Abstract
Primary extra cranial meningioma of the ear and temporal bone are exceedingly rare, making up less than 1% of all meningiomas. Histologically, they are indistinguishable from their intracranial counterpart. The histopathologic diagnosis is often challenging, and the differential diagnosis for neoplasms in this unusual location is quite extensive. We report a 64-year-old male patient with history of seizures who presented with coalescing mastoiditis and sigmoid sinus thrombosis. CT and MRI findings were suggestive of a chronic inflammatory process. Operative findings included a flesh-colored mass extending from the mastoid antrum posteriorly towards the Sigmoid sinus. Intraoperative consultation was requested, yielding a diagnosis favoring squamous cell carcinoma, and the decision was made to end the procedure. Additional tissue was submitted for permanent evaluation, and biopsy demonstrated a grade 1 meningioma, supported by morphologic features and immunophenotype. The clinical and histologic findings of the patient are discussed, and we emphasize the challenging nature of this rare entity, particularly with regards to intraoperative analysis. In addition, English literature is reviewed.
Keywords
Temporal bone, Meningioma, Intraosseous, Extracranial
Introduction
Meningiomas are common, primary brain tumors, accounting for up to 30% of intracranial neoplasms; they are usually slow-growing and benign [1]. Primary extra cranial meningiomas are rare, and approximately 2% of meningiomas are located extra cranially [2]. Furthermore, primary extra cranial meningiomas of the ear and temporal bone are exceedingly rare [2,3]. Determining whether meningiomas presenting within the ear and temporal bone are primary versus secondary is diagnostically challenging, and direct central nervous system (CNS) extension must be radiographically or clinically excluded for the lesion to be considered “primary”. Primary extra cranial meningiomas presumably arise from the neural crest-derived arachnoid cells; arachnoid cells which can be identified both within and without the neuroaxis [4,5]. Clinically, these tumors present with nonspecific symptoms including hearing loss (either conductive or sensorineural), chronic otitis media/otitis externa, vertigo, chronic cough, or headache [2,3]. Histologically, extracranial meningiomas of the ear and temporal bone are indistinguishable from intracranial meningiomas and majority are low grade. However, the histopathologic diagnosis can often be challenging, particularly with small biopsies, as this entity is rare and the differential diagnosis for neoplasms in this unusual location commonly include carcinoma, melanoma, paraganglioma, schwannoma, and middle ear adenoma among others [1,3].
Herein, we present a rare case of primary intraosseous temporal bone meningioma with an acute on chronic presentation and with challenging intraoperative morphology. In addition, accompanying literature review highlights the rarity of primary extracranial meningiomas of the ear and temporal bone, their subtle clinical presentation and the need to distinguish these lesions from histologic mimics due to an excellent long-term prognosis.
Case Report
Clinical history
64-year-old male with history of seizures that first began a year and a half back was transferred from an outside hospital to department of otolaryngology, University of New Mexico for concerns of coalescing mastoiditis and sigmoid sinus thrombosis. He presented with complaints of intermittent double vision and right maxillary dental pain and denied any otalgia, otorrhea, difficulty hearing, jaw pain, headache or blurry vision. In addition, patient had experienced ear pain, drainage, and decreased hearing in the left ear a few months prior to the current presentation, prompting a visit to an urgent care center and was prescribed oral antibiotics followed by some relief of his symptoms.
Imaging
Outside imaging, magnetic resonance angiography (MRA), reportedly revealed left-sided coalescing mastoiditis, petrous apicitis, and sigmoid sinus and internal jugular (IJ) vein thrombosis.
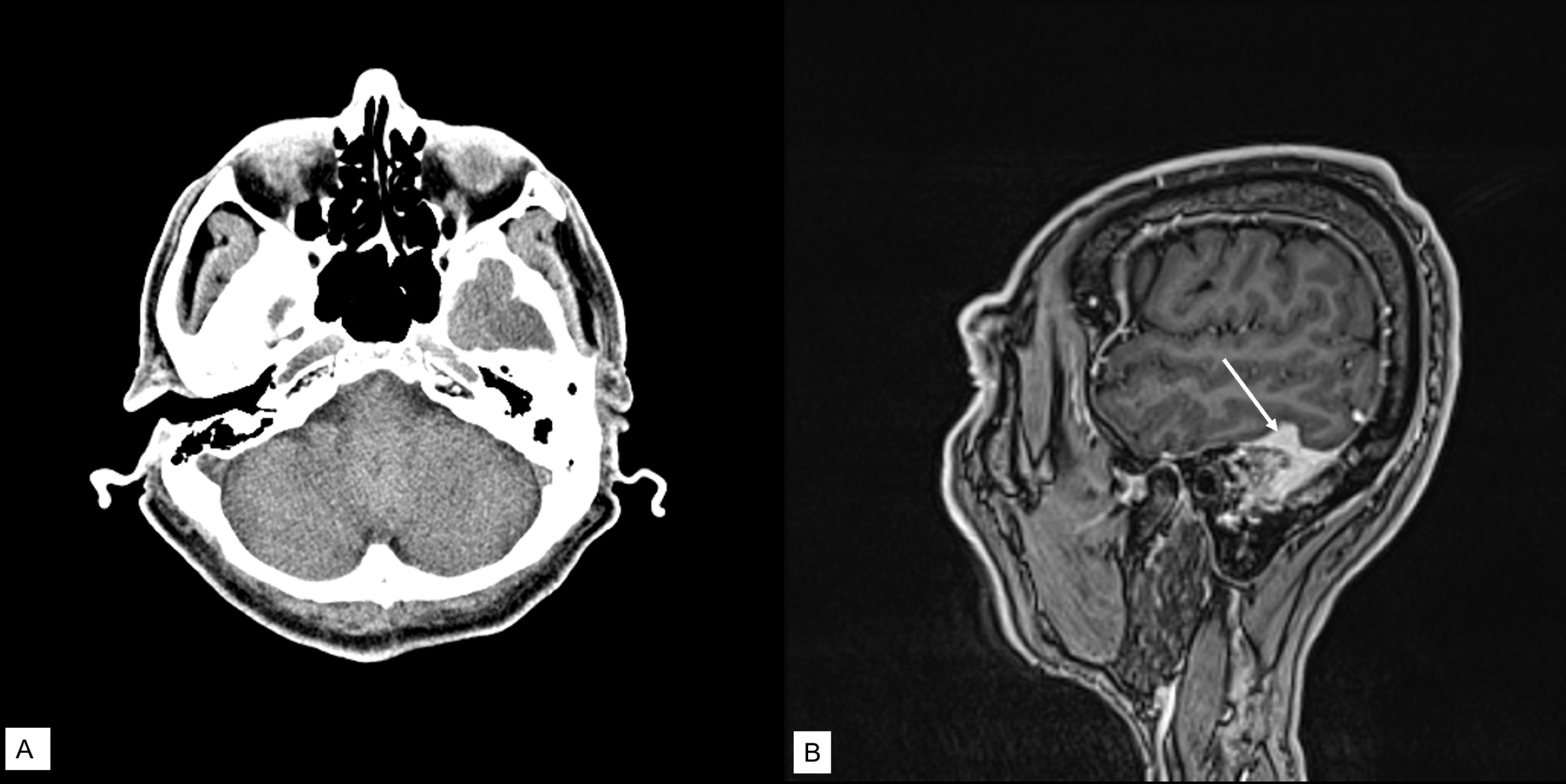
Preoperatively, computed tomography (CT) scan of temporal bones showed partial opacification of left mastoid air cells with osseous destruction including around the sigmoid sinus (Figure 1A). This was concerning for a chronic inflammatory process but the possibility of metastasis, plasmacytoma myeloma, or primary neoplasm were also considered on the radiographic differential diagnosis. CT head venogram was suggestive of dural venous sinus thrombosis. Magnetic resonance imaging (MRI) with gadolinium contrast showed abnormal dural thickening and enhancement overlying the left temporal bone and abnormal opacification of the left mastoid temporal bone (Figure 1B), suggestive of mastoiditis with septic thrombophlebitis. Patient was given IV antibiotic and heparin.
Figure 1. (A) CT scan showed partial opacification of left mastoid air cells with osseous destruction including around the sigmoid sinus; (B) MRI with contrast showed abnormal opacification of left mastoid temporal bone, abnormal signal involving adjacent transverse sinus, sigmoid sinus, and jugular bulb; abnormal dural thickening and enhancement overlying the left temporal bone (arrow). The findings were suggestive of mastoiditis and septic thrombophlebitis.
Operative findings
The ear was examined with binocular microscopy which showed a slightly thickened tympanic membrane but without perforations, effusions or infection. A left cortical mastoidectomy (open approach) was performed. Following incision, a flesh-colored tissue mass was identified, primarily extending from the mastoid antrum posteriorly towards the sigmoid as well as inferiorly.
Representative fragments were removed for pathologic evaluation as well as sent for microbiologic cultures. Frozen section diagnosis was “favor squamous cell carcinoma”.
The decision was made to conclude the procedure.
Pathology
The gross specimen consisted of multiple small fragments of tan-brown soft tissue measuring from 1-5 mm in greatest dimension which were submitted entirely for histopathologic examination.
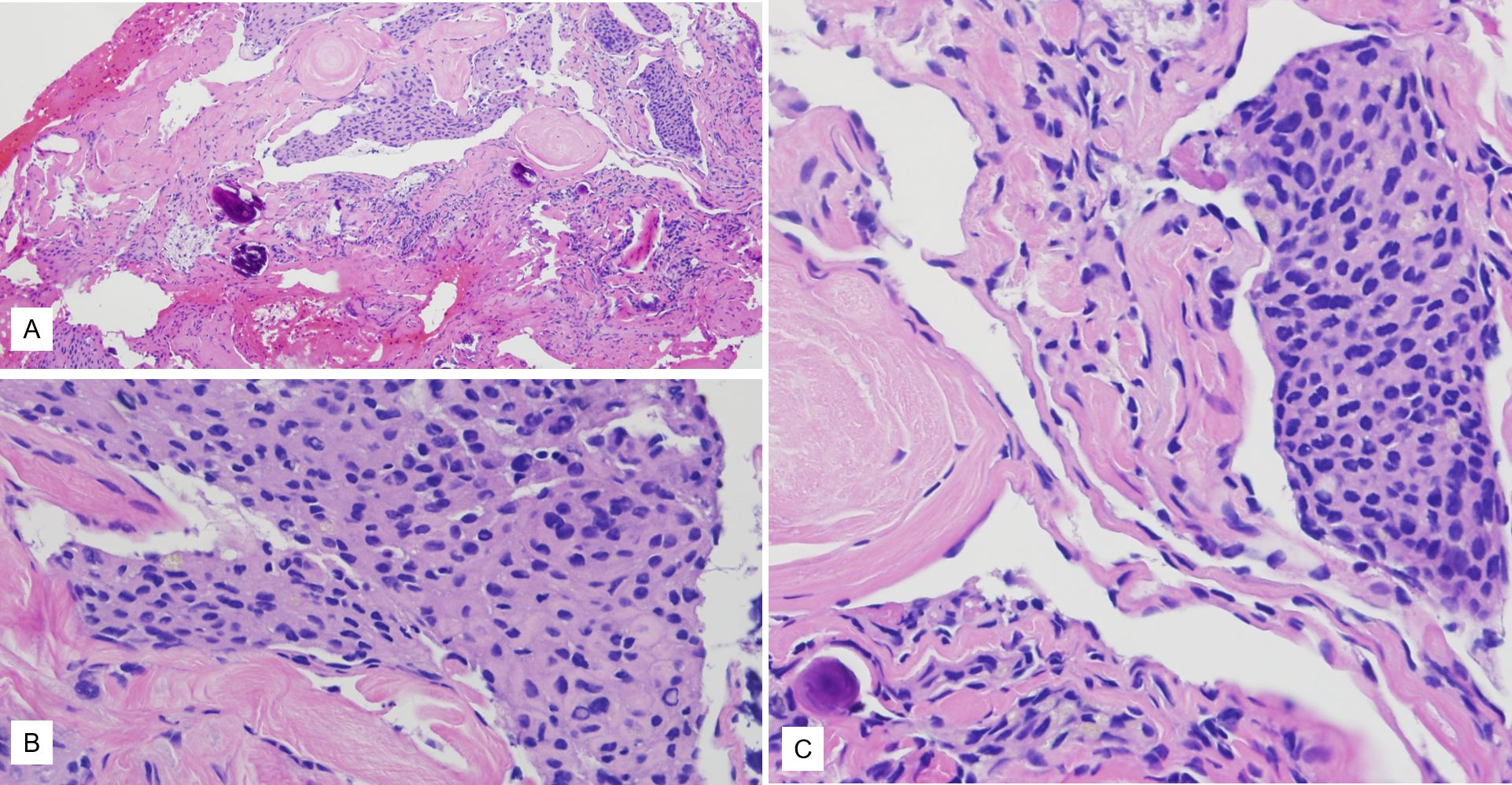
Histology revealed fragments of bone with admixed sheets of epithelioid cells with a syncytial appearance. The sheets were surrounded by areas of fibrosis, in areas with a whorled appearance. The syncytial sheets of neoplastic cells had a relatively monotonous small round-to-ovoid nuclei with occasional intranuclear pseudo-inclusions and abundant eosinophilic cytoplasm. Occasional calcifications (akin to psammoma bodies) were also noted. No significant cytologic atypia or increased mitotic figures were identified (Figures 2A-2C).
Figure 2. (A) Histology showed tight nests of tumor cells punctuated by fibrous bands. (B, C) Higher power view of the tumor cell nests showing syncytial arrangement, bland cytology, occasional intranuclear inclusions, and scattered calcifications. Magnifications in A is 10x while B and C are 40x each.
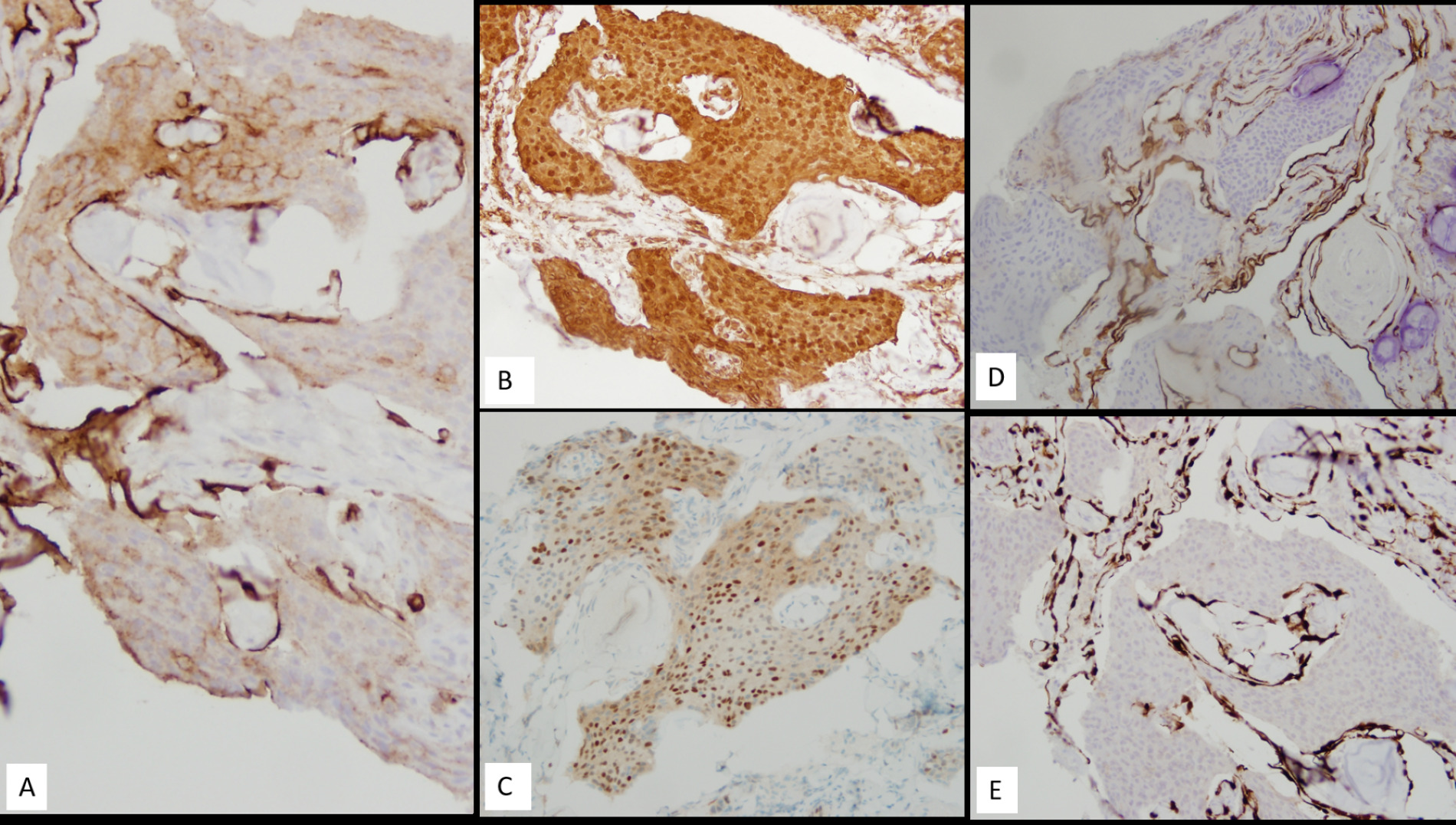
Immunohistochemistry (IHC) revealed patchy membranous staining for epithelial membrane antigen (EMA) (Figure 3A) (Dako/Agilent, Santa Clara, CA, US), strong and diffuse positivity for vimentin (Figure 3B) (Dako/Agilent, Santa Clara, CA, US), and variable nuclear staining for progesterone receptor (PR) (Figure 3C) (Ventana Medical Systems, Oro Valley, AZ, USA). IHC for S-100 (Biocare Medical, Pacheco, CA), cytokeratin 7 (Figure 3D) (Dako/Agilent, Santa Clara, CA, US), CK5/p40 (Figure 3E) (CK5: Leica Biosystems, Buffalo Grove, IL; p40: Biocare Medical, Pacheco, CA), chromogranin (Ventana Medical Systems, Oro Valley, AZ, USA), and synaptophysin (Richard-Allen Scientific/Thermo Fisher Scientific, Kalamazoo, MI USA) were negative. The morphologic features and immunophenotype were consistent with a diagnosis of meningioma, WHO grade 1. All immunohistochemistry was performed at Tricore laboratories using the Ventana/Roche Benchmark Ultra auto stainer.
Figure 3. Immunohistochemistry revealed tumor cells positive for EMA (A), vimentin (B), and PR (C) while negative for CK7 and CK5-p40 (D and E respectively). Magnification in A-E is 20x.
Discussion
DiscussionMeningiomas are common, usually benign, tumors with an indolent clinical course and account for up to 30% of primary brain neoplasms [1]. While they can occur at any age, they generally present during the fifth to seventh decades with a predilection for females over males (F:M= ~2:1) [6]. Extracranial meningiomas are uncommon (<2%) and most commonly involve the head and neck region, especially sinonasal tract, ear and temporal bone and scalp [1,3]. Meningiomas of the external ear and temporal bone are even more rare, and as a primary lesion, they account for less than 1% of all meningioma [3].
More often, intracranial meningiomas show secondary extension (up to 20%) involving the ear and temporal bone, and other sites in the head and neck region [3,7]. Radiographic evidence excluding a primary intracranial component is helpful for diagnosis of a primary extracranial meningioma. Dural thickening can be seen in association with primary intraosseous meningiomas and does not imply existence of an intracranial component [8]. Although CT imaging has its utility, MRI with a T1- weighted, gadolinium diethylene triamine pentoacetic acid (Gd-DTPA) enhancement is the preferred method for detecting meningiomas and evaluating their anatomical origin and extent [1,2,5]. Nager et al. [9] identified two types of temporal bone meningiomas: Type 1, tumors with an associated intracranial component; and Type 2, Tumors without a demonstrable intracranial meningioma. Chang et al. [5] reported type 1 tumors in their case series and discussed the potential pathways of extension of intracranial meningiomas into the middle ear or mastoid, namely: 1. Tegmen tympani, 2. Posterior fossa plate, 3. Internal auditory canal (IAC), and 4. Jugular foramen. In addition, in their review dating back to 1886, the authors found 77 cases of middle ear/ mastoid meningiomas, majority of which were secondary in nature with tegmen being the most common route of spread [5].
The origin of primary extra cranial meningiomas is debatable; however, increasing evidence supports that meningiomas arise from arachnoid cap cells via various mechanisms [1-3,5,7]:
a) From arachnoid cells in the sheaths of nerves or vessels where they emerge from the skull foramina
b) Displaced arachnoid cells during a traumatic event or cerebral hypertension
c) Displaced pacchionian bodies become entrapped in extra cranial location during embryologic development
d) From undifferentiated or multipotential mesenchymal cells
Clinical presentation in these tumors can be nonspecific and primarily depends on the anatomic site of origin. Ear and temporal bone meningiomas usually present with hearing loss (sensorineural or conductive), chronic otitis media, headaches, dizziness, vertigo, tinnitus, otalgia, and bleeding [1-3,10,11]. Kumar et al. [12] described a report of extracranial meningioma arising in the external auditory canal which presented as sagging of the posterior canal wall.
Histologically, ear and temporal bone meningiomas can show a variety of morphologic patterns. The most common pattern is meningothelial, but other patterns including psammomatous and fibroblastic are seen; rarer patterns such as transitional, secretory and atypical meningiomas have also been reported [3,6,11,13,14]. Tumor cells can infiltrate the bone and can also blend with the surface epithelium. The cells are usually arranged in lobules, show a syncytium, and moderate amount of eosinophilic cytoplasm. The tumor cells are usually bland with round nuclei, delicate nuclear chromatin and may have intranuclear cytoplasmic inclusions.
By immunohistochemistry, tumor cells are shown to be strong and diffusely positive for vimentin, focal positive for EMA and PR while negative for GFAP, smooth muscle actin (SMA), synaptophysin, and chromogranin [3,6,13]. Few studies have reported positive expression of cytokeratins particularly in a “psammomatoid” architecture. Ereno et al. [13] reported diffuse positivity for a broad spectrum of cytokeratins in their case of a secretory meningioma of temporal bone. Our case stained strong and diffusely for vimentin, showed focal positive expression of EMA and PR and was negative for CK7, CK5-p40, S100 and synaptophysin.
The pathologic differential diagnosis of ear and temporal bone meningiomas includes benign neoplasms such as paraganglioma, neuroendocrine adenoma of middle ear (NAME) and schwannoma; and malignancies such as primary verses metastatic carcinoma, sarcoma and melanoma [1,3]. Distinction can usually be made based on histomorphology and immunohistochemistry. Positive expression of EMA, vimentin, and PR with negative S100, CD34, SMA and STAT6 can help differentiate meningiomas from schwannoma, solitary fibrous tumor (SFT) and hemangiopericytoma. However, it becomes challenging due to the following reasons: extreme rarity of these lesions and the small, fragmented nature of biopsies from this site [2]. The biopsy in our case was small and fragmented as well (~3 mm in maximum dimension) and had marked crush artifact. The frozen section diagnosis was “favor squamous cell carcinoma”. However, permanent sections showed syncytial nests of tumor cells without distinct cell membranes, an overall bland cytology, and occasional intranuclear inclusions, suggestive of meningioma. Positive immunohistochemistry for EMA, vimentin and PR confirmed the morphologic impression.
Treatment for the primary middle ear and temporal bone meningiomas is wide surgical excision with a reported recurrence rate of 23-28% and a raw survival of 15.5 years [2,12,14,15]. However, complete excision is often not possible especially in larger tumors [15]. Few authors have suggested role of radiation therapy in these tumors, especially when recurrent or unresectable but its role as the first line therapy is controversial. Chemotherapy has no role in the treatment of these tumors. The overall prognosis is generally good with ~85% overall survival rate [2,12,16], with longest survival associated with younger age, and complete resection [1]. In absence of symptoms, a conservative approach with repeat imaging is acceptable [17]. Our patient has been on active surveillance since the diagnostic surgical biopsy and has been symptom free (over a follow up period of 10 months).
We reviewed the English literature using the keywords: extracranial mastoid meningioma, extracranial temporal bone meningioma, extracranial ear meningioma and primary intraosseous meningioma. The largest series of extracranial meningiomas published in 2009 by Rushing et al. [1] reviewed 205 cases from the files of Armed Forces Institute of Pathology (AFIP) out of which 146 were included in the study with adequate clinical information, demographics, and histologic material. Ear and temporal bone were the second most affected site in their review (26%). Majority (36/38) tumors arising in the ear and temporal bone were meningothelial type with the remaining two cases showing psammomatous morphology. All 38 cases were WHO grade I with a larger tumor size seen in temporal bone meningiomas compared to middle ear and EAC. The authors reported a recurrence rate of ~23.6% over an average follow up period of 14.5 years with no statistically significant difference in 5-year survival rates by site [1].
Kwon et al. reported a case of intraosseous osteolytic meningioma located in the skull base, arising from petrous bone, and showing grade II atypical meningioma on histology [18]. Their review of literature revealed 50 cases of primary intraosseous osteolytic meningioma; six of which involved the temporal/temporoparietal bone, and only one showing atypical/ grade II morphology. The atypical type was largely restricted to frontal location. The tumor in our case was in the temporal bone, showed grade I meningothelial meningioma and did not exhibit osteolytic features.
Liu et al. reviewed primary extradural meningiomas in the head, and their literature review identified 34 cases involving the middle ear, 24 involving the temporal bone and 7 in the external auditory canal. Additionally, they reported one original case involving the middle ear [10]. Our review identified additional cases (Table 1), and to date, in the English literature, there are 124 reported cases (including present case) of primary meningiomas involving the ear and temporal bone [1,3,12-15,18-25]. Case reports of temporal bone/middle ear meningiomas that had an intracranial component have been excluded from the table [16,17].
| Author, year | Number of cases (n) | Age (years) | Sex | Location | Type |
| O’Reilly, 1998 [19] | 1 | 59 | M | TB | Unspecified |
| Yadav, 2001 [20] | 1 | 16 | M | TB | Unspecified |
| Uppal, 2003 [21] | 1 | 38 | F | EAC | Transitional Cell |
| Thompson, 2003 [3] and Rushing et al, 2009 [1] |
38# | Mean age= 50 | F> M | MA, TB, EAC | Meningothelial (majority), psammomatous, atypical |
| Ereno et al, 2005 [13] | 1 | 44 | F | ME/EAC | Secretory |
| Mahou et al, 2006 [22] | 1 | 45 | F | TB, SB | Unspecified |
| Kumar et al, 2006 [12] | 1 | 45 | M | EAC | Meningothelial |
| Marcelissen et al, 2008 [11] | 1 | 56 | F | TB | Secretory |
| Stevens, 2014* [15] | 2 | 42 and 47 | F | Mastoid, ME | Unspecified |
| Laconetta, 2012 [23] | 1 | 75 | F | TB | Meningothelial |
| Asil, 2014 [24] | 1 | 64 | M | TB | Unspecified |
| Liu, 2015** [14] | 1 | Unspecified | Unspecified | ME | Unspecified |
| Hu, 2019 [25] | 1 | 80 | M | Mastoid | Meningothelial |
| Kwon, 2019*** [18] | 1 | 80 | M | TB | Atypical |
| Present case | 1 | 64 | M | TB/mastoid | Meningothelial |
|
ME: Middle Ear; EAC: External Auditory Canal; TB: Temporal Bone; SB: Sphenoid Bone #Thompson et al. report 36 cases of TB, ME, EAC and “mixed” meningiomas from 1970-1996; however, definitive evidence that neoplasms were without intracranial component was not available in all cases (except 2). Rushing et al. expanded-on Thompson et al. study, using same cohort but from 1970-1999; two extra cases were identified, one involving ME alone and the other “mixed”, resulting in a combined total of 38 cases. *Stevens et al. presented 10 cases of ME meningiomas; two cases definitively stated they were primary and without an intracranial component. **Liu et al. reviewed primary extradural meningiomas in head, and their literature review identified 34 cases involving ME, 24 involving TB and 7 in EAC; they reported one original case (shown in above table). ***Kwon et al. reviewed primary osteolytic meningiomas, and their literature review identified 8 cases involving TB; they also reported one original case (included in above table). |
|||||
The diagnosis in current case was complicated by patient’s acute on chronic presentation due to mastoiditis and secondary septic thrombophlebitis. Patient possibly suffered from an episode of serous otitis media (prior to his arrival at UNM hospital) when he presented with otalgia, drainage and decreased hearing and was prescribed oral antibiotics at an outside hospital. To our knowledge, there is only one other case of temporal bone meningioma reported in the literature [26] with an acute and critical presentation. However, this patient had a transcranial meningioma with an intracranial en plaque component and an intraosseous temporal bone component, with invasion into the mastoid and middle ear [26]. Additionally, our patient had a one-and-a-half-year history of seizures on lamotrigine with no reports of associated trauma. While meningiomas are largely benign, they can occasionally present with aggressive and obstructive symptoms leading to destruction of tissue, giving rise to further complications including infection and thrombosis, as seen in our patient.
Conclusion
Primary extracranial meningioma of the ear and temporal bone is an extremely rare clinical entity, and its presentation can vary from an innocuous clinical picture to a more pernicious one. Imaging studies may provide some suspicion of diagnosis, but histopathologic examination is required to make a definitive diagnosis. The mainstay of treatment is complete excision of the tumor with debatable radiation therapy for recurrent or unresectable tumors. Distinction from other tumors is essential as extracranial meningioma have an excellent long-term prognosis with only limited recurrence.
Acknowledgements
Guarantor of submission statement
The corresponding author is the guarantor of submission.
Ethical clearance
This is a case report; hence IRB approval is not applicable for this manuscript.
Consent
No consent was obtained in this case as patient’s personal health information (PHI) or any identifiers have not been disclosed in the manuscript.
Author’s contributions
MT researched the literature and wrote the manuscript. SA supervised the work and edited the final manuscript. EF helped with histology images and edited final manuscript.
References
2. Ferlito A, Devaney KO, Rinaldo A. Primary extracranial meningioma in the vicinity of the temporal bone: a benign lesion which is rarely recognized clinically. Acta Otolaryngologica. 2004 Jan 1;124(1):5-7.
3. Thompson LD, Bouffard JP, Sandberg GD, Mena H. Primary ear and temporal bone meningiomas: a clinicopathologic study of 36 cases with a review of the literature. Modern Pathology. 2003 Mar;16(3):236-45.
4. Guzowski J, Paparella MM, Rao KN, Hoshino T. Meningiomas of the temporal bone. The Laryngoscope. 1976 Aug;86(8):1141-6.
5. Chang CJ, Cheung SW, Jackler RK. Meningiomas presenting in the temporal bone: the pathways of spread from an intracranial site of origin. Otolaryngology--Head and Neck Surgery. 1998 Dec;119(6):658-64.
6. Flaman AN, Wasserman JK, Gravel DH, Purgina BM. Ear and temporal bone pathology: neural, sclerosing and myofibroblastic lesions. Head and Neck Pathology. 2018 Sep;12(3):392-406.
7. Friedman CD, Costantino PD, Teitelbaum B, Berktold RE, Sisson Sr GA. Primary extracranial meningiomas of the head and neck. The Laryngoscope. 1990 Jan;100(1):41-8.
8. Bassiouni H, Asgari S, Hübschen U, König HJ, Stolke D. Dural involvement in primary extradural meningiomas of the cranial vault. Journal of Neurosurgery. 2006 Jul 1;105(1):51-9.
9. Nager GT. Meningioma involving the temporal bone. Springfield, IL: Charles C. Thomas, 1964.
10. Evrard D, Daval M, Ayache D. Otitis Media with Effusion Revealing Underlying Meningioma. The Journal of International Advanced Otology. 2018 Apr;14(1):
11. Marcelissen TA, De Bondt RB, Lammens M, Manni JJ. Primary temporal bone secretory meningioma presenting as chronic otitis media. European Archives of Oto-rhinolaryngology. 2008 Jul;265(7):843-6.
12. Kumar G, Basu S, Sen P, Kamal SA, Jiskoot PM. Ectopic meningioma: a case report with a literature review. European Archives of Oto-Rhino-Laryngology and Head & Neck. 2006 May;263(5):426-9.
13. Ereño C, Izquierdo AP, Basurko JM, Bilbao FJ, López JI. Temporal bone secretory meningioma presenting as a middle ear mass. Pathology-Research and Practice. 2006 Jun 12;202(6):481-4.
14. Liu Y, Wang H, Shao H, Wang C. Primary extradural meningiomas in head: a report of 19 cases and review of literature. International Journal of Clinical and Experimental Pathology. 2015;8(5):5624.
15. Stevens KL, Carlson ML, Pelosi S, Haynes DS. Middle ear meningiomas: a case series reviewing the clinical presentation, radiologic features, and contemporary management of a rare temporal bone pathology. American Journal of Otolaryngology. 2014 May 1;35(3):384-9.
16. Ricciardiello F, Fattore L, Liguori ME, Oliva F, Luce A, Abate T, et al. Temporal bone meningioma involving the middle ear: A case report. Oncology Letters. 2015 Oct 1;10(4):2249-52.
17. Nicolay S, De Foer B, Bernaerts A, Van Dinther J, Parizel PM. A case of a temporal bone meningioma presenting as a serous otitis media. Acta Radiologica Short Reports. 2014 Nov 26;3(10):2047981614555048.
18. Kwon SM, Ko Y, Bang SS. Primary intraosseous osteolytic meningioma: a case report and review of the literature. BMC Neurology. 2019 Dec;19(1):1-6.
19. O’Reilly RC, Kapadia SB, Kamerer DB. Primary extracranial meningioma of the temporal bone. Otolaryngology—Head and Neck Surgery. 1998 May;118(5):690-4.
20. Yadav YR, Rahman HH, Tandan JK, Singh BP, Kriplani TC, Mahendra R. Primary ectopic meningioma. Journal of the Indian Medical Association. 2001 Feb 1;99(2):102-3.
21. Uppal HS, Kabbani M, Reddy V, Kaur S. Ectopic extra-cranial meningioma presenting as an aural polyp.European Archives of Oto-rhino-laryngology. 2003 Jul;260(6):322-4.
22. El Mahou S, Popa L, Constantin A, Jamard B, Cantagrel A, Mazieres B, et al. Multiple intraosseous meningiomas. Clinical Rheumatology. 2006 Jul 1;25(4):553-4.
23. Iaconetta G, Santella A, Friscia M, Abbate V, Califano L. Extracranial primary and secondary meningiomas. International Journal of Oral and Maxillofacial surgery. 2012 Feb 1;41(2):211-7.
24. Asil K, Aksoy YE, Yaldiz C, Kahyaglu Z. Primary intraosseous meningioma mimicking osteosarcoma: case report. Turkish Neurosurgery. 2015;25(1):174-6.
25. Hu M, Tang Y, Long G, Zhang D, Kresak JL, Lai J. Primary Extracranial Meningioma of Mastoid in a Patient With History of Skin Squamous Cell Carcinoma, Lung Adenocarcinoma and Prostatic Carcinoma. Anticancer Research. 2019 Jun 1;39(6):3197-201.
26. Qureshi A, Quinones-Hinojosa A, Ziai W. A rare infectious presentation of a temporal bone meningioma. Journal of Nneuro-oncology. 2014 Feb;116(3):633-4.