Abstract
Postmenopausal hyperhidrosis (PMH) is an important differential diagnosis to vasomotor symptoms (VMS) in menopause. The objective is to describe the differences in clinical presentation and treatment of the two conditions.
Patients suffering from PMH represent a unique cohort of patients with primary hyperhidrosis and should therefore not be treated in the same way as those displaying VMS during menopause. Postmenopausal hyperhidrosis is a neglected differential diagnosis to the common VMS in menopause. The two diagnoses have similarities but also distinct differences in presentation. However, the treatments of the diagnoses differ substantially. This review explains the differences in clinical presentation and treatments and suggests botulinum toxin type B in the treatment of vasomotor symptoms due to anti-oestrogen therapy.
Main Message in Bullet Point Form
- Postmenopausal hyperhidrosis (PMH) is primary and hereditary, where oestrogen replacement has no effect.
- Vasomotor symptoms in menopause are due to reduced levels of oestrogen and can be treated with oestrogen replacement.
- Botulinum toxin type B (Btx B) has the best effect and least side effects when treating PMH.
- Vasomotor symptoms secondary to anti-oestrogen could be treated with Btx B to eliminate bothersome sweating.
Introduction
Postmenopausal hyperhidrosis (PMH) is an important and overlooked differential diagnosis of vasomotor symptoms (VMS) during menopause. The diagnoses have similarities but also distinct differences that we want to draw attention to whilst at the same time we discuss treatment options for the different conditions. We experience that many professionals don´t recognise PMH, which leads to doctors’ delay, wrong treatment, risk of side-effects´ from oestrogens, and patients suffering. This review highlight PMH as an important differential diagnosis to VMS in menopause.
Underlying Physiology
Heat regulation of the body is controlled by our ‘thermostat’, the hypothalamus, which responds to afferent nerve signals and humoral substances and maintains a temperature set-point that varies over the day, season, a woman’s menstrual cycle, climate change, pyrogens, osmolality, and carbon dioxide concentration in the blood etc. [1]. Excess heat is eliminated primarily by changed behaviour such as consciously seeking out cooler places or unknowingly kicking off the blanket during sleep [2]. The peripheral thermoregulatory mechanisms involve sweating and redirecting blood flow to the skin.
These are controlled by nuclei in the preoptic area of the hypothalamus. The eccrine sweat glands are innervated by sympathetic fibres with acetylcholine as transmitters. Important co-transmitters in the postganglionic sweat fibres are VIP (vasoactive peptide) and CGRP (calcitonin gene-related peptide) which with vasodilation and increased vasopermeability provide extravasation of plasma to the sweat glands, which via the sweat exits later becomes a hypotonic salt-containing sweat. While sweat eliminates heat by way of evaporation, where one millilitre of evaporated sweat corresponds to 0.58 kcal energy reduction, redirected blood flow to the skin does so by radiating excess heat to a cooler (< 37°Celsius) environment [3]. Thermoregulatory vasodilation is regulated via reduced stimulation of adrenergic receptors, but the neuropeptides in the nerve endings of the sweat glands can also play a role [1].
Primary Hyperhidrosis
Primary hyperhidrosis is an inherited disability in 4.8% of the population and produces a major negative impact on quality of life [4]. The condition is characterised by low thresholds for stimuli such as stress (arousal), heat and exertion, in the sweat-regulating systems of the central nervous system (CNS), which in addition to the hypothalamus also include the limbic system and frontal cortex [5]. Arousal leads to a pathologically increased sympathetic outflow to the sweat glands in patients with hyperhidrosis compared to a normohidrotic control group [6].
Sweat in the palms of hand and soles of the feet, as well as in the paws of many mammals, is crucial for the grip function during “fight and flight”, which explains why nuclei in the limbic system are sweat-regulating and that stress can be a deteriorating factor in patients with mainly thermoregulatory hyperhidrosis which is found on large body surfaces, such as the head and torso. Age of onset varies: hands-feet often debut in childhood, armpits-groin/buttocks/genitalia during adolescence, and head-torso tend to debut later in life. Patients with hyperhidrosis usually sweat abnormally much from more areas of the body, and the process is long-lasting and, for many, lifelong [5].
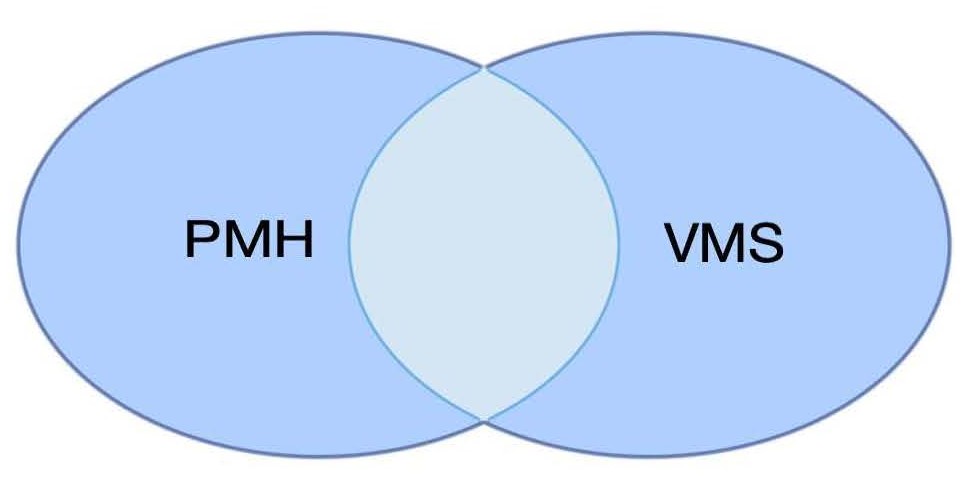
The diagnostic criteria for primary focal hyperhidrosis were established at an international meeting of experts in New York and were defined as bilateral symmetrical excessive sweating for at least 6 months, and at least two of the following bi-criteria: troublesome sweating at least twice a week, heredity, anhidrosis during sleep, reduced quality of life and onset before the age of 25 years [7]. However, the New York meeting did not provide guidelines for thermoregulatory hyperhidrosis, often referred to as general hyperhidrosis, including the head and torso. Of the thousands of patients with thermoregulatory hyperhidrosis that we have treated, the age of onset is often later than 25 years and nocturnal sweating is not uncommon, i.e., contrary to the diagnostic bi-criteria for primary focal hyperhidrosis of the hands, feet and armpits. Thermoregulatory hyperhidrosis has many characteristics similar to those of VMS during menopause (Figure 1).
Figure 1. Postmenopausal hyperhidrosis (PMH) and Vasomotor symptoms in menopause (VMS) have great similarities with probable overlap. Patients with primary hyperhidrosis may be impaired by declining oestrogen levels, and during a period in the course of the illness experience a partial improvement from oestrogen substitution. At the same time, patients with therapy-resistant VMS may instead exhibit strong symptoms of PMH where botulinum toxin type B should be used before oestrogen supplementation.
Secondary hyperhidrosis accounts for a small proportion of all people with hyperhidrosis and must be excluded. The underlying causes can be medication, denervation, endocrine disorders, cancer or infections. It is usually easy, using few anamnestic data, to distinguish primary hyperhidrosis from secondary ditto, but in certain cases further investigation is required (Table 1) [5].
| Primary hyperhidrosis | Secondary hyperhidrosis | |
| Heredity | Yes | No |
| Medical history | Long | Short |
| Onset | Early/late | Late |
| Distribution | Focal/general, symmetrical | Regional/general, asymmetrical |
| B-symptom | No | Occasionally |
| Denervation | No | Occasionally |
Postmenopausal Hyperhidrosis
Postmenopausal hyperhidrosis is a misleading name for primary thermoregulatory hyperhidrosis in postmenopausal women. The name suggests that menopause and declining oestrogen levels have something to do with the diagnosis. Ten percent of all women over the age of 70 years suffer from bothersome sweating, which probably does not only have to do with falling oestrogen levels [8]. In addition, the onset of hyperhidrosis can occur long before or after the onset of menopause. Our experience is that women sense when menopause stops and hyperhidrosis continues: the hot flashes disappear and the sweating becomes situation dependent (stress/ heat/effort). The majority suffering from PMH who seek help at a Sweat Clinic medicate or have medicated with oestrogen despite the effect having diminished or completely disappeared. Many women express “that they understand that their pronounced sweating has nothing to do with menopause” but they continue with oestrogen replacement. This paradox is difficult to comprehend, but patients can sometimes express “that maybe it would be even worse if I stop”. Postmenopausal hyperhidrosis lacks the symptoms of hot flashes and is not helped by oestrogen substitution [9].
Postmenopausal hyperhidrosis often assails the headtorso region, but may also include other body areas. In a minor study, a double onset was noted with hyperhidrosis in adolescence from e.g., the armpits, followed by a troublefree interval and then pronounced sweating starting usually between 40 and 70 years of age [10]. Patients with PMH are often aware that it does not really have anything to do with menopause, and that pronounced sweating is often present in the family. Postmenopausal hyperhidrosis is situation dependent (heat/effort/stress) and lacks hot flashes as in VMS. Nocturnal sweats are common in both conditions. An important dividing line between the diagnoses is the response to oestrogen substitution, where PMH does not respond to treatment, unless the patient has it in combination with VMS (Table 2).
| PMH | VMS | |
| Heredity | Yes | Occasionally |
| Onset | Early/late | Late |
| Duration | Lifelong | Temporary – Long |
| Localisation | Head – Torso + other places | Head – Torso |
| Nocturnal sweating | Occasionally | Often |
| Sweating | Yes | Yes |
| Previous hyperhidrosis | Yes | No |
| Flashes | No | Yes |
| Situation-dependent | Yes (heat/stress/exertion) | Occasionally |
| Effect of oestrogen | No | Yes |
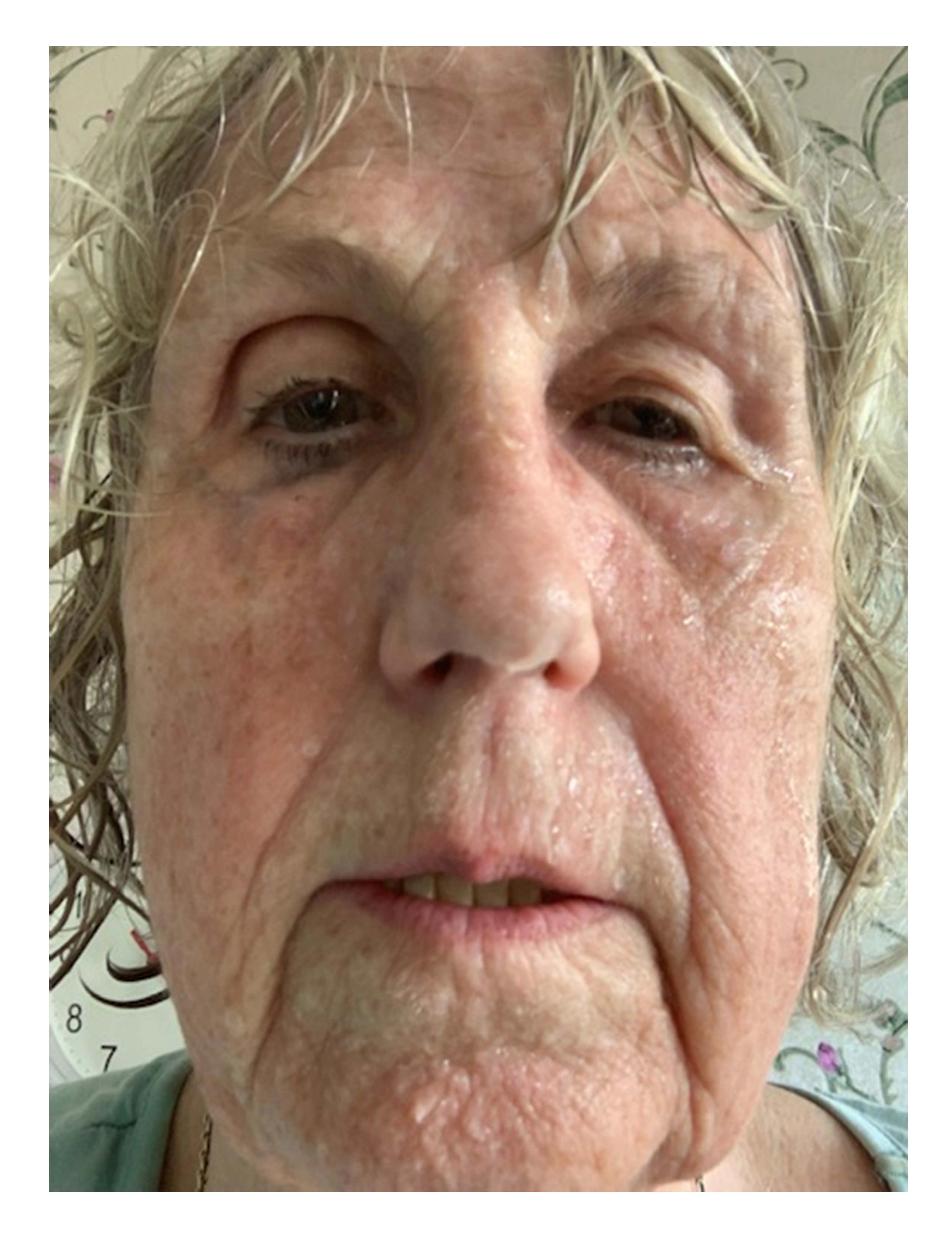
Figure 2. 77-year-old woman who had been sweating profusely since the age of 40 with severe deterioration with the onset of menopause. No flashes. No effect after one year’s oestrogen substitution. Situation-triggered sweating where minor effort induced profuse facial sweating. Her quality of life was strongly negatively affected. Mascara ran, glasses misted up, dripping sweat, hair soaking wet even in winter. Remembers that both parents sweated profusely, father for as long as she can remember, mother following gynaecological surgery in her 40s. Since the age of 67, she has received botulinum toxin type B regularly, initially via County Council-funded healthcare, now privately paid. Does not want to medicate with Oxybutynin given the cognitive side effects. Following treatment, she has been free from sweating of the face and head for 4-6 months. The picture shows her status before treatment.
Vasomotor Symptoms during Menopause
Hot flashes and sweating, i.e., VMS, are a consequence of ovarian insufficiency which can be iatrogenic in cancer treatment or surgery, premature menopause (earlier than age 40 years) or in the physiological menopause that occurs on average at the age of 51 years. The prevalence of VMS in physiological menopause is 75% and in 2018, 11% of Swedish women that aged 45-60 years received oestrogen replacement therapy [11]. The median duration for VMS is five years, but the discomfort experienced by individuals can last for decades. At the same time, the clinician must be aware that long-term VMS may in fact be PMH, especially when oestrogen replacement proves ineffective. Reduced oestrogen levels affect the hypothalamic temperature setpoint, leading to compensatory flashes, such as a wave of heat beginning at the top of the chest and spreading to the head, and followed by sweating. The attacks of flashes/sweating are not uncommonly accompanied by palpitations and often take place without any triggering factors. Night sweats are common and thus is also disturbed night sleep. Untreated, VMS is a major handicap for many women. According to a US study of women with breast cancer being treated with anti-oestrogen, 14% of the subjects discontinued treatment prematurely due to side effects such as VMS [12].
Treatment of Postmenopausal Hyperhidrosis and of Vasomotor Symptoms in Menopause
Postmenopausal hyperhidrosis and VMS are today treated completely differently. This is why it is important to distinguish the diagnoses from each other. At the same time, there may be important commonalities and that treatment of VMS with e.g., Botulinum toxin (Btx) could be an alternative, not least in iatrogenic VMS triggered by anti-oestrogen. While treatment guidelines for VMS are based on large randomised trials and that there is an international consensus, those for PMH, or primary hyperhidrosis, are to a great degree divergent, as small studies, cost aspects and lack of diagnostic criteria play a part. International guidelines primarily advocate the use of Btx and secondarily anti-cholinergics in the treatment of PMH from the head and torso [13,14]. National Swedish guidelines offer only anti-cholinergics (Oxybutynin) and treat with Btx as a second choice in hyperhidrosis localised to the hands and armpits only [15]. The Sweat Clinic has good reasons for recommending Btx B in the treatment of PMH of the head and torso, partly on the basis of preclinical and clinical studies, and partly on the basis of 20 years of proven experience [10,16,17,18]. Botulinum toxin type B can be used on large areas without the risk of general muscle weakness or over areas with underlying small muscles with a low risk of local denervation [10,18,19]. Anti-cholinergics are not recommended for long-term use due to the side effect profile. Cognitive side effects such as memory loss are underreported and are difficult to capture in clinical studies, but in recent years there are more observational studies that indicate an increased risk of dementia development in the elderly who medicate with e.g., Oxybutynin [20,21,22,23]. In dementia such as Alzheimer’s, cholinergics are used for symptom relief, and it seems logical that Oxybutynin – an anti-cholinergic that both crosses the blood-brain barrier and has an affinity for the muscarinic receptors in the hippocampal memory centres – could impair memory function.
Botulinum toxin type A produces excellent results in the treatment of hyperhidrosis, but its powerful effect on muscle tissue means that there exists a great risk of local side effects in the treatment of the face and general side effects in the treatment of larger areas such as the torso. Women generally have less muscle mass than men and this also applies to the muscles of the face. Furthermore, this muscle mass is even less in postmenopausal women. Botulinum toxin type A used in the treatment of the face in postmenopausal women carries a high risk of local denervation of musculus frontalis, eventuating in drooping eyebrows and swollen eyelids (“eyebrow ptosis”). Where injections are given to the cheeks and lips, the denervation, in relevant doses for hyperhidrosis, leads to an image corresponding to facial paralysis. Preclinical dose-response studies show that Btx B and Btx A are close to equipotent in terms of anhidrotic effect, but in terms of the muscle tissue, there is a large difference where Btx B has only 1-2% muscle weakening effect as against comparative doses of Btx A [16,17,24]. The B-toxin’s sudomotor specificity means that it can be used throughout the face, but also over large areas such as the torso, with little risk of both local and general muscle attenuation. Limited studies and the lack of commercial interest in indication studies mean that the use of Btx B for PMH has not had any impact. However, the proven experience in Sweden of using Btx B with PMH is overwhelming and extends over two decades. Btx A and anti-cholinergics are thus unsuitable for PMH, as is topical treatment with aluminium chloride. Sympathectomies can be effective where sweating of the face and head is concerned, but the side effect profile, including compensatory sweating, has meant that the method is no longer used in Sweden [25,26]. In summary, Btx B is the best treatment for PMH and is currently given to insurance patients and private payers in Sweden. Previously, for two decades, it had been given in Swedish healthcare, but has been removed from the therapy arsenal in favour of Oxybutynin.
Vasomotor symptoms treatment during menopause is primarily oestrogen replacement. Previous guidelines for using oestrogen for a maximum of five years and not beyond the age of 60 years have now been challenged, and it has been suggested that the positive effects of systemic oestrogen (reduced risk of osteoporosis and/or cardiovascular disease) may outweigh the negative ones (increased risk of breast cancer and/or thromboembolism) [27].
The second choice when treating VMS is a selective serotonin reuptake inhibitors (SSRI) / selective noradrenaline reuptake inhibitor (SNRI), which does not have the same effect, but can reduce the number of flashes/sweating. Paradoxically, SSRIs/SNRIs may cause increased sweating and may be a cause of secondary hyperhidrosis. Other treatment options for VMS are acupuncture, phytoestrogen and other herbal remedies. When oestrogen replacement proves ineffective with VMS, PMH should be considered.
Iatrogenic VMS in treatment with anti-oestrogen due to hormone-dependent breast or gynaecological cancer constitutes a major problem group because the treatment options are few. Treatment trials with Btx B in individual cases and randomised trials with Btx B are recommended, in order to reduce suffering and so that cancer treatment can be carried out. Botulinum toxin type B would in all probability eliminate the sweating component of VMS, and if the flashes are mainly caused by the co-transmitters of the sudomotor fibres such as VIP and CGRP, this part would also be improved.
Disclosure
Carl Swartling is owner of Svettmottagningen in Stockholm, Sweden.
References
2. Guyton AC. Body temperature, temperature regulation, and fever. In: Guyton AC, editor. Textbook of medical physiology. Philadelphia: WB Saunders, 1986. p. 849-60.
3. Sato K. Normal and abnormal sweat gland function. Clinical autonomic disorders. 1997:97-108.
4. Doolittle J, Walker P, Mills T, Thurston J. Hyperhidrosis: an update on prevalence and severity in the United States. Archives of dermatological research. 2016 Dec;308(10):743-9.
5. Swartling C, Brismar K, Aquilonius SM, Naver H, Rystedt A, Rosell K. KLINIK OCH VETENSKAP-Klinisk översikt-Hyperhidros—det>> tysta<< handikappet. Lakartidningen. 2011;108(47):2428-32.
6. Iwase S, Ikeda T, Kitazawa H, Hakusui S, Sugenoya J, Mano T. Altered response in cutaneous sympathetic outflow to mental and thermal stimuli in primary palmoplantar hyperhidrosis. Journal of the Autonomic Nervous System. 1997 Jun 6;64(2-3):65-73.
7. Hornberger J, Grimes K, Naumann M, Glaser DA, Lowe NJ, Naver H, et al. Recognition, diagnosis, and treatment of primary focal hyperhidrosis. Journal of the American Academy of Dermatology. 2004 Aug 1;51(2):274-86.
8. Berg G, Gottqall T, Hammar M, Lindgren R. Climacteric symptoms among women aged 60–62 in Linköping, Sweden, in 1986. Maturitas. 1988 Oct 1;10(3):193-9.
9. Eustace K, Wilson NJ. Postmenopausal craniofacial hyperhidrosis. Clinical and Experimental Dermatology.2018 Mar;43(2):180-2.
10. Cabreus P, Swartling C, Rystedt A. Postmenopausal craniofacial hyperhidrosis treated with botulinum toxin type B. The Journal of Dermatology. 2019 Oct;46(10):874-8.
11. Kartläggning av vård och behandling vid klimakteriebesvär ur perspektivet jämlik vård. (Mapping of care and treatment for menopausal symptoms from the perspective of equivalent healthcare). Stockholm: The Swedish National Board of Health and Welfare, 2020. Article No: 2020-1-6568.
12. Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, et al. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet. 2005 Jan 1;365(9453):60-2.
13. Sweathelp.org. International Hyperhidrosis Society. Primary Focal Craniofacial and Gustatory Hyperhidrosis (Frey´s Syndrome). 15 Jan 2012 [cited 16 July 2020].
14. Nicholas R, Quddus A, Baker DM. Treatment of primary craniofacial hyperhidrosis: a systematic review. American Journal of Clinical Dermatology. 2015 Oct;16(5):361-70.
15. Swedish Society for Dermatology and Venereology. Behandling av primär lokaliserad hyperhidros i offentlig sjukvård (Treatment of primary localised hyperhidrosis in public healthcare). Dec 2017 [cited 16 July 2020]. https:// ssdv.se/images/HyperhidrosUppdaterat171114.pdf.
16. Rystedt A, Karlqvist M, Bertilsson M, Naver H, Swartling C. Effect of botulinum toxin concentration on reduction in sweating: a randomized, double-blind study. Acta dermato-venereologica. 2013 Nov 1;93(6):674-8.
17. Rystedt A, Swartling C, Naver H. Anhidrotic effect of intradermal injections of botulinum toxin: a comparison of different products and concentrations. Acta Dermatovenereologica. 2008 May 1;88(3):229-33.
18. Karlqvist M, Rosell K, Rystedt A, Hymnelius K, Swartling C. Botulinum toxin B in the treatment of craniofacial hyperhidrosis. Journal of the European Academy of Dermatology and Venereology. 2014 Oct;28(10):1313-7.
19. Karlsson-Groth A, Rystedt A, Swartling C. Treatment of compensatory hyperhidrosis after sympathectomy with botulinum toxin and anticholinergics. Clinical Autonomic Research. 2015 Jun;25(3):161-7.
20. Welk B, McArthur E, Moreno-Palacios J. Editorial Comment: Increased risk of dementia among patients with overactive bladder treated with an anticholinergic medication compared to a beta-3 agonist: a populationbased cohort study. BJU Int. 2020 Jul;126(1):183-90.
21. Gray SL, Anderson ML, Dublin S, Hanlon JT, Hubbard R, Walker R, et al. Cumulative use of strong anticholinergics and incident dementia: a prospective cohort study. JAMA Internal Medicine. 2015 Mar 1;175(3):401-7.
22. Risacher SL, McDonald BC, Tallman EF, West JD, Farlow MR, Unverzagt FW, et al. Association between anticholinergic medication use and cognition, brain metabolism, and brain atrophy in cognitively normal older adults. JAMA Neurology. 2016 Jun 1;73(6):721-32.
23. Chancellor M, Boone T. Anticholinergics for overactive bladder therapy: central nervous system effects. CNS Neuroscience & Therapeutics. 2012 Feb;18(2):167-74.
24. Bentivoglio AR, Del Grande A, Petracca M, Ialongo T, Ricciardi L. Clinical differences between botulinum neurotoxin type A and B. Toxicon. 2015 Dec 1;107: 77-84.
25. Moon DH, Kang DY, Kim DW, Kang MK, Lee S. Early results of new endoscopic thoracic sympathectomy for craniofacial hyperhidrosis. Journal of Thoracic Disease. 2018 Jun;10(6):3627-31.
26. Drott C, Claes G, Rex L, Dalman P, Göthberg G, Fahlén T. Long-term results after surgery for hand sweat and flushing. Läkartidningen. 2001; 98: 1766-72.
27. North American Menopause Society. The 2017 hormone therapy position statement of the North American Menopause Society. Menopause. 2017 Jul 1;24(7):728-53.