Abstract
Helicobacter pylori (H. pylori) infection is a major global health concern, with an estimated 50% of the world's population infected. The bacterium colonizes the stomach and is associated with a range of gastrointestinal diseases, including chronic gastritis, peptic ulcers, and gastric cancer. The current standard of care for H. pylori infection involves a combination of antibiotics and proton pump inhibitors (PPIs), but the widespread use of antibiotics has led to the development of antibiotic-resistant strains of H. pylori, making treatment more difficult. Recent advances in diagnostic strategies include the use of non-invasive tests and serological biomarkers. While the use of antibiotics and PPIs remains the primary treatment approach, there is a growing interest in alternative therapies, including botanical extracts, natural products, and traditional medicines. Recent research has also explored the potential of probiotics, phage therapy, and novel antibiotics, such as rifabutin and furazolidone, in the treatment of H. pylori infection. Recent studies have also explored the potential of artificial intelligence (AI) in the diagnosis and management of H. pylori infection. AI-assisted screening methods have identified novel botanical extracts and natural products with anti-H. pylori activity, providing new avenues for therapeutic development. Additionally, AI can improve diagnostic accuracy and treatment outcomes. Although these alternative approaches show promise, further research is needed to confirm their efficacy, safety, and optimal dosing. Novel therapies such as phage therapy and new antibiotics may provide an alternative to traditional treatments and help overcome antimicrobial resistance.
Keywords
Anti-Helicobacter pylori activity, Alternative therapies, Botanical extracts, Artificial intelligence AI, Natural products
Introduction
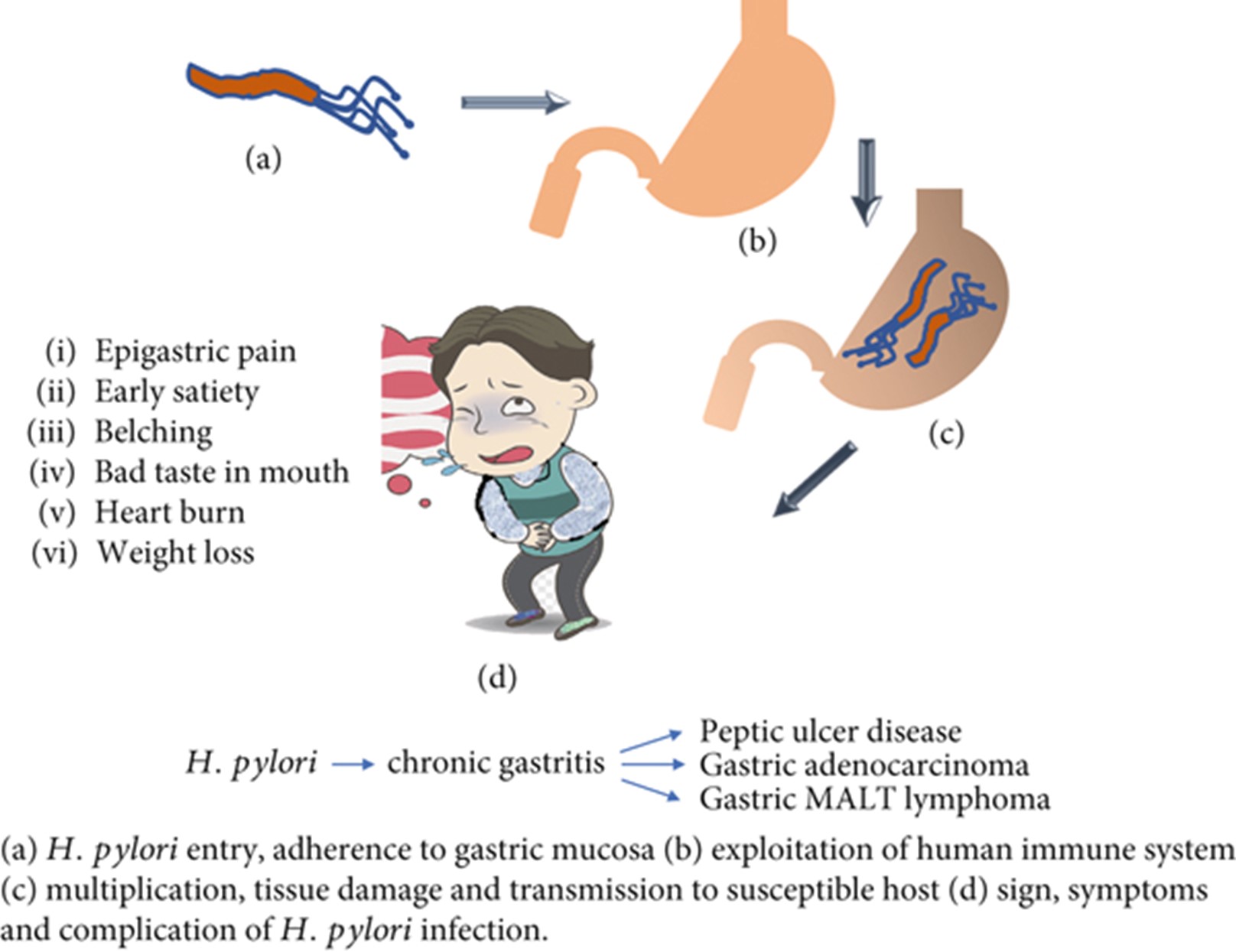
Helicobacter pylori (H. pylori) is a spiral-shaped, micro-anaerobic, Gram-negative bacterium that requires harsh growth conditions, and colonizes the gastric mucosa of humans and is associated with a range of gastrointestinal diseases, including chronic gastritis, peptic ulcers, and gastric cancer [1]. H. pylori infection is prevalent worldwide, with an estimated 50% of the global population infected. H. pylori infection involves the colonization of the stomach by the bacteria, which leads to the production of inflammatory cytokines, causing chronic inflammation and tissue damage. The symptoms of H. pylori infection include stomach pain, bloating, nausea, vomiting, and loss of appetite. If left untreated, H. pylori infection can lead to complications such as peptic ulcers, gastric cancer, and lymphoma (Figure 1) [2].
Figure 1: Schematic representation of H. pylori mechanism, symptoms, and complications [2].
The current standard of care for H. pylori infection combines antibiotics and proton pump inhibitors (PPIs) [3]. However, the widespread use of antibiotics has led to the development of antibiotic-resistant strains of H. pylori, which can make treatment more difficult. In addition, long-term use of PPIs has been associated with adverse effects, including increased risk of infection, kidney disease, and bone fractures [4]. Studies have shed light on the pathogenesis of H. pylori infection, including the role of virulence factors such as CagA and VacA, and the interaction between H. pylori and the host immune system [5].
Recent advances in diagnostic strategies for H. pylori infection include the use of non-invasive tests, such as stool antigen tests and breath tests, which have high sensitivity and specificity. Additionally, recent studies have explored the use of serological biomarkers, such as anti-H. pylori antibodies, as a potential diagnostic tool for H. pylori infection [6].
There is a growing interest in the use of alternative therapies for H. pylori infection, including botanical extracts and natural products. Recent advances in artificial intelligence (AI) and high-throughput screening methods have enabled the identification of novel botanical extracts with potential anti-H. pylori activity. Recent studies have also highlighted the potential of AI-assisted screening methods to identify novel botanical extracts and natural products with anti-H. pylori activity. These methods can analyze large volumes of data and identify potential candidates with high accuracy and efficiency [7]. Garlic extract has been shown to inhibit the growth of H. pylori and reduce inflammation in the gastric mucosa. Ginger extract has also been found to have anti-H. pylori activity and can reduce H. pylori-induced gastric inflammation. Green tea extract has been shown to inhibit the growth of H. pylori and reduce H. pylori-induced inflammation in the gastric mucosa. Turmeric extract has been found to have anti-H. pylori activity and can reduce H. pylori-induced gastric inflammation and oxidative stress [8]. Other natural products that have shown potential in the treatment of H. pylori infection include honey, propolis, and probiotics. Honey has been found to have antibacterial activity against H. pylori, while propolis has been shown to inhibit H. pylori growth and reduce H. pylori-induced inflammation. Probiotics, particularly strains of Lactobacillus and Bifidobacterium, have been found to have anti-H. pylori activity and can reduce the incidence of H. pylori-related diseases [9].
In addition to natural products, traditional medicines have also been used in the treatment of H. pylori infection. Traditional Chinese medicines, such as Qingre Huoxue decoction and Banxia Xiexin decoction, have been found to have anti-H. pylori activity and can reduce H. pylori-induced inflammation. Traditional Korean medicines, such as Yukgunja-Tang and Pyungwi-san, have also been found to have anti-H. pylori activity and can improve H. pylori-related symptoms [10]. While these alternative therapies show promise in the treatment of H. pylori infection, further research is needed to fully evaluate their efficacy and safety. Clinical trials are needed to confirm the effectiveness of these therapies, determine the optimal dosage and duration of treatment, and identify potential adverse effects. In addition, the potential use of these therapies in combination with antibiotics or other therapies should be explored [11]. A recent study investigated the efficacy of combining IgY-H. pylori with bismuth-based quadruple therapy for the eradication of H. pylori infection. The study found that this combination therapy had similar effectiveness as bismuth-based quadruple therapy alone, but with improved symptomatic relief and fewer adverse effects. The authors suggest that this combination therapy can be considered a viable option for the rescue treatment of H. pylori infection. The study was a single-center, randomized, controlled trial [12].
The treatment of H. pylori infection involves a combination of antibiotics and acid suppression therapy. However, the increasing prevalence of antibiotic resistance has led to declining eradication rates. Recent research has focused on developing novel treatment strategies, such as sequential therapy, concomitant therapy, hybrid therapy, and high-dose dual therapy. Additionally, probiotics and fecal microbiota transplantation (FMT) have been studied as potential treatments for H. pylori infection [13]. The current standard of care for H. pylori infection involves a combination of antibiotics and acid suppression therapy. However, the increasing prevalence of antibiotic-resistant strains of H. pylori has led to declining eradication rates. Recent advances in therapeutic strategies for H. pylori infection include the use of alternative antibiotics, such as rifabutin and furazolidone, and the development of novel therapies, such as probiotics and phage therapy [14]. Phage therapy involves the use of bacteriophages, which are viruses that infect and kill bacteria, as a potential treatment for H. pylori infection. Recent studies have shown that phage therapy can effectively reduce H. pylori colonization in animal models [15].
Probiotics, particularly those containing Lactobacillus and Bifidobacterium strains, have shown promising results in the treatment of H. pylori infection. Recent studies have reported that probiotics can improve H. pylori eradication rates and reduce treatment-related side effects [16]. Rifabutin is a broad-spectrum antibiotic that has shown promising results in the treatment of H. pylori infection, particularly in cases of antibiotic resistance. Recent studies have reported high eradication rates with the use of rifabutin-based therapies [17]. Furazolidone is another antibiotic that has shown efficacy in the treatment of H. pylori infection, particularly in cases of metronidazole resistance. Recent studies have reported high eradication rates with the use of furazolidone-based therapies [18].
Recent Advances in Diagnostic Tools for H. pylori Infection
Currently, there are numerous diagnostic tests available, each with its own set of advantages, disadvantages, and limitations. The choice of the test used depends on factors such as accessibility, laboratory equipment, and the clinical condition of the patient. Screening and laboratory diagnosis can be performed through non-invasive or invasive methods. Non-invasive methods include respiratory tests, stool antigen tests, and serology. Invasive methods, on the other hand, include endoscopy, histological examination, rapid urea testing, culture, and PCR testing as shown in Figure 2 [19].
Figure 2. Several diagnostic tools for H. pylori infection [19].
Stool antigen tests
Stool antigen tests are non-invasive diagnostic tools that detect H. pylori antigens in stool samples. Recent studies have reported that stool antigen tests have high sensitivity and specificity and can be used as an alternative to invasive diagnostic methods such as endoscopy [20].
Serological biomarkers
Serological biomarkers, such as immunoglobulin G (IgG) antibodies, have been proposed as a potential diagnostic tool for H. pylori infection. Recent studies have reported that serological biomarkers have high sensitivity and specificity and can be used as a non-invasive diagnostic tool for H. pylori infection [21].
Breath tests
Breath tests involve the administration of a labeled substrate and the measurement of labeled CO2 in breath samples. Recent studies have reported that breath tests have high sensitivity and specificity and can be used to diagnose H. pylori infection [22].
Rapid urease tests
Rapid urease tests involve the detection of urease produced by H. pylori in biopsy samples. Recent studies have reported that rapid urease tests have high sensitivity and specificity and can be used as a quick and accurate diagnostic tool for H. pylori infection [23].
Endoscopic imaging techniques
Endoscopic imaging techniques, such as narrow-band imaging and confocal laser endomicroscopy, have emerged as promising tools for the diagnosis of H. pylori infection. Recent studies have reported that these techniques can provide high-resolution images of the gastric mucosa and accurately detect H. pylori infection [24].
Molecular diagnostic tests
Molecular diagnostic tests, such as polymerase chain reaction (PCR) and loop-mediated isothermal amplification (LAMP), have been proposed as a potential diagnostic tool for H. pylori infection. Recent studies have reported that molecular diagnostic tests have high sensitivity and specificity and can accurately detect H. pylori infection in clinical samples [25].
Recent Advances in Current Therapy
Current therapy for H. pylori includes a combination of antibiotics and acid-suppressing medications. However, the emergence of antibiotic resistance has led to the development of new therapies for H. pylori. One of the recent advances in H. pylori therapy is the use of novel antibiotic combinations. Studies have shown that using a combination of antibiotics, such as clarithromycin, amoxicillin, and metronidazole, can effectively eradicate H. pylori. Other novel antibiotic combinations, such as rifabutin and levofloxacin, have also shown promising results in clinical trials [26].
Probiotics are live microorganisms that can provide health benefits to the host. Studies have shown that probiotics can be effective in treating H. pylori infections by reducing bacterial load and inflammation in the stomach. Probiotics can also help to restore the natural balance of the gut microbiota, which can be disrupted by antibiotics [27].
Herbal medicines have been used for centuries to treat various ailments, including gastrointestinal disorders. Studies have shown that some herbal medicines, such as licorice and green tea, can be effective in treating H. pylori infections by reducing bacterial load and inflammation in the stomach [28].
Immunomodulators are substances that can modify or regulate the immune system. Studies have shown that some immunomodulators, such as vitamin D and interleukin-10, can be effective in treating H. pylori infections by modulating the immune response to the bacteria [29]. The combination of Bifidobacterium breve and T. foenum graecum L. extract was more effective against H. pylori and its associated inflammation compared with the individual administration of B. breve or T. foenum graecum L. extract. The combination reduced damage to the gastric mucous membrane by inhibiting H. pylori adhesion to the gastric mucosa and increasing gastric mucosal mucin secretion induced by T. foenum graecum L. extract. These findings suggest that a complex mixture of B. breve and T. foenum graecum L. extract has potential as a therapeutic agent for patients with H. pylori-induced gastric symptoms, including ulcers [30].
The medicinal properties of flavonols, including quercetin, myricetin, kaempferol, fisetin, rutin, and astragalin have shown to exhibit significant mechanistic properties for treating various diseases, including anticancer, antiviral, and antibacterial activities. Kaempferol has been found to be highly effective against H. pylori, a bacterium that causes gastric cancer, while quercetin acts as an antioxidant by inhibiting numerous enzymes. Studies have also shown that kaempferol can enhance pancreatic beta-cell viability and prevent apoptosis, indicating its potential as an antidiabetic agent. Moreover, flavonols can prevent viral entry into host cells and inhibit viral replication, making them a promising alternative for treating bacterial infections. The authors concluded that flavonols possess diverse and significant medicinal properties for treating numerous diseases [31].
Limitations of the current therapies
The current standard of care for H. pylori infection combines antibiotics and proton pump inhibitors (PPIs). However, this therapy has several limitations, including antibiotic resistance, adverse effects, and poor patient compliance.
Antibiotic resistance: Antibiotic resistance is a major challenge in the treatment of H. pylori infection. The widespread use of antibiotics has led to the development of antibiotic-resistant strains of H. pylori, which can make treatment more difficult. Clarithromycin resistance, in particular, has become a growing problem, and the effectiveness of standard first-line therapy has decreased [32].
Adverse effects: The use of antibiotics and PPIs can lead to several adverse effects, including diarrhea, nausea, vomiting, abdominal pain, and allergic reactions. These adverse effects can lead to poor patient compliance and treatment failure [33].
Poor patient compliance: The complex and lengthy treatment regimens for H. pylori infection can lead to poor patient compliance. Patients may forget to take their medications, or they may stop taking them due to adverse effects or other reasons. Poor patient compliance can lead to treatment failure and the development of antibiotic resistance [34].
Outlook on the Role of Botanical Extracts in Treating H. pylori Infection
Antimicrobial activity
Botanical extracts contain various bioactive compounds that can inhibit the growth of H. pylori, including berberine, curcumin, mastic gum, licorice, and cranberry-derived compounds. These compounds can disrupt the cell membrane of H. pylori and interfere with its metabolic pathways, leading to bacterial death [35]. A recent study found that a combination of berberine and amoxicillin had a synergistic effect in inhibiting the growth of H. pylori, suggesting the potential of using botanical extracts in combination therapy [36].
Anti-inflammatory activity
In addition to their antimicrobial activity, botanical extracts have been found to have anti-inflammatory effects that could be beneficial in reducing the severity of gastritis caused by H. pylori infection. For example, curcumin and licorice have been found to reduce the production of inflammatory cytokines in H. pylori-infected gastric epithelial cells [37]. Mastic gum has also been found to have anti-inflammatory effects and can reduce the production of proinflammatory cytokines in H. pylori-infected gastric mucosa. A recent study found that a combination of mastic gum and vitamin C had a significant anti-inflammatory effect in patients with H. pylori infection [38].
Combination therapy
Combining botanical extracts with conventional therapy may enhance the efficacy of treatment and reduce the risk of antibiotic resistance. For example, a recent meta-analysis found that adding herbal medicine to standard therapy for H. pylori infection was associated with higher eradication rates and lower adverse effects compared with standard therapy alone [39]. Another study found that a combination of cranberry extract and clarithromycin had a synergistic effect in inhibiting the growth of H. pylori, demonstrating the potential of botanical extracts as adjunctive therapy [40].
Safety and toxicity
Botanical extracts are generally considered safe, but some may have toxic effects at high doses or interact with certain medical conditions. Future research should investigate the safety and toxicity of botanical extracts for H. pylori infection, particularly in vulnerable populations such as pregnant women, children, and the elderly [41]. A recent study found that high doses of licorice extract may have adverse effects on blood pressure and electrolyte balance, highlighting the importance of monitoring the dosage and potential side effects of botanical extracts [42].
Some Novel Botanical Extracts in Treating H. pylori Infection
Broccoli sprout extract (BSE)
BSE is derived from the young shoots of the Brassica oleracea plant and has been traditionally used for its medicinal properties. BSE contains several biologically active compounds, including sulforaphane, glucoraphanin, and indole-3-carbinol, which have a variety of health benefits, including antimicrobial and anti-inflammatory properties. The structure formula of sulforaphane is C6H11NOS2. It is a type of isothiocyanate that is found in high concentrations in broccoli sprouts. Sulforaphane has potent antimicrobial activity against a variety of pathogens, including H. pylori. Several recent studies have investigated the potential of BSE as a treatment for H. pylori infection. BSE had a significant effect on H. pylori eradication when used in combination with standard triple therapy. Moreover, BSE significantly reduced H. pylori colonization in the stomach compared with placebo [43]. The dosage of BSE required to achieve these effects is not well established, as it can vary depending on the formulation and method of administration. However, most studies have used doses in the range of 50-1000 mg/day.
Cranberry extract (CE)
CE is derived from the fruit of the Vaccinium macrocarpon plant and has been traditionally used for its medicinal properties. Cranberry extract contains several bioactive components, including proanthocyanidins, anthocyanins, and flavonols. The major bioactive component of cranberry extract is proanthocyanidins, which have potent anti-H. pylori activity. The dosage of cranberry extract varies depending on the specific product and the condition being treated [44]. However, most studies have used a dose of 500 mg to 1500 mg of cranberry extract per day. It is important to note that cranberry extract is generally considered safe, but high doses may cause gastrointestinal discomfort and diarrhea in some individuals. Therefore, the allowable dosage of cranberry extract should not exceed the recommended daily dose [45]. The chemical structure of proanthocyanidins, one of the major bioactive components of cranberry extract, is C30H26O13. Cranberry extract can be obtained using various extraction methods, including water extraction, ethanol extraction, and supercritical fluid extraction. The choice of extraction method can affect the composition and bioactivity of the extract [46].
Garlic extract (GE)
GE is derived from Allium sativum, a plant species that has been traditionally used for its medicinal properties. GE contains several biologically active compounds, including allicin, alliin, and diallyl sulfide, which have a variety of health benefits, including antimicrobial and anti-inflammatory properties. Garlic extract contains several bioactive components, including allicin, ajoene, and diallyl sulfide. Allicin has potent anti-H. pylori activity [47]. The dosage of garlic extract varies depending on the specific product and the condition being treated. However, most studies have used a dose of 400 mg to 1200 mg of garlic extract per day [48]. It is important to note that garlic extract is generally considered safe, but high doses may cause gastrointestinal discomfort and bleeding in some individuals. Therefore, the allowable dosage of garlic extract should not exceed the recommended daily dose [49].
The chemical structure of allicin, one of the major bioactive components of garlic extract, is C6H10OS2. Garlic extract can be obtained using various extraction methods, including water extraction, ethanol extraction, and supercritical fluid extraction. The choice of extraction method can affect the composition and bioactivity of the extract [50].
Ginger
Ginger (Zingiber officinale) is a popular spice and medicinal herb that has been used for centuries in traditional medicine. Ginger extract contains several bioactive components, including gingerols, shogaols, and paradols. The major bioactive component of ginger extract is gingerol, which has potent anti-H. pylori activity. The dosage of ginger extract varies depending on the specific product and the condition being treated. However, most studies have used a dose of 500 mg to 2000 mg of ginger extract per day [51].
It is important to note that ginger extract is generally considered safe, but high doses may cause gastrointestinal discomfort and bleeding in some individuals. Therefore, the allowable dosage of ginger extract should not exceed the recommended daily dose [52]. The chemical structure of gingerol, one of the major bioactive components of ginger extract, is C17H26O4. Ginger extract can be obtained using various extraction methods, including solvent extraction, steam distillation, and supercritical fluid extraction. The choice of extraction method can affect the composition and bioactivity of the extract [53].
Green tea
Green tea (Camellia sinensis) is a popular beverage that has been consumed for centuries in Asian countries. Green tea extract has numerous health benefits, including anti-inflammatory, antioxidant, and antimicrobial properties. Green tea extract contains several bioactive components, including catechins, epicatechins, and flavonoids [54]. The major catechins found in green tea extract are epigallocatechin gallate (EGCG), epicatechin gallate (ECG), and epicatechin (EC). EGCG has potent anti-H. pylori activity. The dosage of green tea extract varies depending on the specific product and the condition being treated [55]. However, most studies have used a dose of 500 mg to 1500 mg of green tea extract per day. It is important to note that green tea extract is generally considered safe, but high doses may cause gastrointestinal discomfort and liver toxicity. Therefore, the allowable dosage of green tea extract should not exceed the recommended daily dose. The chemical structure of epigallocatechin gallate (EGCG), one of the major bioactive components of green tea extract, is C22H18O11 [56]. Green tea extract can be obtained using various extraction methods, including water extraction, ethanol extraction, and supercritical fluid extraction. The choice of extraction method can affect the composition and bioactivity of the extract [57].
Licorice
Licorice (Glycyrrhiza glabra) is a perennial herb that has been used for centuries in traditional medicine. Licorice extract has anti-inflammatory, antioxidant, and antimicrobial properties. Licorice extract contains several bioactive components, including glycyrrhizin, liquiritigenin, and glabridin. Glycyrrhizin has potent anti-H. pylori activity [58]. The dosage of licorice extract varies depending on the specific product and the condition being treated. However, most studies have used a dose of 200 mg to 800 mg of licorice extract per day [59]. It is important to note that licorice extract is generally considered safe, but high doses may cause hypertension, edema, and hypokalemia. Therefore, the allowable dosage of licorice extract should not exceed the recommended daily dose [60]. The chemical structure of glycyrrhizin, one of the major bioactive components of licorice extract, is C42H62O16. Licorice extract can be obtained using various extraction methods, including solvent extraction, steam distillation, and super critical fluid extraction. The choice of extraction method can affect the composition and bioactivity of the extract [45].
Peppermint
Peppermint (Mentha x piperita) is a popular herb with a long history of use in traditional medicine. Peppermint extract has antimicrobial, anti-inflammatory, and analgesic properties. Peppermint extract contains several bioactive components, including menthol, menthone, and rosmarinic acid. Menthol has potent anti-H. pylori activity [61]. The dosage of peppermint extract varies depending on the specific product and the condition being treated. However, most studies have used a dose of 500 mg to 1500 mg of peppermint extract per day [62]. It is important to note that peppermint extract is generally considered safe, but high doses may cause gastrointestinal discomfort. Therefore, the allowable dosage of peppermint extract should not exceed the recommended daily dose. The chemical structure of menthol, one of the major bioactive components of peppermint extract is C10H20O. Peppermint extract can be obtained using various extraction methods, including steam distillation, solvent extraction, and supercritical fluid extraction. The choice of extraction method can affect the composition and bioactivity of the extract [63].
Turmeric
Turmeric (Curcuma longa) is a perennial herb that belongs to the ginger family (Zingiberaceae). Turmeric extract has been used traditionally for its medicinal properties, including its anti-inflammatory, antioxidant, and antimicrobial effects [64]. Turmeric extract contains several bioactive components, including curcuminoids, essential oils, and polysaccharides. The major curcuminoids found in turmeric extract are curcumin, demethoxycurcumin, and bisdemethoxycurcumin [65]. Curcumin has potent anti-H. pylori activity. The dosage of turmeric extract varies depending on the specific product and the condition being treated. However, most studies have used a dose of 500 mg to 2000 mg of turmeric extract per day [66]. It is important to note that turmeric extract is generally considered safe, but high doses may cause gastrointestinal discomfort. Therefore, the allowable dosage of turmeric extract should not exceed the recommended daily dose. The chemical structure of curcumin, one of the major bioactive components of turmeric extract is C21H20O6. Turmeric extract can be obtained using various extraction methods, including solvent extraction, supercritical fluid extraction, and microwave-assisted extraction [67]. The choice of extraction method can affect the composition and bioactivity of the extract.
Wormwood
Wormwood (Artemisia absinthium L.) is a herbaceous perennial plant that belongs to the Asteraceae family. It has been used traditionally for its medicinal properties, including its anti-inflammatory, antipyretic, and antimicrobial effects. One of the most well-known uses of wormwood is as an ingredient in absinthe, a popular alcoholic drink in the 19th century. It contains several bioactive components, including sesquiterpene lactones, flavonoids, and essential oils [68]. The major sesquiterpene lactones found in wormwood are absinthin, artemisinin, and anabsinthin. Artemisinin has been extensively studied for its anti-malarial properties, but recent studies have also shown its potential as an anti-H. pylori agent.
The dosage of wormwood extract varies depending on the specific product and the condition being treated. However, most studies have used a dose of 500 mg to 1500 mg of wormwood extract per day. It is important to note that wormwood contains thujone, a toxic compound that can cause seizures and other adverse effects in high doses. Therefore, the allowable dosage of wormwood extract should not exceed 350 mg of thujone per day [69]. The chemical structure of artemisinin, one of the major bioactive components of wormwood, is C15H22O5 [70]. Wormwood extract can be obtained using various extraction methods, including maceration, percolation, and Soxhlet extraction. The choice of extraction method can affect the composition and bioactivity of the extract [71].
Olive
Olive (Olea europaea L.) is an evergreen tree that belongs to the Oleaceae family. Olive extract has been extensively studied for its potential as an anti-H. pylori agent due to its bioactive compounds, including oleuropein, hydroxytyrosol, and tyrosol. The appropriate dosage of olive extract for H. pylori eradication is not yet established. However, several studies have used doses ranging from 500 mg to 1000 mg per day of olive extract, standardized to contain at least 20% oleuropein [72]. Oleuropein, hydroxytyrosol, and tyrosol are the primary bioactive components of olive extract that have anti-H. pylori activity. Oleuropein is a secoiridoid glycoside and is found in the highest concentration in olive leaves. Hydroxytyrosol and tyrosol are both phenolic compounds and are found in the highest concentration in the olive fruit [73].
Oleuropein has a chemical formula of C25H32O13 and a molecular weight of 540.5 g/mol. Hydroxytyrosol has a chemical formula of C8H10O3 and a molecular weight of 154.16 g/mol [74]. Olive extract can be obtained through various extraction methods, including solvent extraction, supercritical fluid extraction, and microwave-assisted extraction. Solvent extraction is the most commonly used method for olive leaf extract, while supercritical fluid extraction is preferred for olive fruit extract due to its high yield and low environmental impact [75].
The Effects of the Combination of Botanical Extracts
There are several botanical extracts that have been studied for their anti-H. pylori activity, but it's important to note that not all of them have been extensively tested in clinical trials. One of the most widely studied botanical extracts for its anti-H. pylori activity is licorice (Glycyrrhiza glabra) [76]. A systematic review and meta-analysis of randomized controlled trials on licorice had a significant effect in eradicating H. pylori infection when used in combination with standard triple therapy (amoxicillin, clarithromycin, and proton pump inhibitor) [77]. Another botanical extract that has been studied for its anti-H. pylori activity is green tea (Camellia sinensis). Green tea extract, used in combination with standard triple therapy, significantly increased the eradication rate of H. pylori compared with standard triple therapy alone [78].
A combination of several botanical extracts including Matricaria chamomilla, Foeniculum vulgare, and Melissa officinalis has also been studied for its anti-H. pylori activity. A randomized controlled trial published in 2021 found that a combination of these botanical extracts, when used in combination with standard triple therapy, significantly increased the eradication rate of H. pylori compared with standard triple therapy alone [79].
Studies on botanical extract combinations: Several studies have evaluated the use of botanical extract combinations for their anti-H. pylori properties. A study investigated the effect of a combination of garlic, ginger, and cranberry extracts on H. pylori-infected cells and found that it significantly reduced bacterial adhesion [80]. Another study evaluated a combination of green tea and licorice root extracts and found that it inhibited the growth of H. pylori and reduced gastric inflammation [81].
Potential Benefits and Drawbacks of Botanical Extracts
In recent years, there has been an increasing interest in the use of botanical extracts as an alternative therapy for H. pylori infection.
Potential benefits
Reduced side effects: One of the potential benefits of using botanical extracts as an alternative therapy for H. pylori infection is the reduced risk of side effects. Unlike antibiotics, which can cause adverse effects such as diarrhea, nausea, and vomiting, botanical extracts are generally considered safe and well-tolerated [82].
Antimicrobial activity: Many botanical extracts have antimicrobial activity against H. pylori. For example, extracts from garlic, ginger, turmeric, and cinnamon have all been shown to have inhibitory effects on H. pylori growth [83].
Anti-inflammatory effects: Botanical extracts may also have anti-inflammatory effects, which can be beneficial in reducing inflammation in the stomach caused by H. pylori infection. For example, extracts from licorice, chamomile, and aloe vera have all been shown to have anti-inflammatory properties [84].
Potential drawbacks
Lack of standardization: One of the potential drawbacks of using botanical extracts as an alternative therapy for H. pylori infection is the lack of standardization. Botanical extracts can vary in composition and potency depending on the source, preparation, and extraction method, which can make it difficult to ensure consistent and effective doses [85].
Limited clinical evidence: While some studies have shown promising results, there is limited clinical evidence to support the use of botanical extracts as a treatment option for H. pylori infection. Many of the studies have been conducted in vitro or in animal models, and more clinical trials are needed to fully understand the effectiveness and safety of botanical extracts in humans [86].
Potential interactions with other medications: Botanical extracts may also interact with other medications, which can be a concern for patients taking multiple medications. For example, extracts from St. John's wort and Ginkgo biloba can interact with anticoagulant medications, while extracts from grapefruit can interact with certain statins [87].
Comparison between Chemical Therapy and Herbal Alternatives
Efficacy
Standard therapy for H. pylori infection using a combination of antibiotics and proton pump inhibitors has been shown to be effective in eradicating the infection in up to 90% of cases [88]. However, the emergence of antibiotic-resistant strains has reduced the efficacy of standard therapy in some regions.
Side effects
Chemical therapy using antibiotics and proton pump inhibitors can cause side effects such as diarrhea, nausea, vomiting, and abdominal pain. Long-term use of proton pump inhibitors has been associated with an increased risk of bone fractures, kidney disease, and infections [89].
Herbal alternatives
Herbal alternatives such as mastic gum, licorice, and cranberry-derived compounds have shown promising results in in vitro and animal studies as an alternative therapy for H. pylori infection. However, clinical evidence is limited, and the efficacy of herbal alternatives for H. pylori infection remains uncertain [90]. Herbal alternatives are generally considered safe, but some may have toxic effects at high doses or interact with certain medical conditions. For example, licorice can cause hypertension and hypokalemia in some individuals [91].
Chemical therapy
Chemical therapy using a combination of antibiotics and proton pump inhibitors is the standard therapy for H. pylori infection and has been shown to be effective in eradicating the infection. However, the emergence of antibiotic-resistant strains has reduced the efficacy of standard therapy in some regions, and chemical therapy can cause side effects. Herbal alternatives have shown promising results in in vitro and animal studies and may have fewer side effects. However, clinical evidence is limited, and the efficacy of herbal alternatives for H. pylori infection remains uncertain.
Management of H. pylori by Artificial Intelligence (AI)
Artificial intelligence (AI) has opened up new possibilities for the discovery and development of botanical extracts for the treatment of H. pylori infection. Machine learning algorithms can be trained on large datasets of chemical compounds and their biological activities to identify novel compounds with potential therapeutic properties. For example, a recent study used a machine learning approach to identify plant-derived compounds with potential antimicrobial activity against H. pylori and identified several promising candidates for further study [92]. In addition to identifying potential compounds, AI can also be used to optimize the extraction and purification of bioactive compounds from botanical sources. Traditional extraction methods often involve trial-and-error optimization, but AI can be used to design experiments that systematically explore the effects of different extraction parameters on the yield and purity of target compounds. For example, a recent study used a machine learning approach to optimize the extraction of flavonoids from Scutellaria baicalensis and found that the combination of ultrasound-assisted extraction and enzymatic hydrolysis resulted in the highest yield and purity of target compounds [93]. Furthermore, AI can be used to predict the biological activities of botanical extracts based on their chemical composition. This can help to guide the selection of extracts for further study, and to identify the specific compounds responsible for their therapeutic effects. For example, a recent study used a machine learning approach to predict the anti-inflammatory and anti-H. pylori activity of botanical extracts based on their chemical composition and identified several compounds with potent activity against H. pylori, including epigallocatechin gallate and quercetin. 94 AI can also be used to evaluate the synergistic effects of botanical extracts. A recent study used AI to evaluate the synergistic effects of a combination of Chinese herbal medicines, including Rhizoma coptidis, Cortex phellodendri, and Radix scutellariae, on H. pylori infection [95]. The study used a support vector machine algorithm to predict the potential synergistic effects of the combination of extracts and found that the combination displayed significantly greater antimicrobial activity against H. pylori compared with individual extracts [96].
Future Research Directions in the Field of Herbal Medicine
Many herbal medicines contain multiple bioactive compounds, making it difficult to identify the active compounds responsible for their antimicrobial and anti-inflammatory effects. Future research should focus on identifying and characterizing the active compounds in herbal medicines that are effective against H. pylori [97].
Standardization of herbal preparations
Herbal preparations can vary in their composition and potency, depending on factors such as plant species, extraction method, and geographical location. Standardization of herbal preparations is critical to ensure consistent efficacy and safety [98].
Clinical trials
There is limited clinical evidence to support the use of herbal medicine for H. pylori infection. Future research should focus on conducting well-designed, randomized controlled trials to evaluate the efficacy and safety of herbal medicines for H. pylori infection [99].
Mechanistic studies
Mechanistic studies are needed to better understand the antimicrobial and anti-inflammatory mechanisms of herbal medicine against H. pylori. These studies can provide insights into the molecular targets and pathways involved in the therapeutic effects of herbal medicine [100].
Combination therapy
Combining herbal medicine with conventional therapy may enhance the efficacy of treatment and reduce the risk of antibiotic resistance. Future research should investigate the efficacy and safety of combination therapy for H. pylori infection [101].
Safety and toxicity
Herbal medicine is generally considered safe, but some may have toxic effects at high doses or interact with certain medical conditions. Future research should investigate the safety and toxicity of herbal medicines for H. pylori infection, particularly in vulnerable populations such as pregnant women, children, and the elderly [102].
Conclusion
Recent advances in botanical extracts have shown promise as potential alternative therapies for Helicobacter pylori infection. Green tea, broccoli sprout extract, cranberry extract, garlic, ginger, Peppermint oil, Turmeric root, artemisinin, and licorice are examples of botanical extracts that have been studied for their potential health benefits, including antibacterial, anti-inflammatory, and antioxidant properties. However, more research is needed to fully understand the effects and limitations of these extracts, including their potential adverse effects and interactions with other medications. In addition to alternative therapies, diagnostic tools for H. pylori infection have also advanced, including non-invasive methods such as serology and stool antigen tests. Current therapy for H. pylori includes a combination of antibiotics and proton pump inhibitors, but increasing antibiotic resistance has prompted the need for alternative therapies.
The next direction for research in this area is to develop more effective and targeted therapies for H. pylori infection. This can include developing standardized methods for evaluating the efficacy of botanical extracts and exploring the potential of combination therapies. Moreover, AI can be used to identify potential compounds, predict their biological activities, optimize extraction and purification processes, and evaluate the synergistic effects of different botanical extracts. Several recent studies have demonstrated the potential of AI as a powerful tool for the discovery and development of botanical extracts for the treatment of H. pylori infection. By combining AI with traditional methods, researchers can more efficiently identify effective extracts and optimize their extraction and purification processes. This approach could lead to the development of more effective and targeted therapies for H. pylori infection, and ultimately improve the health outcomes of patients with this condition. In the future, botanical extracts may provide a viable alternative to traditional antibiotic therapy for H. pylori infection.
Recommendations
Further research is needed on clinical efficacy, optimal dosing, and safety of botanical extracts against H. pylori, particularly combination therapy with standard antibiotics. Use of AI for compound screening and extraction process optimization shows promise. Phage therapy and novel antibiotics like furazolidone warrant expanded investigation to address antibiotic resistance. Standardization of extracts and broader clinical trials remain key to validating plant-based therapies.
List of Abbreviations
HP: Helicobacter Pylori; RRA: Review of Recent Advances; AI: Artificial intelligence; BE: Botanical Extracts; PI: Potential Alternative Therapies; SE: Standard Extracts; RC: Randomized Controlled; RCT: Randomized Controlled Trial; MIC: Minimum Inhibitory Concentration; MBC: Minimum Bactericidal Concentration; ROS: Reactive Oxygen Species; IL: Interleukin; TNF: Tumor Necrosis Factor; Nrf2: Nuclear factor erythroid 2-related factor 2; H. pylori: Heliobacter pylori; GI: gastrointestinal; NSAID: Nonsteroidal Anti-inflammatory Drug; UBT: Urea Breath Test; PCR: Polymerase Chain Reaction; Cag-A: Cytotoxin-associated gene-A; TLR-2: Toll-like Receptor 2; TLR-4: Toll-like Receptor 4; NF-κB: Nuclear Factor kappa B.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
All data is publicly available for sharing and publication. The manuscript does not have any other associated data, and all necessary data has been declared within the original manuscript.
Competing interests
The authors hereby declare that they have no competing interests.
Funding
Corresponding author supplied all study materials. There was no further funding for this study.
Author's contributions
The authors completed the study protocol and were the primary organizers of data collection, as well as the draft and revision process of the manuscript. TA wrote the article and ensured its accuracy. All authors contributed to the discussion, assisted in designing the study and protocol, and engaged in critical discussions of the draft manuscript. Lastly, the authors (TA, MA, IE, YW) reviewed and confirmed the final version of the manuscript.
Acknowledgements
Authors thank all the researchers who have made great efforts on their studies. Moreover, we are grateful to the editors, reviewers, and readers of this journal.
References
2. Bashir SK, Khan MB. Overview of Helicobacter pylori infection, prevalence, risk factors, and its prevention. Adv Gut Microbiome Res. 2023;2023:9747027.
3. Liu D, Wang Q, Li P, Wu DH, Pan J, Chen ZY, et al. Comparing high-dose dual therapy with bismuth-containing quadruple therapy for the initial eradication of Helicobacter pylori infection on Hainan Island: A randomized, multicenter clinical trial. Clin Res Hepatol Gastroenterol. 2023;47(5):102125.
4. Li R, Xu J, Wang X, Liao LJ, Wei X, Xie P, et al. Therapeutic effect of demethylated hydroxylated phillygenin derivative on Helicobacter pylori infection. Front Microbiol. 2023;14.
5. Reyes VE. Helicobacter pylori and its role in gastric cancer. Microorganisms. 2023;11(5):1312.
6. Addissouky TA, Wang Y, El Sayed IE, Baz AE, Ali MM, Khalil AA. Recent trends in Helicobacter pylori management: harnessing the power of AI and other advanced approaches. Beni-Suef University Journal of Basic and Applied Sciences. 2023 Sep 2;12(1):80.
7. Shi Y, Wei N, Wang K, Tao T, Yu F, Lv B, et al. Diagnostic value of artificial intelligence-assisted endoscopy for chronic atrophic gastritis: A systematic review and meta-analysis. Front Med. 2023;10.
8. Dehzad MJ, Ghalandari H, Amini MR, Askarpour M. Effects of curcumin/turmeric supplementation on liver function in adults: A GRADE-assessed systematic review and dose-response meta-analysis of randomized controlled trials. Complement Ther Med. 2023;74:102952.
9. Jiang X, Xu C, Liu B, Chen P, Xu Q, Zhang L, et al. Efficacy and safety of bifidobacterium quadruple viable tablets in the treatment of Helicobacter pylori-infected peptic ulcer or gastritis patients: A systematic review and meta-analysis. BMC Infect Dis. 2023;23(1):313.
10. Lee B, Ha N, Park H, Kim AR, Kwon OJ, Cho JH, et al. Herbal Medicine Yukgunja-Tang for Functional Dyspepsia: A Protocol for a Randomized, Controlled, Multicenter Clinical Trial. Healthcare (Basel). 2023;11(10):1456.
11. Mosaddad SA, Hussain A, Tebyaniyan H. Green alternatives as antimicrobial agents in mitigating periodontal diseases: A narrative review. Microorganisms. 2023;11(5):1269.
12. Cheng S, Li H, Luo J, Chi J, Zhao W, Lin J, et al. Egg yolk antibody combined with bismuth-based quadruple therapy in Helicobacter pylori infection rescue treatment: A single-center, randomized, controlled study. Front Microbiol. 2023;14.
13. Goodoory VC, Ford AC. Antibiotics and probiotics for irritable bowel syndrome. Drugs. 2023;83(6):687-699.
14. Syam AF, Miftahussurur M, Makmun D, Abdullah M, Rani AA, Siregar GA, et al. Management of dyspepsia and Helicobacter pylori infection: the 2022 Indonesian Consensus Report. Gut Pathog. 2023;15(1):25.
15. Johnson MC, Laderman E, Huiting E, Zhang C, Davidson A, Bondy-Denomy J. Core defense hotspots within Pseudomonas aeruginosa are a consistent and rich source of anti-phage defense systems. Nucleic Acids Research. 2023 Jun 9;51(10):4995-5005.
16. Ngashangva L, Chattopadhyay S. Biosensors for point-of-care testing and personalized monitoring of gastrointestinal microbiota. Front Microbiol. 2023;14:1114707.
17. Syam AF, Miftahussurur M, Makmun D, Abdullah M, Rani AA, Siregar GA, et al. Management of dyspepsia and Helicobacter pylori infection: the 2022 Indonesian Consensus Report. Gut Pathog. 2023;15:25.
18. Rattanachaisit P, Burana C, Jaroenlapnopparat A, Vongseenin S, Chaithongrat S, Rerknimitr R, et al. The prevalence and treatment outcomes of Helicobacter pylori infection in a tertiary hospital in Thailand, 2018-2021. JGH Open. 2023 May 26;7(6):439-444.
19. Cardos AI, Maghiar A, Zaha DC, Pop O, Fritea L, Miere Groza F, et al. Evolution of Diagnostic Methods for Helicobacter pylori Infections: From Traditional Tests to High Technology, Advanced Sensitivity and Discrimination Tools. Diagnostics. 2022;12(2):508.
20. Olson L, Christensen E. For patients with Helicobacter pylori infection, is whole-family treatment more effective than single-patient treatment at preventing recurrence?. Evidence-Based Practice. 2023 Oct 1;26(10):7-8.
21. Jaradat H, Ibbini M, Fourati N, Kanoun O. Novel Sensitive Electrochemical Immunosensor Development for the Selective Detection of HopQ H. pylori Bacteria Biomarker. Biosensors. 2023;13(5):527.
22. Chen MJ, Chen PY, Fang YJ, Bair MJ, Chen CC, Chen CC, et al. Molecular testing-guided therapy versus susceptibility testing-guided therapy in first-line and third-line Helicobacter pylori eradication: two multicentre, open-label, randomised controlled, non-inferiority trials. Lancet Gastroenterol Hepatol. 2023 Jul;8(7):623-634.
23. Wang X, Gong Y, He L, Zhao L, Wang Y, Zhang J, et al. Clinical relevance and distribution of Helicobacter pylori virulence factors in isolates from Chinese patients. Ann Transl Med. 2023;11(8):301.
24. Garcés-Durán R, Llach J, Da Fieno A, Córdova H, Fernández-Esparrach G. Endoscopic diagnosis of H. pylori infection. Gastroenterol Hepatol. 2023 Jun-Jul;46(6):483-488. English, Spanish.
25. Kim I, Maeng LS, Kim JS, Kim BW, Cheung DY, Kim JI, et al. Quantitative multiplex real-time polymerase chain reaction assay for the detection of Helicobacter pylori and clarithromycin resistance. BMC Microbiol. 2023;23:155.
26. Chen J, Guo Y, Huang Y, Ding Z, Wang J, Liang X, et al. Rifabutin-Containing Triple Therapy Versus Bismuth Quadruple Therapy for Helicobacter pylori Rescue Treatment: A Multicenter, Randomized Controlled Trial. J Infect Dis. 2023 Aug 31;228(5):511-518.
27. Zhang L, Zhao M, Fu X. Gastric microbiota dysbiosis and Helicobacter pylori infection. Front Microbiol. 2023;14:1153269.
28. Wu L, Jin X, Zheng C, Ma F, Zhang X, Gao P, et al. Bidirectional Effects of Mao Jian Green Tea and Its Flavonoid Glycosides on Gastrointestinal Motility. Foods. 2023;12(4):854.
29. Malli C, Pandit L, Sudhir A. Helicobacter pylori infection may influence prevalence and disease course in myelin oligodendrocyte glycoprotein antibody associated disorder (MOGAD) similar to MS but not AQP4-IgG associated NMOSD. Front Immunol. 2023;14:1162248.
30. Khalaf SS, Shalaby OA, Hassan AR, El-Kherbetawy MK, Mehanna ET. Acacia nilotica stem bark extract ameliorates obesity, hyperlipidemia, and insulin resistance in a rat model of high fat diet-induced obesity. J Tradit Complement Med. 2023 Mar 8;13(4):397-407.
31. Mahmud AR, Ema TI,Hossain MS, et al. Association of Helicobacter pylori infection with non-alcoholic fatty liver disease: a systematic review and meta-analysis. BMC Gastroenterol. 2023;23:92.
32. Boyanova L, Hadzhiyski P, Gergova R, Markovska R. Evolution of Helicobacter pylori Resistance to Antibiotics: A Topic of Increasing Concern. Antibiotics (Basel). 2023;12(2):332.
33. Godavarthy PK, Puli C. From Antibiotic Resistance to Antibiotic Renaissance: A New Era in Helicobacter pylori Treatment. Cureus. 2023;15(3):e36041.
34. Manfredi M, Gargano G, Gismondi P, Ferrari B, Iuliano S. Therapeutic eradication choices in Helicobacter pylori infection in children. Therap Adv Gastroenterol. 2023;16:17562848231170052.
35. Gharbani P, Jam N, Doshmanfekan H, et al. Optimization of synergic antibacterial activity of Punica granatum L. and Areca nut (P.G.L.A.N) extracts through response surface methodology. Sci Rep. 2023;13:6098.
36. Luo Q, Liu N, Pu S, Zhuang Z, Gong H, Zhang D. A review on the research progress on non-pharmacological therapy of Helicobacter pylori. Front Microbiol. 2023;14:1134254.
37. Hegde M, Girisa S, Chetty BB, Vishwa R, Kunnumakkara AB. Curcumin formulations for better bioavailability: What we learned from clinical trials thus far? ACS Omega. 2023;8(12):10713-10746.
38. Mosaddad SA, Hussain A, Tebyaniyan H. Green Alternatives as Antimicrobial Agents in Mitigating Periodontal Diseases: A Narrative Review. Microorganisms. 2023;11(5):1269.
39. Kim K, Ko S, Cho SH, Kim J, Park J. Herbal medicine, Banxia-xiexin tang, for functional dyspepsia: A systematic review and meta-analysis. Front Pharmacol. 2023;14:1130257.
40. Bodie AR, A., C., Olson EG, Ricke SC. Natural Antimicrobials for Listeria monocytogenes in Ready-to-Eat Meats: Current Challenges and Future Prospects. Microorganisms. 2023;11(5):1301.
41. Zhong Y, Li MY, Han L, et al. Galangin inhibits programmed cell death-ligand 1 expression by suppressing STAT3 and MYC and enhances T cell tumor-killing activity. Phytomedicine. 2023;154877.
42. Xu J, He J, Xu S, Wang R, Peng N, Zhang M, et al. Gitelman syndrome with Graves’ disease leading to rhabdomyolysis: a case report and literature review. BMC Nephrol. 2023;24(1):123.
43. Mohammed A, Mohammed HA. Beneficial role of broccoli and its active ingredient, sulforaphane in the treatment of diabetes. Phytomedicine Plus. 2023;3(2):100431.
44. Da-Ming L, Chen C. Oral and physiological benefits of cranberries. J Dent Probl Solut. 2023;10(1):001-004.
45. Abdi S, Ataei S, Abroon M, Majma Sanaye P, Abbasinazari M, Farrokhian A. A Comprehensive Review of the Role of Complementary and Dietary Medicines in Eradicating Helicobacter pylori. Innov J Pharm Res. 2022;21(1):e127030.
46. Amin R, Thalluri C, Docea AO, Sharifi-Rad J, Calina D. Therapeutic potential of cranberry for kidney health and diseases. EFood. 2022;3(5):e33.
47. Wang Q, Yao C, Li Y, Luo L, Xie F, Xiong Q, et al. Effect of polyphenol compounds on Helicobacter pylori eradication: a systematic review with meta-analysis. BMJ Open. 2023 Jan 5;13(1):e062932.
48. Verma T, Aggarwal A, Dey P, Chauhan AK, Rashid S, Chen T, et al. Medicinal and therapeutic properties of garlic, garlic essential oil, and garlic-based snack food: An updated review. Front Nutr. 2023;10.
49. Pandey P, Khan F, Alshammari N, Saeed A, Aqil F, Saeed M. Updates on the anticancer potential of garlic organosulfur compounds and their nanoformulations: Plant therapeutics in cancer management. Front Pharmacol. 2023;14.
50. Sathianarayanan S, Ammanath AV, Biswas R, B A, Sukumaran S, Venkidasamy B. A new approach against Helicobacter pylori using plants and its constituents: A review study. Microb Pathog. 2022;168:105594.
51. Moradi V, Esfandiary E, Ghanadian M, Ghasemi N, Rashidi B. The effect of Zingiber Officinale Extract on Preventing Demyelination of Corpus Callosum in a Rat Model of Multiple Sclerosis. Iran Biomed J. 2022;26(4):330-339.
52. Sabry ME-B, Elzoghby RR, Alfattah MA, Kandeel M, Hamouda AF. Aqueous Ginger (Zingiber officinale) Extract Ameliorates the Harmful Effects of High-Dose Lornoxicam in Albino Male Rats. BioMed Res Int. 2022;2022:1546734.
53. Sharma S, Shukla MK, Sharma KC, Tirath, Kumar L, Anal JMH, et al. Revisiting the therapeutic potential of gingerols against different pharmacological activities. Naunyn Schmiedebergs Arch Pharmacol. 2023;396(3):633-647.
54. Habbash F, Alalwan TA, Perna S, Ahmed N, Sharif O, Al Sayyad A, et al. Association between Dietary Habits and Helicobacter pylori Infection among Bahraini Adults. Nutrients. 2022 Oct 10;14(19):4215
55. Ivyna de Araújo Rêgo R, Guedes Silvestre GF, Ferreira de Melo D, Albino SL, Pimentel MM, Silva Costa Cruz SB, et al. Flavonoids-Rich Plant Extracts Against Helicobacter pylori Infection as Prevention to Gastric Cancer. Front Pharmacol. 2022 Aug 31;13:951125.
56. Zhao T, Li C, Wang S, Song X. Green Tea (Camellia sinensis): A Review of Its Phytochemistry, Pharmacology, and Toxicology. Molecules. 2022;27(12):3909.
57. Wu L, Jin X, Zheng C, Ma F, Zhang X, Gao P, et al. Bidirectional Effects of Mao Jian Green Tea and Its Flavonoid Glycosides on Gastrointestinal Motility. Foods. 2023;12(4):854.
58. Khan U, Karmakar BC, Basak P, Paul S, Gope A, Sarkar D, et al. Glycyrrhizin, an inhibitor of HMGB1 induces autolysosomal degradation function and inhibits Helicobacter pylori infection. Mol Med. 2023;29:51.
59. Lenka S, Bhuyan R. Management of H. pylori Induced Pepticulcer – A Phytotherapeutic Approach. J Pure Appl Microbiol. 2022;16(3):1530-1537.
60. Wang Q, Yao C, Li Y, Luo L, Xie F, Xiong Q, et al. Effect of polyphenol compounds on Helicobacter pylori eradication: a systematic review with meta-analysis. BMJ Open. 2023 Jan 5;13(1):e062932.
61. Madisch A, Frieling T, Zimmermann A, Hollenz M, Labenz J, Stracke B, et al. Menthacarin, a proprietary peppermint oil and caraway oil combination, improves multiple complaints in patients with functional gastrointestinal disorders: A systematic review and meta-analysis. Digestion. 2022:1-11.
62. Saqib S, Fazal Ullah, Muhammad Naeem, Younas M, Ayaz A, Ali S, et al. Mentha: Nutritional and Health Attributes to Treat Various Ailments Including Cardiovascular Diseases. Molecules. 2022;27(19):6728.
63. Matera R, Lucchi E, Valgimigli L. Plant Essential Oils as Healthy Functional Ingredients of Nutraceuticals and Diet Supplements: A Review. Molecules. 2023;28(2):901.
64. El-Saadony MT, Yang T, Korma SA, Sitohy M, Abd El-Mageed TA, Selim S, et al. Impacts of turmeric and its principal bioactive curcumin on human health: Pharmaceutical, medicinal, and food applications: A comprehensive review. Front Nutr. 2022;9:1040259.
65. Kunnumakkara AB, Hegde M, Parama D, Girisa S, Kumar A, Daimary UD, et al. Role of turmeric and curcumin in prevention and treatment of chronic diseases: Lessons learned from clinical trials. ACS Pharmacol Transl Sci. 2023;6(4):447-518.
66. Sadeghi M, Dehnavi S, Asadirad A, Xu S, Majeed M, Jamialahmadi T, et al. Curcumin and chemokines: mechanism of action and therapeutic potential in inflammatory diseases. Inflammopharmacol. 2023 Jun;31(3):1069-1093.
67. Iweala EJ, Uche ME, Dike ED, Etumnu LR, Dokunmu TM, Oluwapelumi AE, et al. Curcuma longa (Turmeric): Ethnomedicinal uses, phytochemistry, pharmacological activities and toxicity profiles—A review. Pharmacol Res Mod Chin Med. 2023;6:100222.
68. Bordean ME, Ungur RA, Toc DA, Borda IM, Marțiș GS, Pop CR, et al. Antibacterial and Phytochemical Screening of Artemisia Species. Antioxidants. 2023;12(3):596.
69. Qanash H, Bazaid AS, Aldarhami A, Alharbi B, Almashjary MN, Hazzazi MS, et al. Phytochemical Characterization and Efficacy of Artemisia judaica Extract Loaded Chitosan Nanoparticles as Inhibitors of Cancer Proliferation and Microbial Growth. Polymers. 2023;15(2):391.
70. Kim YB, Cho HJ, Yi YS. Anti-inflammatory role of Artemisia argyi methanol extract by targeting the caspase-11 non-canonical inflammasome in macrophages. J Ethnopharmacol. 2023;307:116231.
71. Gazim ZC, Valle JS, Rahal IL, Silva GC, Lopes AD, Ruiz SP, et al. Ethnomedicinal, phytochemical and pharmacological investigations of Baccharis dracunculifolia DC. (ASTERACEAE). Front Pharmacol. 2022 Nov 28;13:1048688.
72. Tamer A. Addissouky, Fayed Attia Koutb Megahed, Ayman E. Elagroudy and Ibrahim El Tantawy El Sayed, EFFICIENCY OF MIXTURE OF OLIVES OIL AND FIGS AS AN ANTIVIRAL AGENT: A REVIEW AND PERSPECTIVE, International Journal of Medical Science and Health Research. Aug 2020;4(4):107-11.
73. Villalva M, Silvan JM, Guerrero-Hurtado E, Gutierrez-Docio A, Navarro Del Hierro J, Alarcón-Cavero T, et al. Influence of In Vitro Gastric Digestion of Olive Leaf Extracts on Their Bioactive Properties against H. pylori. Foods. 2022;11(13):1832.
74. Silvan JM, Michalska-Ciechanowska A, Villalva M, Brzezowska J, Díaz S, Martinez-Rodriguez AJ. Bioactive Properties of Blueberry Extracts Obtained by Different Drying Techniques against Helicobacter pylori. Biol Life Sci Forum. 2022;18(1):20.
75. Palos-Hernández A, Gutiérrez Fernández MY, Escuadra Burrieza J, Pérez-Iglesias JL, González-Paramás AM. Obtaining green extracts rich in phenolic compounds from underexploited food by-products using natural deep eutectic solvents. Opportunities and challenges. Sustain Chem Pharm. 2022;29:100773.
76. Reddy KV, Sree NRS, Ranjit P, Maddela NR, Kumar V, Jha P, et al. Essential oils, herbal extracts and propolis for alleviating Helicobacter pylori infections: A critical view. S Afr J Bot. 2023;157:138-150.
77. Gharbani P, Jam N, Doshmanfekan H, Mehrizad A. Optimization of synergic antibacterial activity of Punica granatum L. and Areca nut (P.G.L.A.N) extracts through response surface methodology. Sci Rep. 2023;13(1):1-8.
78. Vieira Neves NC, Smith SM, Boylan F, Caliari MV, Castilho RO. Chemical Composition and In Vitro Anti-Helicobacter pylori Activity of Campomanesia lineatifolia Ruiz & Pavón (Myrtaceae) Essential Oil. Plants. 2022 Jul 27;11(15):1945.
79. Ivyna de Araújo Rêgo R, Guedes Silvestre GF, Albino SL, Pimentel MM, Silva Costa Cruz SB et al. Flavonoids-Rich Plant Extracts Against Helicobacter pylori Infection as Prevention to Gastric Cancer. Front Pharmacol. 2022;13:951125.
80. Ali DE, Abd el-Aziz MM, Ibrahim SSA, Sheta E, Abdel-Sattar E. Gastroprotective and anti-Helicobacter pylori potentials of essential oils from the oleoresins of Araucaria bidwillii and Araucaria heterophylla. Inflammopharmacol. 2023;31(2):465-483.
81. Liang J, Huang X, Ma G. Antimicrobial activities and mechanisms of extract and components of herbs in East Asia. RSC Adv. 2022;12:29197-29213.
82. Inada I, Kiuchi F, Urushihara H. Comparison of Regulations for Arsenic and Heavy Metals in Herbal Medicines Using Pharmacopoeias of Nine Counties/Regions. Ther Innov Regul Sci 2023 Sep;57(5):963-974.
83. Salari Sedigh S, Gholipour A, Zandi M, Qubais Saeed B, Al-Naqeeb BZT, Abdullah Al-Tameemi NM, et al. The role of bismuth nanoparticles in the inhibition of bacterial infection. World J Microbiol Biotechnol. 2023;39(10):190.
84. Miron A, Giurcaneanu C, Mihai MM, Beiu C, Voiculescu VM, Popescu MN, et al. Antimicrobial Biomaterials for Chronic Wound Care. Pharmaceutics. 2023;15(6):1606.
85. Widelski J, Okińczyc P, Suśniak K, Malm A, Bozhadze A, Jokhadze M, et al. Correlation between Chemical Profile of Georgian Propolis Extracts and Their Activity against Helicobacter pylori. Molecules. 2023;28(3):1374.
86. Kim H, Ha J, Park HY, Choung YH, Jang JH. Efficacy and Safety of Co-Administered St. John's Wort and Ginkgo biloba Extracts in Patients with Subjective Tinnitus: A Preliminary Prospective Randomized Controlled Trial. J Clin Med. 2023;12(9):3261.
87. Lai Y, Wei W, Du Y, Gao J, Li Z. Biomaterials for Helicobacter pylori Therapy: Therapeutic Potential and Future Perspectives. Gut Microbes. 2023;14(1):2120747.
88. Sukri A, Hanafiah A, Patil S, Lopes BS. The Potential of Alternative Therapies and Vaccine Candidates against Helicobacter pylori. Pharmaceuticals. 2023;16(4):552.
89. Roberts LT, Issa PP, Sinnathamby ES, Granier M, Mayeux H, Eubanks TN, et al. Helicobacter Pylori: A Review of Current Treatment Options in Clinical Practice. Life. 2022;12(12):2038.
90. Malfertheiner P, Camargo MC, Liou J, Liou JM, Peek R, Schulz C, et al. Helicobacter pylori infection. Nat Rev Dis Primers. 2023;9(1):1-24.
91. Yin X, Lai Y, Du Y, Zhang T, Gao J, Li Z. Metal-Based Nanoparticles: A Prospective Strategy for Helicobacter pylori Treatment. Int J Nanomedicine. 2023;18:2413-2429.
92. Afrash MR, Shafiee M, Kazemi-Arpanahi H. Establishing Machine Learning Models to Predict the Early Risk of Gastric Cancer Based on Lifestyle Factors. BMC Gastroenterol. 2023;23(1):6.
93. Yang H, Guan L, Hu B. Detection and Treatment of Helicobacter pylori: Problems and Advances. Gastroenterol Res Pract. 2022;2022:4710964.
94. Zhong Z, Wang X, Li J, Zhang B, Yan L, Xu S, et al. Study on the Diagnosis of the Helicobacter pylori Coccoid Form with Artificial Intelligence Technology. Front Microbiol. 2022;13.
95. Zhang M, Pan J, Lin J, Xu M, Zhang L, Shang R, et al. An explainable artificial intelligence system for diagnosing Helicobacter pylori infection under endoscopy: A case–control study. Ther Adv Gastroenterol. 2023;14:17562848231155023.
96. Luo Q, Yang H, Hu B. Application of artificial intelligence in the endoscopic diagnosis of early gastric cancer, atrophic gastritis, and Helicobacter pylori infection. J Dig Dis. 2022;23(12):666-674.
97. Elbehiry A, Marzouk E, Aldubaib M, Abalkhail A, Anagreyyah S, Anajirih N, et al. Helicobacter pylori Infection: Current Status and Future Prospects on Diagnostic, Therapeutic and Control Challenges. Antibiotics. 2023;12(2):191.
98. Yao P, Kartsonaki C, Butt J, Jeske R, de Martel C, Plummer M, et al. Helicobacter pylori multiplex serology and risk of non-cardia and cardia gastric cancer: a case-cohort study and meta-analysis. Int J Epidemiol. 2023 Aug 2;52(4):1197-1208.
99. Miri AH, Kamankesh M, Rad-Malekshahi M, Yadegar A, Banar M, Hamblin MR, et al. Factors associated with treatment failure, and possible applications of probiotic bacteria in the arsenal against Helicobacter pylori. Expert Rev Anti Infect Ther. 2023:1-23.
100. Duran N, Barriuso J. Microbial degradation of herbicides: A review. Arch Agron Soil Sci. 2023;86(1):145-174.
101. Yu C, Qiu J, Xiong M, Ou C, Zeng M, Song H. Trends in Helicobacter pylori-related gastric ulcer research from 2012 to 2022: A bibliometric and visual analysis. Front Med (Lausanne). 2022 Nov 23;9:1027534.
102. Sukri A, Hanafiah A, Patil S, Lopes BS. The Potential of Alternative Therapies and Vaccine Candidates against Helicobacter pylori. Pharmaceuticals (Basel). 2023 Apr 6;16(4):552.