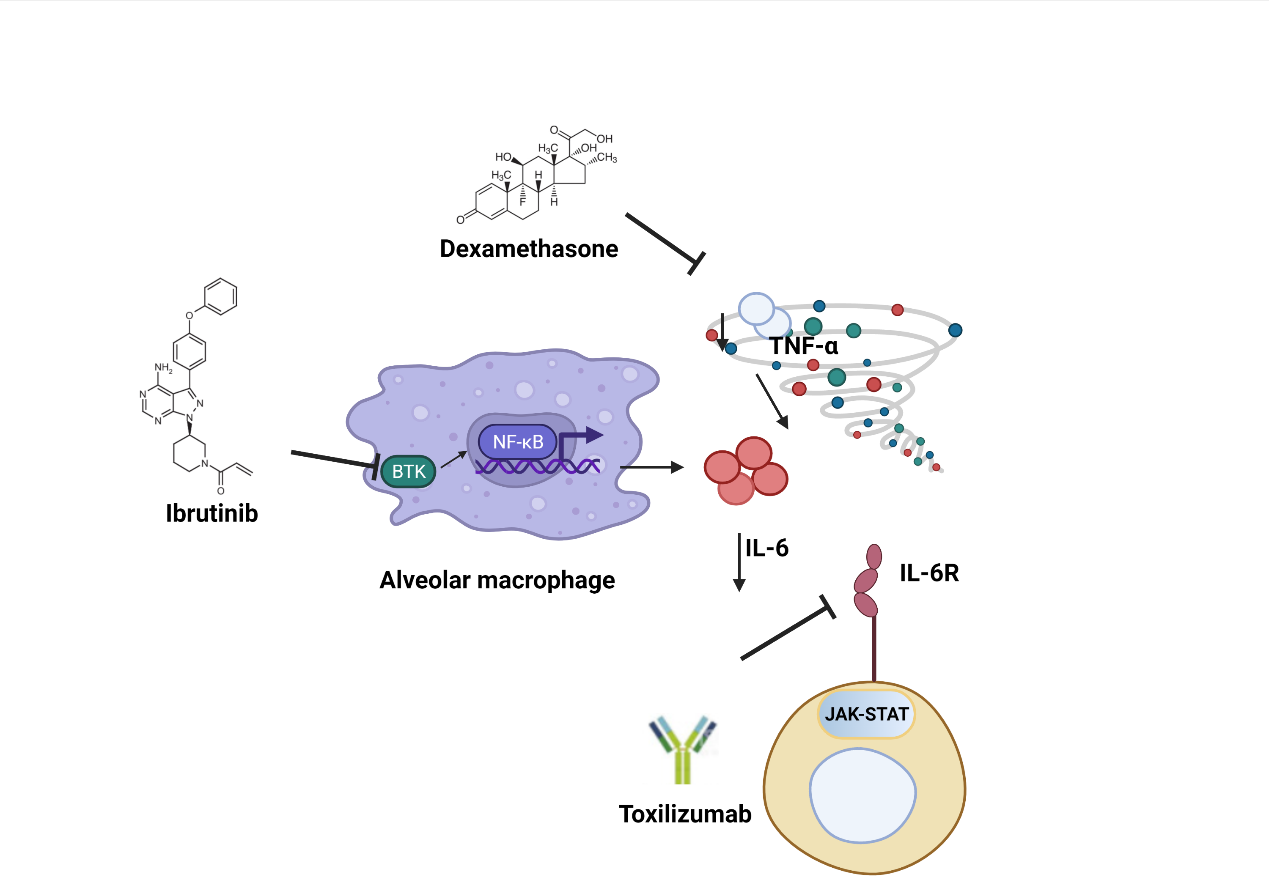
Figure 1: IL-6 Blockers Therapeutics Approaches for TB. Inhibition of BKT (Ibrutinib) impedes NF-κB signaling and is a consequence of diminished proliferation of IL-6. Corticosteroids (Dexamethasone) impede protein secretion and expression of TNF-α-mediated IL-6 mRNA by lowering the stability of IL-6 mRNA. Tocilizumab (IL-6R monoclonal antibodies) dampen both the trans-signaling and classic IL-6 pathways to repress IL-6-JAK-STAT signaling. Figure created with Biorender.
| Repurposed Anti-IL-6 | Clinical trials | Treatment | Affected pathways |
|---|---|---|---|
| IL-6 inhibitors | |||
| Tocilizumab | A phase III randomized controlled trial, COVACTA (NCT01232569) | TB | JAK-STAT signaling |
| Corticosteroids | |||
| Dexamethasone | phase-III and IV multicenter trials (NCT0310 0786; NCT03092817; NCT02588196) | TB meningitis | TNF-a and HDT |
| Prednisolone | a phase-III trial (NCT00810849) | TB pericarditis in HIV infection | |
| BTK inhibitors | |||
| Ibrutinib | M. tuberculosis in macrophages | NF-κB signaling | |
Table 1: Repurposed Anti-IL-6 therapeutics.
IL-6 inhibitors
Tocilizumab is a humanized anti-IL-6 receptor IgG1 monoclonal antibody which represses both the trans-signaling and the classic pathways and is approved for rheumatoid arthritis treatment and other chronic inflammatory diseases. The antimycobacterial activity of IL-6 can be decreased by tocilizumab [29]. In the treatment of TB patients, the safeness and effectiveness of tocilizumab was identified in a recent finding [30]. Tocilizumab is undergoing a phase III randomized controlled clinical trial, COVACTA (NCT01232569) [31].
Bruton tyrosine kinase inhibitors
Controlled by toll-like receptors that recognize viral genomes, bruton tyrosine kinase (BTK) provoke NF-κB signaling which end up at the production of chemokine and cytokine, including IL-6. Ibrutinib is a BTK inhibitor which was used for the treatment of specific B cell malignancies. Findings revealed that inhibition of BTK in lymphoma leads to the side effect of an invasion of aspergillosis infection, however it is usually regulated by monocytes, neutrophils, and macrophages. This raised the probability that BTK inhibitors contribute to the regulation of the inflammatory response of these cell types that are preponderant in TB [32].
In macrophages derived from patients with chronic lymphocytic leukemia, ibrutinib modifies the secretion of TNF-α and influences the polarization towards the pro-inflammatory profile against irradiated M. tuberculosis. In addition, ibrutinibtreated γδ T cells revealed remarkably decreased activation, as indicated by the weak expression of the activation marker CD69 and weak secretion of IFN-γ, furnishing a better sapience of the risk of contagious complications in ibrutinib-treated chronic lymphocytic leukemia patients [33]. The target of BTK inhibition are the activation of pathological monocyte and macrophage which reduce the “cytokine storm,” conducting to better results. Ibrutinib represses intracellular M. tuberculosis growth by provoking the autophagy of macrophages [34].
Corticosteroids
Belonging to a class of steroid hormones, corticosteroids exhibit an anti-inflammatory activity via the connection of the cytoplasmic receptor of corticosteroid, which contribute to the regulation of anti-inflammatory genes transcription. The reduction of IL-6 mRNA stability is done by corticosteroids, which hinder the expression and protein secretion of TNF-α- mediated IL-6 mRNA [35].
As immunoadjuvants to standard TB therapy, numerous researches have shown corticosteroids to be beneficial. In particular, trials testing the efficacy of adjunctive dexamethasone treatment on the risk of death or disability in TB meningitis demonstrated improved patient survival rate [36,37]. Recent study suggest that dexamethasone impede necrotic cell death of cells infected with M. tuberculosis by facilitating mitogen-activated protein kinase phosphatase 1 (MKP-1)-dependent dephosphorylation of p38 MAPK [38].
The phase-III and IV multicenter trials are underway (NCT02588196; NCT03100786; NCT03092817). Prednisolone was investigated for TB pericarditis treatment in HIV infection, in a phase-III trial (NCT00810849). Prednisolone remarkably decreased the levels of IL-6 in plasma by 8 hours of treatment [39]. In general, it is clear that further research is needed to obtain conclusive results concerning the risks and benefits of corticosteroids as an adjunctive therapy for TB disease.
Conclusion & Future Perspective
Considering the role of IL-6 in the immune response to TB, it is important to understand the release of IL-6 mediated by monocytes and macrophages as part of the "cytokine storm" has played a role in a better comprehension of TB. The cytokine storm is real in the development of severe form of the disease in TB patients. Identification of the repurposed drugs that block IL-6 proliferation may contribute to the attenuation of the “cytokine storm” from TB. The incidence of disease might be decreased in TB patients, as TB researches are making IL-6 blocker therapeutic approaches including tocilizumab.
Declaration of Competing Interest
The authors declare that there are no conflicts of interest.
References
2. World Health Organization. Global tuberculosis report 2013. World Health Organization; 2013.
3. Choy EH, De Benedetti F, Takeuchi T, Hashizume M, John MR, Kishimoto T. Translating IL-6 biology into effective treatments. Nature Reviews Rheumatology. 2020 Jun;16(6):335-45.
4. Heinrich PC, Behrmann I, Haan S, Hermanns HM, Müller- Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochemical Journal. 2003 Aug 15;374(1):1-20.
5. Domingo-Gonzalez R, Das S, Griffiths KL, Ahmed M, Bambouskova M, Gopal R, et al. Interleukin-17 limits hypoxiainducible factor 1α and development of hypoxic granulomas during tuberculosis. JCI insight. 2017 Oct 5;2(19):e92973.
6. Engels EA, Shen M, Chapman RS, Pfeiffer RM, Yu YY, He X, Lan Q. Tuberculosis and subsequent risk of lung cancer in Xuanwei, China. International Journal of Cancer. 2009 Mar 1;124(5):1183-7.
7. Fried LE, Arbiser JL. Honokiol, a multifunctional antiangiogenic and antitumor agent. Antioxidants & Redox Signaling. 2009 May 1;11(5):1139-48.
8. Liang HY, Li XL, Yu XS, Guan P, Yin ZH, et al. Facts and fiction of the relationship between preexisting tuberculosis and lung cancer risk: a systematic review. International Journal of Cancer. 2009 Dec 15;125(12):2936-44.
9. Liuzzo G, Trotta F, Pedicino D. Interleukin-17 in atherosclerosis and cardiovascular disease: the good, the bad, and the unknown. European Heart Journal. 2013 Feb 21;34(8):556-9.
10. Ordonez AA, Tucker EW, Anderson CJ, Carter CL, Ganatra S, Kaushal D, et al. Visualizing the dynamics of tuberculosis pathology using molecular imaging. The Journal of Clinical Investigation. 2021 Mar 1;131(5).
11. Wang K, Ren D, Qiu Z, Li W. Clinical analysis of pregnancy complicated with miliary tuberculosis. Annals of Medicine. 2022 Dec 31;54(1):71-9.
12. Xu H, Yang HM, Liu JR, Liu H, Shen YL, Zhao SY, et al. Clinical features of children with post-primary tuberculosis. Zhonghua er ke za zhi= Chinese Journal of Pediatrics. 2022 Apr 1;60(4):307-10.
13. Nasiri MJ, Dabiri H, Darban-Sarokhalil D, Hashemi Shahraki A. Prevalence of non-tuberculosis mycobacterial infections among tuberculosis suspects in Iran: systematic review and meta-analysis. PloS One. 2015 Jun 8;10(6):e0129073.
14. Song YH, Li Q, Ma LP, Liu RM, Jiang GL, Li Q, et al. Performance of the Xpertreg; MTB/RIF assay in the rapid diagnosis of tracheobronchial tuberculosis using bronchial washing fluid. Journal of International Medical Research. 2020 Oct;48(10):0300060520921640.
15. Li J, Cao C, Xiang Y, Hong Z, He D, Zhong H, et al. TLT2 suppresses Th1 response by promoting IL-6 production in monocyte through JAK/STAT3 signal pathway in tuberculosis. Frontiers in Immunology. 2020:2031.
16. Dienz O, Rincon M. The effects of IL-6 on CD4 T cell responses. Clinical Immunology. 2009 Jan 1;130(1):27-33.
17. Linge I, Tsareva A, Kondratieva E, Dyatlov A, Hidalgo J, Zvartsev R, Apt A. Pleiotropic Effect of IL-6 Produced by B-Lymphocytes During Early Phases of Adaptive Immune Responses Against TB Infection. Frontiers in Immunology. 2022;13:750068.
18. Gupte AN, Kumar P, Araújo-Pereira M, Kulkarni V, Paradkar M, Pradhan N, et al. Baseline IL-6 is a biomarker for unfavourable tuberculosis treatment outcomes: a multisite discovery and validation study. European Respiratory Journal. 2022 Apr 1;59(4).
19. Del Valle DM, Kim-Schulze S, Huang HH, Beckmann ND, Nirenberg S, Wang B, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nature Medicine. 2020 Oct;26(10):1636-43.
20. Martinez AN, Mehra S, Kaushal D. Role of interleukin 6 in innate immunity to Mycobacterium tuberculosis infection. The Journal of Infectious Diseases. 2013 Apr 15;207(8):1253-61.
21. Saunders BM, Frank AA, Orme IM, Cooper AM. Interleukin-6 induces early gamma interferon production in the infected lung but is not required for generation of specific immunity to Mycobacterium tuberculosis infection. Infection and Immunity. 2000 Jun 1;68(6):3322-6.
22. Nagabhushanam V, Solache A, Ting LM, Escaron CJ, Zhang JY, Ernst JD. Innate inhibition of adaptive immunity: Mycobacterium tuberculosis-induced IL-6 inhibits macrophage responses to IFN-γ. The Journal of Immunology. 2003 Nov 1;171(9):4750-7.
23. Huang J, Hume AJ, Abo KM, Werder RB, Villacorta-Martin C, Alysandratos KD, et al. SARS-CoV-2 infection of pluripotent stem cell-derived human lung alveolar type 2 cells elicits a rapid epithelial-intrinsic inflammatory response. Cell Stem Cell. 2020 Dec 3;27(6):962-73.
24. Bost P, Giladi A, Liu Y, Bendjelal Y, Xu G, David E, et al. Hostviral infection maps reveal signatures of severe COVID-19 patients. Cell. 2020 Jun 25;181(7):1475-88.
25. Al-Aska AI, Al-Anazi AR, Al-Subaei SS, Al-Hedaithy MA, Barry MA, Somily AM, et al. CD4+ T-lymphopenia in HIV negative tuberculous patients at King Khalid University Hospital in Riyadh, Saudi Arabia. European Journal of Medical Research. 2011 Dec;16(6):285-8.
26. Obeagu EI, Okoroiwu IL, Nwanjo HU, Nwosu DC. Evaluation of interferon-gamma, interleukin 6 and interleukin 10 in tuberculosis patients in Umuahia. Ann Clin Lab Res. 2019;7(2):307.
27. Schinnerling K, Aguillón JC, Catalán D, Soto L. The role of interleukin-6 signalling and its therapeutic blockage in skewing the T cell balance in rheumatoid arthritis. Clinical & Experimental Immunology. 2017 Jul;189(1):12-20.
28. Singh PP. Interleukin-6: a potential biomarker of the success of tuberculosis treatment. International Journal of Infectious Diseases. 2016 Apr 1;45:413.
29. Cantini F, Nannini C, Niccoli L, Petrone L, Ippolito G, Goletti D. Risk of tuberculosis reactivation in patients with rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis receiving non-anti-TNF-targeted biologics. Mediators of Inflammation. 2017 Oct;2017.
30. Lin CT, Huang WN, Hsieh CW, Chen YM, Chen DY, Hsieh TY, et al. Safety and effectiveness of tocilizumab in treating patients with rheumatoid arthritis–A three-year study in Taiwan. Journal of Microbiology, Immunology and Infection. 2019 Feb 1;52(1):141- 50.
31. Kivitz A, Olech E, Borofsky M, Zazueta BM, Navarro-Sarabia F, Radominski SC, et al. Subcutaneous tocilizumab versus placebo in combination with disease-modifying antirheumatic drugs in patients with rheumatoid arthritis. Arthritis Care & Research. 2014 Nov;66(11):1653-61.
32. Chamilos G, Lionakis MS, Kontoyiannis DP. Call for action: invasive fungal infections associated with ibrutinib and other small molecule kinase inhibitors targeting immune signaling pathways. Clinical Infectious Diseases. 2018 Jan 1;66(1):140-8.
33. Ye B, Zhou C, Guo H, Zheng M. Effects of BTK signalling in pathogenic microorganism infections. Journal of Cellular and Molecular Medicine. 2019 Oct;23(10):6522-9.
34. Hu Y, Wen Z, Liu S, Cai Y, Guo J, Xu Y, et al. Ibrutinib suppresses intracellular mycobacterium tuberculosis growth by inducing macrophage autophagy. Journal of Infection. 2020 Jun 1;80(6):e19-26.
35. Quante T, Ng YC, Ramsay EE, Henness S, Allen JC, Parmentier J, et al. Corticosteroids reduce IL-6 in ASM cells via up-regulation of MKP-1. American Journal of Respiratory Cell and Molecular Biology. 2008 Aug;39(2):208-17.
36. Schutz C, Davis AG, Sossen B, Lai RP, Ntsekhe M, Harley YX, et al. Corticosteroids as an adjunct to tuberculosis therapy. Expert Review of Respiratory Medicine. 2018 Oct 3;12(10):881-91.
37. Viarasilpa T, Tongyoo S, Permpikul C. Effect of adjunctive corticosteroid therapy on outcomes in pulmonary tuberculosis patients with acute respiratory failure: a cohort study. Clinical Critical Care. 2021 Aug 31;29.
38. Gräb J, Suárez I, van Gumpel E, Winter S, Schreiber F, Esser A, et al. Corticosteroids inhibit Mycobacterium tuberculosis-induced necrotic host cell death by abrogating mitochondrial membrane permeability transition. Nature Communications. 2019 Feb 8;10(1):1-4.
39. Shenje J, Lai RP, Ross IL, Mayosi BM, Wilkinson RJ, Ntsekhe M, et al. Effect of prednisolone on inflammatory markers in pericardial tuberculosis: A pilot study. IJC Heart & Vasculature. 2018 Mar 1;18:104-8.
