Abstract
The outbreak of severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) has led to coronavirus disease-19 (COVID-19); a pandemic disease that has resulted in devastating social, economic, morbidity and mortality burdens. SARS-CoV-2 infects cells following receptor-mediated endocytosis and priming by cellular proteases. Following uptake, SARS-CoV-2 replicates in autophagosome-like structures in the cytosol following its escape from endolysosomes. Accordingly, the greater endolysosome pathway including autophagosomes and the mTOR sensor may be targets for therapeutic interventions against SARS-CoV-2 infection and COVID-19 pathogenesis. Naturally existing compounds (phytochemicals) through their actions on endolysosomes and mTOR signaling pathways might provide therapeutic relief against COVID-19. Here, we discuss evidence that some natural compounds through actions on the greater endolysosome system can inhibit SARS-CoV-2 infectivity and thereby might be repurposed for use against COVID-19.
Keywords
SARS-CoV-2, COVID-19, Endolysosomes, Autophagy, Natural compounds
Introduction
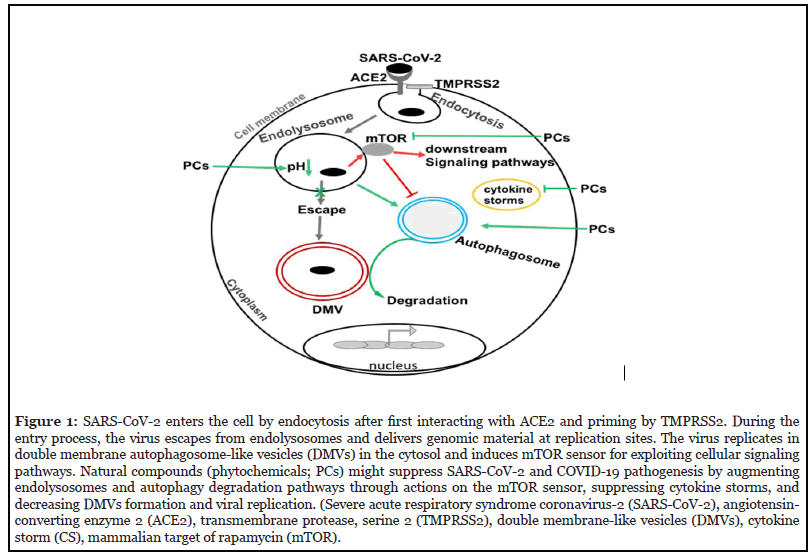
Severe acute respiratory syndrome coronavirus-2 (SARSCoV- 2) is an enveloped virus containing single-stranded RNA genomic material [1,2]. Coronavirus infectious disease-2019 (COVID-19) is a pandemic disease in humans caused by SARS-CoV-2 infection; symptoms and consequences include cardiovascular disorders, acute respiratory distress syndrome (ARDS), and death [3-5]. SARS-CoV-2 infects cells by viral spike proteins interacting with host cells expressing angiotensinconverting enzyme 2 (ACE2) receptors; the virus enters host cells following transmembrane protease serine 2 (TMPRSS2)-mediated priming [6-8]. To infect cells, the virus must be endocytosed into and then released from endolysosomes; a feature common to enveloped viruses [9,10]. In so doing, coronaviruses hijack the endocytic machinery such that they deliver their genomic material at replication sites without initiating host immune detection and host-pathogen responses [8,11-14]. Once released from endolysosomes into the cytosol, coronaviruses replicate in double membrane vesicles that resemble autophagosomes [15-18] and when viral levels are sufficiently high pathological conditions develop including cytokine storms [19-22]. Because endolysosomes are acidic organelles that contain ~60 acid hydrolases capable of catalyzing the degradation of viral particles, enhancing endolysosome acidification might suppress SARS-CoV-2 infection [15,23,24]. The acidic nature of lysosomes regulates the functions of endolysosomes and the autophagy system and multiple endolysosome-associated ion channels and proteins regulate lysosome acidity including vacuolar- ATPase, TRPML1, BK [25], SLC38A9 [26-29], and mammalian target of rapamycin (mTOR) [30-34].
mTOR downstream signaling pathways regulate fundamental cellular processes such as protein synthesis, metabolism, transcription, cell cycle, apoptosis, endolysosomes, autophagy, and immune regulation and tolerance [35-39]. Aberrant mTOR signaling is involved in various pathological conditions such as cancer and inflammation as well as cardiovascular and metabolic disorders [40,41]. In addition, multiple viruses can hijack the mTOR signaling system for the purpose of completing viral replication including influenza [42] and HIV-1 [43,44] as well as the coronaviruses MERS-CoV [45, 46] and SARS-CoV-2 [15, 47, 48].
The mTOR signaling pathway can be targeted to block the infection and replication of viruses other than coronaviruses by inducing autophagy and inhibiting viral protein synthesis [15,45-47,49,50]. Hence, mTOR might be targeted to suppress SARS-CoV-2 infection and COVID-19 using synthetic and natural compounds [51-57]. Natural compounds (phytochemicals) can enhance endolysosome acidification and autophagy by inhibiting mTOR-signaling pathways [49,58-64]. It has been suggested that increased consumption of phytochemicals or foods rich in phytochemicals might decrease the prevalence and severity of cancer, osteoporosis, and cardiovascular diseases [63]. Fruits, legumes, vegetables, and cereals contain high levels of phytochemicals including carotenoids, terpenoids, phytosterols, flavonoids, isoflavones, isothiocyanates, and fibers; substances shown to have anti-inflammatory, anti-oxidant and anti-infectious properties [64]. Phytochemicals can also enhance the degradative properties of endolysosomes and thereby suppress microbial infections as well as human metabolic and aging-related diseases [15,63,64]. Here, we briefly discuss natural compounds that affect endolysosomes and autophagy, the mTOR sensor, and as such, might find therapeutic use against SARS-CoV-2 infection and the pathogenesis of COVID-19.
Natural Compounds
Spermidine and spermine
Polyamines are generated endogenously from arginine and ornithine, and they are ingested as components of various plants [65,66]. Endogenously, putrescine synthesis from ornithine is catalyzed by ornithine decarboxylase [67-69] and from ornithine, the polyamines spermidine and spermine are generated [68]. Exogenously, ingestion of polyamines protected against age-related memory loss [70,71] and rescued memory performance [71,72]. The cardio-protective [73], anti-inflammatory, and antioxidant [74-76], actions of the polyamine spermidine may be mediated by the induction of autophagy [71,77]. Moreover, spermidine and spermine induce 5’-AMP-activated protein kinase (AMPK) and inhibit the mTOR signaling pathway to induce autophagy and suppress functions of inflammatory dendritic cells [78-80]. Spermidine and spermine both inhibited SARS-CoV-2 infection and appeared to do so by inducing viral degradation in endolysosomes [15].
Resveratrol
Resveratrol is a polyphenol with antioxidant and antiinflammatory properties, and resveratrol has been found to protect against oxidative damage in high-risk conditions like cancer, diabetes, heart diseases, neurodegenerative diseases, and microbial infections [81]. Resveratrol is enriched in peanuts, berries, and red grapes [81,82], and it can be ingested in capsules containing Polygonum cuspidatum plant extracts [83,84]. Resveratrol has an ability to enhance autophagy and kill cancer cells by suppressing the phosphoinositide 3-kinase (PI3K)/A serine/threonine protein kinase (Akt)/mTOR signaling pathway and enhancing AMPK and sirtuin (SIRT1) pathways [85-88]. Resveratrol can exert antiviral effects against various viral infections [89] including herpes simplex virus [90], enterovirus 71, Epstein-Barr virus, respiratory syncytial virus, influenza, and Middle East Respiratory Syndrome-coronavirus (MERS-CoV) [49]; MERS-CoV is a family member of SARS-CoV-2 virus [91,92]. Co-administration of resveratrol with copper may be useful in suppressing SARS-CoV-2 replication and diminishing SARS-CoV-2-induced cytokine storms [93,94].
Phytoestrogen
Phytoestrogens are natural compounds found in plants such as tofu, flaxseed, soybean, sesame seeds, and garlic [95,96]. Phytoestrogens exert estrogen-like effects [95] and have antioxidant, anti-inflammatory [97-100] and neuroprotective [101,102] properties as well as the ability to induce autophagy [103]. Phytoestrogens restrict PI3K/ Akt/mTOR signaling pathways and this mechanism has been implicated in their ability to induce autophagy and kill cancer cells [104-106]. One estrogen, 17β-estradiol, is known already to suppress multiple viral infections including influenza [107], rubella [108], HIV-1 [109], HSV-1 [110], SARS-CoV [111], and SARS-CoV-2 [112-114].
Trehalose
Trehalose, also known as tremalose and mycose, is a stable disaccharide assembled from two molecules of d-glucose [115]. Some plants, fungi, bacteria, and invertebrate animals can produce trehalose and use it as an energy source as well as to survive freezing and lack of water [116-118]. Trehalose has antioxidant [119] and neuroprotective properties [119-122], and it has been shown to inhibit HIV- 1 and mycobacterium tuberculosis (Mtb) co-infection by inducing the endolysosomal degradation pathway [123]. Further, trehalose induced mTOR-independent autophagy and suppressed cytomegalovirus infection in different cell types [124].
Baicalin
Baicalin, a component of Scutellaria baicalensis and Scutellaria lateriflora [125], can protect against amyloid-β protein-, hydrogen peroxide [H2O2]-, middle cerebral artery occlusion-, and oxygen/glucose deprivationinduced neurotoxicity [126-131]. At least some of these protective effects might be mediated through its actions on endolysosomes because baicalin can attenuate high-fat diet-induced endolysosome deacidification [132]. Baicalin can also induce apoptosis in cancer cells by downregulating mTOR signaling pathways [133-135]. The anti-influenza [136] effects of baicalin suggests its possible use against SARS-CoV-2 by targeting its 3CL protease enzyme [137].
Curcumin
Turmeric is a spice with many purported medicinal properties [138] and is a rich source of curcumin [139,140]. Curcumin (1,7-bis (4-hydroxy-3-methoxyphenyl)-1,6- heptadiene-3,5-dione) is also known as diferuloylmethane; a natural polyphenol present in the rhizome of turmeric (Curcuma longa) [140,141]. Curcumin has antioxidative and anti-inflammatory properties, and it has been used against arthritis, bacterial infections, metabolic syndrome, anxiety, and hyperlipidemia [142-147]. Curcumin has antiviral effects against a broad spectrum of viruses including herpes simplex virus-2 (HSV-2) [148], HIV-1, zikavirus [149], influenza virus [149], hepatitis virus [150], and human papillomavirus (HPV) [151]. Moreover, curcumin increases endolysosomal functions by promoting lysosomal acidification and suppressing the mTOR sensor [152-154].
Quercetin
Quercetin is a flavonoid that is present in many plants and foods including onions, red wine, berries, green tea, apples, ginkgo biloba, and buckwheat [155]. Quercetin has a broad range of biological activities including being anti-inflammatory, attenuating lipid peroxidation, inhibiting platelet aggregation [156-159], inducing cell death in cancer cells by enhancing autophagic flux and lysosomal activity [160], and suppressing PI3K/Akt/ mTOR signaling pathways [161-163]. Quercetin displays a broad range of antiviral properties; it interferes with virus entry, replication, and assembly [164-167]. Quercetin can suppress SARS-CoV-2 infection but has yet to be tested against COVID-19 [168].
Coumarin
Coumarin is a phenolic substance that is a fusion of benzene and α-pyrone rings [169,170]. Coumarin is present in Tonka bean (D. odorata) and Cinnamomum aromaticum and has also been isolated from various plants [171]. Coumarins have anti-oxidant, anti-bacterial, anti-fungal, anti-viral, and anti-cancer properties [172-175]. A hybrid of phenylsulfonylfuroxan and coumarin induced caspase-dependent cell death, autophagy, and suppressed PI3K/Akt/mTOR signaling pathway to kill cancer cells [176-178]. Accordingly, it has been suggested that coumarin might protect against COVID-19 by blocking the protease enzyme of SARS-CoV-2 [179,180].
Epigallocatechin 3-gallate (EGCG)
EGCG is a component of tea leaves [181]. EGCG has antioxidant properties and may prevent autoimmune diseases and cytokine storms [182-186] by blocking downstream inflammatory signaling pathways of the transcription factors STAT (signal transducer and activator of transcription 1/3) and NF-κB (nuclear factor kappa-lightchain- enhancer of activated B cells) [187-190]. EGCG upregulates AMPK activity in a dose-dependent manner and suppresses mTOR signaling in hepatoma cells [191]. A computer-based study has shown that EGCG is an ATP-competitive inhibitor of Akt/mTOR and enhances autophagy by AMPK activation [192-194]. Moreover, EGCG synergistically enhanced curcumin’s effects on cancer cells by inducing autophagy through suppression of the Akt/mTOR signaling pathway [195].
Naringenin
Naringenin is a flavorless flavanone; a predominant flavanone in various herbs and fruits including grapefruits, citrus, and tomatoes [196-198]. Naringenin has hepatoprotective, anti-inflammatory, anti-mutagenic, anti-cancer, and anti-microbial [199-204] effects and may control neurological, metabolic, rheumatological, and cardiovascular diseases [205-207]. Moreover, naringenin is an inhibitor of endolysosome two-pore channels (TPCs) [208-210]; channels involved in SARS-CoV-2 and Ebola virus infections [211-213] as well as the ability of HIV-1 protein Tat to escape endolysosomes [214]. Naringenin can induce cancer cell death by promoting autophagy and downregulate the Akt/mTOR signaling pathway [215-219]. These finding suggest a possible use of naringenin against COVID-19 by targeting TPCs and the Akt/mTOR signaling pathway [220-222].
Conclusion
The COVID-19 pandemic is a global disaster with devasting social, behavioral, economic and health ramifications. Endolysosomes play important roles in regulating SARSCoV- 2 infection and thus might be targeted therapeutically against COVID-19.
| Compounds | mTOR inactivation |
Endolysosomes and autophagy | Anti-inflammatory | Anti-SARS-CoV-2 activity [References] | Scoring |
|---|---|---|---|---|---|
| Spermidine and spermine | Negatively regulates mTOR signaling pathway [15,78,79] | Autophagy inducer [15] | Potential anti- inflammatory [74,75] |
Restricts SARS-CoV-2 infec- tion by SKP2 modulation (in vitro) [15] | ++ |
| Resveratrol | Negatively regulates mTOR signaling pathways [85-88] |
Autophagy inducer [86,230,231] | Potential anti- inflammatory [232] |
MERS-CoV inhibition in vitro [49] SARS-CoV-2 inhibition in vitro [93,94] Proposed for clinical trials (NCT04542993) | +++ |
| Phytoestro-gen | Negatively regulates mTOR signaling pathways [104- 106] |
Autophagy inducer [103,104] | Potential anti- inflammatory [97,98,100,233] | Restricts SARS-CoV in vivo [111] Suggested as a suppressor of COVID-19 [112,114,224,234- 236] Estrogen therapy (NCT04539626) |
+++ |
| Trehalose | No effect on mTOR [124] |
Induces autophagy and lysosomal biogenesis by TFEB activation [120,122] Induces lysosomes acidification and autophagy by mucolipin-1 (TRPML1) activation to protect mycobacterium tuberculosis infection [123] |
Potential anti- inflammatory [237] |
Potential target against COVID-19 [238] | ++ |
| Baicalin | Negatively regulates mTOR signaling pathways [133- 135] |
Autophagy inducer [133] Induces lysosomes acidification by promoting assembly of v-ATPase pump [132] | Potential anti- inflammatory [239- 241] |
Suppresses COVID-19 patho- logical condition in vivo, in vitro [137,242-244] Proposed for clinical trial (NCT03830684) | +++ |
| Curcumin | Negatively regulates mTOR signaling pathways [152- 154] |
Autophagy inducer [59,60] | Potential anti- inflammatory [142,143,245] | Proposed against COVID-19 [245-248] Proposed for clinical trial against COVID-19 (NCT04353310) | +++ |
| Quercetin | Negatively regulates mTOR signaling pathways [161,162] | Autophagy inducer [160,161] | Potential anti- inflammatory [156,249] | Potential target against COVID-19 [168,250-253] Proposed for clinical trial against COVID-19 (NCT04377789) | +++ |
| Coumarin | Negatively regulates mTOR signaling pathways [177] | Autophagy inducer [176,178] | Potential anti- inflammatory [254] |
Potential target against COVID-19 (in silico) 179,180] | ++ |
| Epigallocat-echin 3-gal- late [EGCG] | Negatively regulates mTOR signaling pathways [192,194,195] | Autophagy inducer [194,255] | Potential anti- inflammatory [183,185,187,189] | Potential target against COVID-19 and Proposed as previfenon (NCT04446065) [186,256-258] | +++ |
| Naringenin | Negatively regu- lates mTOR sig- naling pathways [217] | Autophagy inducer [217,219,259] A blocker of Two pore channels (TPCs). TPCs are highly involved in SARS-CoV-2’s entry into cells [260] | Potential anti- inflammatory [200,261,262] | Suppresses SARS- CoV-2 infection in vitro [221,222,263] |
+++ |
Table 1: Potential natural compounds against SARS-CoV-2 infection and COVID-19 pathogenesis (Scoring according to evidence; +++ (high confidence), ++ (moderate confidence).
Relevant to COVID-19, endolysosomes are important regulators of innate immune responses and antigen presentation and phytochemicals have purported antiinflammatory, anti-oxidant, and anti-viral properties. These properties might play protective roles in blocking SARS-CoV-2 replication and infection at least in part by enhancing endolysosome acidification, increasing autophagy, and inhibiting mTOR-signaling pathways. Several natural compounds have shown promise in suppressing SARS-CoV-2 infection in humans, but these compounds may be toxic at higher concentrations and doses [223-229]. Accordingly, a great deal more work is necessary to have confidence that phytochemicals can provide therapeutic benefit against SARS-CoV-2 infection and alter positively the clinical course of COVID-19.
Conflict of Interest
No conflict of interest.
Author Contribution
All authors contributed equally to the writing of this manuscript.
Funding
This work was partly supported by NIH grant RO1 (MH119000).
References
2. Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565-74.
3. Contini C, Di Nuzzo M, Barp N, Bonazza A, De Giorgio R, Tognon M, et al. The novel zoonotic COVID-19 pandemic: An expected global health concern. Journal of Infection in Developing Countries. 2020;14(3):254-64.
4. Gudbjartsson DF, Helgason A, Jonsson H, Magnusson OT, Melsted P, Norddahl GL, et al. Spread of SARS-CoV-2 in the Icelandic Population. New England Journal of Medicine. 2020.
5. Geiger JD, Khan N, Murugan M, Boison D. Possible Role of Adenosine in COVID-19 Pathogenesis and Therapeutic Opportunities. Front Pharmacol. 2020;11(1901).
6. Hoffmann M, Kleine-Weber H, Pöhlmann S. A Multibasic Cleavage Site in the Spike Protein of SARSCoV- 2 Is Essential for Infection of Human Lung Cells. Molecular Cell. 2020;78(4):779-84.e5.
7. Shang J, Ye G, Shi K, Wan Y, Luo C, Aihara H, et al. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581(7807):221-4.
8. Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181(2):271-80.e8.
9. White JM, Whittaker GR. Fusion of Enveloped Viruses in Endosomes. Traffic (Copenhagen, Denmark). 2016;17(6):593-614.
10. Yang Z-Y, Huang Y, Ganesh L, Leung K, Kong W-P, Schwartz O, et al. pH-dependent entry of severe acute respiratory syndrome coronavirus is mediated by the spike glycoprotein and enhanced by dendritic cell transfer through DC-SIGN. Journal of Virology. 2004;78(11):5642- 50.
11. Coutard B, Valle C, de Lamballerie X, Canard B, Seidah NG, Decroly E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antiviral Res. 2020;176:104742-.
12. Pranesh P, Rajat D, Narendra D. Targeting TMPRSS2 and Cathepsin B/L Together May Be Synergistic Against SARS-CoV-2 Infection. PLoS Comput Biol. 2020 Dec 8;16(12):e1008461.
13. Follis KE, York J, Nunberg JH. Furin cleavage of the SARS coronavirus spike glycoprotein enhances cellcell fusion but does not affect virion entry. Virology. 2006;350(2):358-69.
14. Bosch BJ, van der Zee R, de Haan CAM, Rottier PJM. The Coronavirus Spike Protein Is a Class I Virus Fusion Protein: Structural and Functional Characterization of the Fusion Core Complex. Journal of Virology. 2003;77(16):8801-11.
15. Gassen NC, Papies J, Bajaj T, Dethloff F, Emanuel J, Weckmann K, et al. Analysis of SARS-CoV-2- controlled autophagy reveals spermidine, MK-2206, and niclosamide as putative antiviral therapeutics. bioRxiv. 2020:2020.04.15.997254.
16. Snijder EJ, Limpens RW, De Wilde AH, De Jong AW, Zevenhoven-Dobbe JC, Maier HJ, et al. A unifying structural and functional model of the coronavirus replication organelle: tracking down RNA synthesis. Plos Biology. 2020;18(6):e3000715.
17. Wolff G, Limpens RWAL, Zevenhoven-Dobbe JC, Laugks U, Zheng S, de Jong AWM, et al. A molecular pore spans the double membrane of the coronavirus replication organelle. Science. 2020:eabd3629.
18. Khan N, Chen X, Geiger JD. Role of Endolysosomes in Severe Acute Respiratory Syndrome Coronavirus-2 Infection and Coronavirus Disease 2019 Pathogenesis: Implications for Potential Treatments. Front Pharmacol. 2020;11(1739).
19. Chau VQ, Oliveros E, Mahmood K, Singhvi A, Lala A, Moss N, et al. The Imperfect Cytokine Storm: Severe COVID-19 with ARDS in Patient on Durable LVAD Support. JACC: Case Reports. 2020:10.1016/j.jaccas.2020.04.001.
20. Daneshkhah A, Agrawal V, Eshein A, Subramanian H, Roy HK, Backman V. The Possible Role of Vitamin D in Suppressing Cytokine Storm and Associated Mortality in COVID-19 Patients. medRxiv. 2020:2020.04.08.20058578.
21. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(10223):497-506.
22. Minakshi R, Padhan K, Rani M, Khan N, Ahmad F, Jameel S. The SARS Coronavirus 3a Protein Causes Endoplasmic Reticulum Stress and Induces Ligand- Independent Downregulation of the Type 1 Interferon Receptor. PLoS One. 2009;4(12):e8342.
23. Gassen NC, Niemeyer D, Muth D, Corman VM, Martinelli S, Gassen A, et al. SKP2 attenuates autophagy through Beclin1-ubiquitination and its inhibition reduces MERS-Coronavirus infection. Nature Communications. 2019;10(1):5770.
24. Khan N, Haughey NJ, Nath A, Geiger JD. Involvement of organelles and inter-organellar signaling in the pathogenesis of HIV-1 associated neurocognitive disorder and Alzheimer's disease. Brain Research. 2019;1722:146389.
25. Khan N, Lakpa KL, Halcrow PW, Afghah Z, Miller NM, Geiger JD, et al. BK channels regulate extracellular Tat-mediated HIV-1 LTR transactivation. Scientific Reports. 2019;9(1):12285.
26. Rebsamen M, Pochini L, Stasyk T, de Araújo ME, Galluccio M, Kandasamy RK, et al. SLC38A9 is a component of the lysosomal amino acid sensing machinery that controls mTORC1. Nature. 2015;519(7544):477-81.
27. Shen K, Sabatini DM. Ragulator and SLC38A9 activate the Rag GTPases through noncanonical GEF mechanisms. Proceedings of the National Academy of Sciences of the United States of America. 2018;115(38):9545-50.
28. Wang S, Tsun Z-Y, Wolfson RL, Shen K, Wyant GA, Plovanich ME, et al. Metabolism. Lysosomal amino acid transporter SLC38A9 signals arginine sufficiency to mTORC1. Science (New York, NY). 2015;347(6218):188- 94.
29. Wang S, Tsun Z-Y, Wolfson RL, Shen K, Wyant GA, Plovanich ME, et al. Lysosomal amino acid transporter SLC38A9 signals arginine sufficiency to mTORC1. Science. 2015;347(6218):188-94.
30. Collins MP, Forgac M. Regulation of V-ATPase Assembly in Nutrient Sensing and Function of V-ATPases in Breast Cancer Metastasis. Frontiers in Physiology. 2018;9:902-.
31. Zoncu R, Bar-Peled L, Efeyan A, Wang S, Sancak Y, Sabatini DM. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science. 2011;334(6056):678-83.
32. Hu Y, Carraro-Lacroix LR, Wang A, Owen C, Bajenova E, Corey PN, et al. Lysosomal pH Plays a Key Role in Regulation of mTOR Activity in Osteoclasts. Journal of Cellular Biochemistry. 2016;117(2):413-25.
33. Cang C, Zhou Y, Navarro B, Seo Y-j, Aranda K, Shi L, et al. mTOR Regulates Lysosomal ATP-Sensitive Two- Pore Na+ Channels to Adapt to Metabolic State. Cell. 2013;152(4):778-90.
34. Rabanal-Ruiz Y, Korolchuk VI. mTORC1 and Nutrient Homeostasis: The Central Role of the Lysosome. International journal of Molecular Sciences. 2018;19(3):818.
35. Saxton RA, Sabatini DM. mTOR Signaling in Growth, Metabolism, and Disease. Cell. 2017;168(6):960-76.
36. Laplante M, Sabatini DM. mTOR signaling at a glance. Journal of Cell Science. 2009;122(20):3589-94.
37. Weichhart T. mTOR as Regulator of Lifespan, Aging, and Cellular Senescence: A Mini-Review. Gerontology. 2018;64(2):127-34.
38. Powell JD, Pollizzi KN, Heikamp EB, Horton MR. Regulation of immune responses by mTOR. The Annual Review of Immunology. 2012;30:39-68.
39. Wang P, Zhang Q, Tan L, Xu Y, Xie X, Zhao Y. The Regulatory Effects of mTOR Complexes in the Differentiation and Function of CD4+ T Cell Subsets. Journal of Immunology Research. 2020;2020:3406032.
40. Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149(2):274-93.
41. Das A, Reis F, Mishra PK. mTOR Signaling in Cardiometabolic Disease, Cancer, and Aging 2018. Oxidative Medicine and Cellular Longevity. 2019;2019:9692528.
42. Ranadheera C, Coombs KM, Kobasa D. Comprehending a Killer: The Akt/mTOR Signaling Pathways Are Temporally High-Jacked by the Highly Pathogenic 1918 Influenza Virus. EBioMedicine. 2018;32:142-63.
43. Taylor HE, Calantone N, Lichon D, Hudson H, Clerc I, Campbell EM, et al. mTOR Overcomes Multiple Metabolic Restrictions to Enable HIV-1 Reverse Transcription and Intracellular Transport. Cell Reports. 2020;31(12):107810.
44. Akbay B, Shmakova A, Vassetzky Y, Dokudovskaya S. Modulation of mTORC1 Signaling Pathway by HIV-1. Cells. 2020;9(5):1090.
45. Kindrachuk J, Ork B, Hart BJ, Mazur S, Holbrook MR, Frieman MB, et al. Antiviral potential of ERK/MAPK and PI3K/AKT/mTOR signaling modulation for Middle East respiratory syndrome coronavirus infection as identified by temporal kinome analysis. Antimicrobial Agents and Chemotherapy. 2015;59(2):1088-99.
46. Lehrer S. Inhaled biguanides and mTOR inhibition for influenza and coronavirus (Review). World Acad Sci J. 2020;2(3):1.
47. Appelberg S, Gupta S, Svensson Akusjärvi S, Ambikan AT, Mikaeloff F, Saccon E, et al. Dysregulation in Akt/mTOR/HIF-1 signaling identified by proteotranscriptomics of SARS-CoV-2 infected cells. Emerging Microbes & Infections. 2020;9(1):1748-60.
48. Bouhaddou M, Memon D, Meyer B, White KM, Rezelj VV, Correa Marrero M, et al. The Global Phosphorylation Landscape of SARS-CoV-2 Infection. Cell. 2020;182(3):685-712.e19.
49. Lin S-C, Ho C-T, Chuo W-H, Li S, Wang TT, Lin C-C. Effective inhibition of MERS-CoV infection by resveratrol. BMC Infectious Diseases. 2017;17(1):144.
50. Martin AR, Pollack RA, Capoferri A, Ambinder RF, Durand CM, Siliciano RF. Rapamycin-mediated mTOR inhibition uncouples HIV-1 latency reversal from cytokineassociated toxicity. Journal of Clinical Investigation. 2017;127(2):651-6.
51. Zheng Y, Li R, Liu S. Immunoregulation with mTOR inhibitors to prevent COVID-19 severity: A novel intervention strategy beyond vaccines and specific antiviral medicines. Journal of Medical Virology. 2020:10.1002/ jmv.26009.
52. Ramaiah MJ. mTOR inhibition and p53 activation, microRNAs: The possible therapy against pandemic COVID-19. Gene Reports. 2020;20:100765-.
53. Terrazzano G, Rubino V, Palatucci AT, Giovazzino A, Carriero F, Ruggiero G. An Open Question: Is It Rational to Inhibit the mTor-Dependent Pathway as COVID-19 Therapy? Frontiers in Pharmacology. 2020;11(856).
54. Sharma S, Ray A, Sadasivam B. Metformin in COVID-19: A possible role beyond diabetes. Diabetes Research and Clinical Practice. 2020;164:108183-.
55. Scheen AJ. Metformin and COVID-19: From cellular mechanisms to reduced mortality. Diabetes & Metabolism. 2020;46(6):423-6.
56. Husain A, Byrareddy SN. Rapamycin as a potential repurpose drug candidate for the treatment of COVID-19. Chemico-Biological Interactions. 2020;331:109282-.
57. Maiese K. The Mechanistic Target of Rapamycin (mTOR): Novel Considerations as an Antiviral Treatment. Current Neurovascular Research. 2020;17(3):332-7.
58. Moosavi MA, Haghi A, Rahmati M, Taniguchi H, Mocan A, Echeverría J, et al. Phytochemicals as potent modulators of autophagy for cancer therapy. Cancer Letters. 2018;424:46-69.
59. Limanaqi F, Biagioni F, Busceti CL, Ryskalin L, Polzella M, Frati A, et al. Phytochemicals Bridging Autophagy Induction and Alpha-Synuclein Degradation in Parkinsonism. International Journal of Molecular Sciences. 2019;20(13):3274.
60. Deng S, Shanmugam MK, Kumar AP, Yap CT, Sethi G, Bishayee A. Targeting autophagy using natural compounds for cancer prevention and therapy. Cancer. 2019;125(8):1228-46.
61. Francini-Pesenti F, Spinella P, Calò LA. Potential role of phytochemicals in metabolic syndrome prevention and therapy. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy. 2019;12:1987-2002.
62. Cicero AFG, Colletti A. Role of phytochemicals in the management of metabolic syndrome. Phytomedicine. 2016;23(11):1134-44.
63. Dahiya P, Purkayastha S. Phytochemical Screening and Antimicrobial Activity of Some Medicinal Plants Against Multi-drug Resistant Bacteria from Clinical Isolates. Indian Journal of Pharmaceutical Sciences. 2012;74(5):443-50.
64. Newman DJ, Cragg GM. Natural Products as Sources of New Drugs from 1981 to 2014. Journal of Natural Products. 2016;79(3):629-61.
65. Muñoz-Esparza NC, Latorre-Moratalla ML, Comas- Basté O, Toro-Funes N, Veciana-Nogués MT, Vidal Carou MC. Polyamines in Food. Frontiers in Nutrition. 2019;6(108).
66. Jobgen WS, Fried SK, Fu WJ, Meininger CJ, Wu G. Regulatory role for the arginine–nitric oxide pathway in metabolism of energy substrates. The Journal of Nutritional Biochemistry. 2006;17(9):571-88.
67. Jackson LK, Brooks HB, Osterman AL, Goldsmith EJ, Phillips MA. Altering the Reaction Specificity of Eukaryotic Ornithine Decarboxylase. Biochemistry. 2000;39(37):11247-57.
68. Seiler N. Polyamine Metabolism. Digestion. 1990;46(suppl 2)(Suppl. 2):319-30.
69. Ramos-Molina B, Queipo-Ortuño MI, Lambertos A, Tinahones FJ, Peñafiel R. Dietary and Gut Microbiota Polyamines in Obesity- and Age-Related Diseases. Frontiers in Nutrition. 2019;6(24).
70. Xu T-T, Li H, Dai Z, Lau GK, Li B-Y, Zhu W-L, et al. Spermidine and spermine delay brain aging by inducing autophagy in SAMP8 mice. Aging (Albany NY). 2020;12(7):6401-14.
71. Sigrist SJ, Carmona-Gutierrez D, Gupta VK, Bhukel A, Mertel S, Eisenberg T, et al. Spermidine-triggered autophagy ameliorates memory during aging. Autophagy. 2014;10(1):178-9.
72. Wirth M, Schwarz C, Benson G, Horn N, Buchert R, Lange C, et al. Effects of spermidine supplementation on cognition and biomarkers in older adults with subjective cognitive decline (SmartAge)-study protocol for a randomized controlled trial. Alzheimer's Research & Therapy. 2019;11(1):36-.
73. Tong D, Hill JA. Spermidine Promotes Cardioprotective Autophagy. Circulation Research. 2017;120(8):1229-31.
74. Choi YH, Park HY. Anti-inflammatory effects of spermidine in lipopolysaccharide-stimulated BV2 microglial cells. Journal of Biomedical Science. 2012;19(1):31-.
75. Lagishetty CV, Naik SR. Polyamines: Potential antiinflammatory agents and their possible mechanism of action. Indian J Pharmacol. 2008;40(3):121-5.
76. Skatchkov SN, Woodbury-Fariña MA, Eaton M. The role of glia in stress: polyamines and brain disorders. Psychiatric Clinics of North America. 2014;37(4):653-78.
77. Yang Y, Chen S, Zhang Y, Lin X, Song Y, Xue Z, et al. Induction of autophagy by spermidine is neuroprotective via inhibition of caspase 3-mediated Beclin 1 cleavage. Cell Death and Disease. 2017;8(4):e2738-e.
78. Li G, Ding H, Yu X, Meng Y, Li J, Guo Q, et al. Spermidine Suppresses Inflammatory DC Function by Activating the FOXO3 Pathway and Counteracts Autoimmunity. iScience. 2020;23(1):100807.
79. Tirupathi Pichiah PB, Suriyakalaa U, Kamalakkannan S, Kokilavani P, Kalaiselvi S, SankarGanesh D, et al. Spermidine may decrease ER stress in pancreatic beta cells and may reduce apoptosis via activating AMPK dependent autophagy pathway. Medical Hypotheses. 2011;77(4):677- 9.
80. Ghosh I, Sankhe R, Mudgal J, Arora D, Nampoothiri M. Spermidine, an autophagy inducer, as a therapeutic strategy in neurological disorders. Neuropeptides. 2020;83:102083.
81. Salehi B, Mishra AP, Nigam M, Sener B, Kilic M, Sharifi-Rad M, et al. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines. 2018;6(3):91.
82. Romero-Pérez AI, Ibern-Gómez M, Lamuela- Raventós RM, de La Torre-Boronat MC. Piceid, the major resveratrol derivative in grape juices. Journal of Agricultural and Food Chemistry. 1999;47(4):1533-6.
83. Singh CK, George J, Ahmad N. Resveratrol-based combinatorial strategies for cancer management. Annals of the New York Academy of Sciences. 2013;1290(1):113- 21.
84. Yang F, Zhang T, Ito Y. Large-scale separation of resveratrol, anthraglycoside A and anthraglycoside B from Polygonum cuspidatum Sieb. et Zucc by highspeed counter-current chromatography. Journal of Chromatography A. 2001;919(2):443-8.
85. Jiang H, Shang X, Wu H, Gautam SC, Al-Holou S, Li C, et al. Resveratrol downregulates PI3K/Akt/mTOR signaling pathways in human U251 glioma cells. Journal of Experimental Therapeutics & Oncology. 2009;8(1):25- 33.
86. Tian Y, Song W, Li D, Cai L, Zhao Y. Resveratrol As A Natural Regulator Of Autophagy For Prevention And Treatment Of Cancer. OncoTargets and Therapy. 2019;12:8601-9.
87. Wang J, Li J, Cao N, Li Z, Han J, Li L. Resveratrol, an activator of SIRT1, induces protective autophagy in non-small-cell lung cancer via inhibiting Akt/mTOR and activating p38-MAPK. OncoTargets and Therapy. 2018;11:7777.
88. Liu M, Wilk SA, Wang A, Zhou L, Wang R-H, Ogawa W, et al. Resveratrol inhibits mTOR signaling by promoting the interaction between mTOR and DEPTOR. Journal of Biological Chemistry. 2010;285(47):36387-94.
89. Abba Y, Hassim H, Hamzah H, Noordin MM. Antiviral Activity of Resveratrol against Human and Animal Viruses. Advances in Virology. 2015;2015:184241.
90. Annunziata G, Maisto M, Schisano C, Ciampaglia R, Narciso V, Tenore GC, et al. Resveratrol as a Novel Anti- Herpes Simplex Virus Nutraceutical Agent: An Overview. Viruses. 2018;10(9):473.
91. Zaki AM, Van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. New England Journal of Medicine. 2012;367(19):1814-20.
92. Li F, Du L. MERS Coronavirus: An Emerging Zoonotic Virus. Viruses. 2019;11:663.
93. Ellen ter BM, Dinesh Kumar N, Bouma EM, Troost B, Pol van de DPI, Ende van der-Metselaar HH, et al. Resveratrol And Pterostilbene Potently Inhibit SARS-CoV-2 Infection In Vitro. bioRxiv. 2020:2020.09.24.285940.
94. Marinella MA. Indomethacin and resveratrol as potential treatment adjuncts for SARS-CoV-2/ COVID-19. International Journal of Clinical Practice. 2020;74(9):e13535.
95. Patisaul HB, Jefferson W. The pros and cons of phytoestrogens. Front Neuroendocrinol. 2010;31(4):400- 19.
96. Mazur W. 11 Phytoestrogen content in foods. Baillière’s Clinical Endocrinology and Metabolism. 1998;12(4):729- 42.
97. Ito A, Bebo BF, Matejuk A, Zamora A, Silverman M, Fyfe-Johnson A, et al. Estrogen Treatment Down- Regulates TNF-α Production and Reduces the Severity of Experimental Autoimmune Encephalomyelitis in Cytokine Knockout Mice. The Journal of Immunology. 2001;167(1):542-52.
98. Khan D, Ansar Ahmed S. The Immune System Is a Natural Target for Estrogen Action: Opposing Effects of Estrogen in Two Prototypical Autoimmune Diseases. Frontiers in Immunology. 2016;6:635-.
99. Monteiro R, Teixeira D, Calhau C. Estrogen Signaling in Metabolic Inflammation. Mediators of Inflammation. 2014;2014:615917.
100. Speyer CL, Rancilio NJ, McClintock SD, Crawford JD, Gao H, Sarma JV, et al. Regulatory effects of estrogen on acute lung inflammation in mice. American Journal of Physiology-Cell Physiology. 2005;288(4):C881-C90.
101. Harrison RF, Bonnar J. Clinical uses of estrogens. Pharmacology & Therapeutics. 1980;11(2):451-67.
102. Raghava N, Das BC, Ray SK. Neuroprotective effects of estrogen in CNS injuries: insights from animal models. Neuroscience and Neuroeconomics. 2017;6:15-29.
103. Xiang J, Liu X, Ren J, Chen K, Wang H-L, Miao Y-Y, et al. How does estrogen work on autophagy? Autophagy. 2019;15(2):197-211.
104. Maxwell T, Lee KS, Kim S, Nam KS. Arctigenin inhibits the activation of the mTOR pathway, resulting in autophagic cell death and decreased ER expression in ERpositive human breast cancer cells. International Journal of Oncology. 2018;52(4):1339-49.
105. Nehybova T, Smarda J, Daniel L, Brezovsky J, Benes P. Wedelolactone induces growth of breast cancer cells by stimulation of estrogen receptor signalling. The Journal of Steroid Biochemistry and Molecular Biology. 2015;152.
106. Hsieh C-J, Kuo P-L, Hou M-F, Hung J-Y, Chang F-R, Hsu Y-C, et al. Wedelolactone inhibits breast cancer-induced osteoclastogenesis by decreasing Akt/ mTOR signaling. International Journal of Oncology. 2015;46(2):555-62.
107. Peretz J, Pekosz A, Lane AP, Klein SL. Estrogenic compounds reduce influenza A virus replication in primary human nasal epithelial cells derived from female, but not male, donors. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2016;310(5):L415-L25.
108. Roehrig JT, Brawner TA, Riggs HG, Jr. Effects of 17beta-estradiol on the replication of rubella virus in an estrogen-responsive, continuous cell line. Journal of Virology. 1979;29(1):417-20.
109. Heron P, Turchan-Cholewo J, Bruce-Keller A, Wilson M. Estrogen Receptor Alpha Inhibits the Estrogen-Mediated Suppression of HIV Transcription in Astrocytes: Implications for Estrogen Neuroprotection in HIV Dementia. AIDS Research and Human Retroviruses. 2009;25:1071-81.
110. Anipindi VC, Bagri P, Roth K, Dizzell SE, Nguyen PV, Shaler CR, et al. Estradiol Enhances CD4+ T-Cell Anti-Viral Immunity by Priming Vaginal DCs to Induce Th17 Responses via an IL-1-Dependent Pathway. PLOS Pathogens. 2016;12(5):e1005589.
111. Channappanavar R, Fett C, Mack M, Ten Eyck PP, Meyerholz DK, Perlman S. Sex-Based Differences in Susceptibility to Severe Acute Respiratory Syndrome Coronavirus Infection. Journal of Immunology. 2017;198(10):4046-53.
112. Shabbir S, Hafeez A, Rafiq MA, Khan MJ. Estrogen shields women from COVID-19 complications by reducing ER stress. Medical Hypotheses. 2020;143:110148-.
113. Suba Z. Prevention and therapy of COVID-19 via exogenous estrogen treatment for both male and female patients: Prevention and therapy of COVID-19. Journal of Pharmacy & Pharmaceutical Sciences. 2020;23(1):75-85.
114. Khan N. Possible protective role of 17β-estradiol against COVID-19. Journal of Allergy and Infectious Diseases. 2020;1(2):38-48.
115. Elbein AD, Pan YT, Pastuszak I, Carroll D. New insights on trehalose: a multifunctional molecule. Glycobiology. 2003;13(4):17R-27R.
116. Grennan AK. The role of trehalose biosynthesis in plants. Plant Physiology. 2007;144(1):3-5.
117. Ruhal R, Kataria R, Choudhury B. Trends in bacterial trehalose metabolism and significant nodes of metabolic pathway in the direction of trehalose accumulation. Microbial Biotechnology. 2013;6(5):493-502.
118. Tang B, Wang S, Wang S-G, Wang H-J, Zhang J-Y, Cui S-Y. Invertebrate Trehalose-6-Phosphate Synthase Gene: Genetic Architecture, Biochemistry, Physiological Function, and Potential Applications. Frontiers in Physiology. 2018;9:30-.
119. Mizunoe Y, Kobayashi M, Sudo Y, Watanabe S, Yasukawa H, Natori D, et al. Trehalose protects against oxidative stress by regulating the Keap1-Nrf2 and autophagy pathways. Redox Biology. 2018;15:115-24.
120. Lee H-J, Yoon Y-S, Lee S-J. Mechanism of neuroprotection by trehalose: controversy surrounding autophagy induction. Cell Death & Disease. 2018;9(7):712.
121. Khalifeh M, Barreto GE, Sahebkar A. Trehalose as a promising therapeutic candidate for the treatment of Parkinson's disease. British Journal of Pharmacology. 2019;176(9):1173-89.
122. Rusmini P, Cortese K, Crippa V, Cristofani R, Cicardi ME, Ferrari V, et al. Trehalose induces autophagy via lysosomal-mediated TFEB activation in models of motoneuron degeneration. Autophagy. 2019;15(4):631-51.
123. Sharma V, Makhdoomi M, Singh L, Kumar P, Khan N, Singh S, et al. Trehalose limits opportunistic mycobacterial survival during HIV co-infection by reversing HIVmediated autophagy block. Autophagy. 2020:1-20.
124. Belzile J-P, Sabalza M, Craig M, Clark E, Morello CS, Spector DH. Trehalose, an mTOR-Independent Inducer of Autophagy, Inhibits Human Cytomegalovirus Infection in Multiple Cell Types. Journal of Virology. 2016;90(3):1259- 77.
125. Li H-B, Chen F. Isolation and purification of baicalein, wogonin and oroxylin A from the medicinal plant Scutellaria baicalensis by high-speed countercurrent chromatography. Journal of Chromatography A. 2005;1074(1-2):107-10.
126. Sowndhararajan K, Deepa P, Kim M, Park SJ, Kim S. Neuroprotective and cognitive enhancement potentials of baicalin: a review. Brain Sciences. 2018;8(6):104.
127. Hannan MA, Dash R, Sohag AAM, Haque M, Moon IS. Neuroprotection against oxidative stress: Phytochemicals targeting TrkB signaling and the Nrf2-ARE antioxidant system. Frontiers in Molecular Neuroscience. 2020;13:116.
128. Chen C, Li X, Gao P, Tu Y, Zhao M, Li J, et al. Baicalin attenuates alzheimer-like pathological changes and memory deficits induced by amyloid β1-42 protein. Metabolic Brain Disease. 2015;30(2):537-44.
129. Pan Y, Chen D, Lu Q, Liu L, Li X, Li Z. Baicalin prevents the apoptosis of endplate chondrocytes by inhibiting the oxidative stress induced by H2O2. Molecular Medicine Reports. 2017;16(3):2985-91.
130. Liang W, Huang X, Chen W. The Effects of Baicalin and Baicalein on Cerebral Ischemia: A Review. Aging and Disease. 2017 Dec;8(6):850-67.
131. Liu LY, Wei EQ, Zhao YM, Chen FX, Wang ML, Zhang WP, et al. Protective effects of baicalin on oxygen/ glucose deprivation- and NMDA-induced injuries in rat hippocampal slices. The Journal of Pharmacy and Pharmacology. 2005;57(8):1019-26.
132. Zhu X, Yao P, Liu J, Guo X, Jiang C, Tang Y. Baicalein attenuates impairment of hepatic lysosomal acidification induced by high fat diet via maintaining V-ATPase assembly. Food and Chemical Toxicology. 2020;136:110990.
133. Zhang JA, Luan C, Huang D, Ju M, Chen K, Gu H. Induction of Autophagy by Baicalin Through the AMPKmTOR Pathway Protects Human Skin Fibroblasts from Ultraviolet B Radiation-Induced Apoptosis. Drug design, Development and Therapy. 2020;14:417-28.
134. Wang Y, Han E, Xing Q, Yan J, Arrington A, Wang C, et al. Baicalein upregulates DDIT4 expression which mediates mTOR inhibition and growth inhibition in cancer cells. Cancer Letters. 2015;358(2):170-9.
135. Guo Z, Hu X, Xing Z, Xing R, Lv R, Cheng X, et al. Baicalein inhibits prostate cancer cell growth and metastasis via the caveolin-1/AKT/mTOR pathway. Molecular and Cellular Biochemistry. 2015;406(1-2):111- 9.
136. Ding Y, Dou J, Teng Z, Yu J, Wang T, Lu N, et al. Antiviral activity of baicalin against influenza A (H1N1/ H3N2) virus in cell culture and in mice and its inhibition of neuraminidase. Archives of Virology. 2014;159(12):3269- 78.
137. Su H, Yao S, Zhao W, Li M, Liu J, Shang W, et al. Discovery of baicalin and baicalein as novel, natural product inhibitors of SARS-CoV-2 3CL protease in vitro. bioRxiv. 2020:2020.04.13.038687.
138. Zdrojewicz Z, Szyca M, Popowicz E, Michalik T, Śmieszniak B. [Turmeric - not only spice]. Polski merkuriusz lekarski : organ Polskiego Towarzystwa Lekarskiego. 2017;42(252):227-30.
139. Soleimani V, Sahebkar A, Hosseinzadeh H. Turmeric (Curcuma longa) and its major constituent (curcumin) as nontoxic and safe substances: Review. Phytotherapy Research : PTR. 2018;32(6):985-95.
140. Eke-Okoro UJ, Raffa RB, Pergolizzi JV, Jr., Breve F, Taylor R, Jr. Curcumin in turmeric: Basic and clinical evidence for a potential role in analgesia. Journal of Clinical Pharmacy and Therapeutics. 2018;43(4):460-6.
141. Kocaadam B, Şanlier N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Critical Reviews in Food Science and Nutrition. 2017;57(13):2889-95.
142. Esatbeyoglu T, Ulbrich K, Rehberg C, Rohn S, Rimbach G. Thermal stability, antioxidant, and antiinflammatory activity of curcumin and its degradation product 4-vinyl guaiacol. Food & function. 2015;6(3):887- 93.
143. Menon VP, Sudheer AR. Antioxidant and antiinflammatory properties of curcumin. Advances in Experimental Medicine and Biology. 2007;595:105-25.
144. Patel SS, Acharya A, Ray RS, Agrawal R, Raghuwanshi R, Jain P. Cellular and molecular mechanisms of curcumin in prevention and treatment of disease. Critical Reviews in Food Science and Nutrition. 2020;60(6):887-939.
145. Bachmeier BE, Killian PH, Melchart D. The Role of Curcumin in Prevention and Management of Metastatic Disease. International Journal of Molecular Sciences. 2018;19(6).
146. Bavarsad K, Riahi MM, Saadat S, Barreto G, Atkin SL, Sahebkar A. Protective effects of curcumin against ischemia-reperfusion injury in the liver. Pharmacological Research. 2019;141:53-62.
147. Rahmani AH, Alsahli MA, Aly SM, Khan MA, Aldebasi YH. Role of Curcumin in Disease Prevention and Treatment. Adv Biomed Res. 2018;7:38.
148. Kutluay SB, Doroghazi J, Roemer ME, Triezenberg SJ. Curcumin inhibits herpes simplex virus immediateearly gene expression by a mechanism independent of p300/CBP histone acetyltransferase activity. Virology. 2008;373(2):239-47.
149. Mounce BC, Cesaro T, Carrau L, Vallet T, Vignuzzi M. Curcumin inhibits Zika and chikungunya virus infection by inhibiting cell binding. Antiviral Research. 2017;142:148- 57.
150. Kim K, Kim KH, Kim HY, Cho HK, Sakamoto N, Cheong J. Curcumin inhibits hepatitis C virus replication via suppressing the Akt-SREBP-1 pathway. FEBS Letters. 2010;584(4):707-12.
151. Mishra A, Das BC. Curcumin as an anti-human papillomavirus and anti-cancer compound. Future Oncology. 2015;11(18):2487-90.
152. Beevers CS, Chen L, Liu L, Luo Y, Webster NJG, Huang S. Curcumin disrupts the Mammalian target of rapamycinraptor complex. Cancer Research. 2009;69(3):1000-8.
153. Beevers CS, Li F, Liu L, Huang S. Curcumin inhibits the mammalian target of rapamycin-mediated signaling pathways in cancer cells. International Journal of Cancer. 2006;119(4):757-64.
154. Beevers CS, Zhou H, Huang S. Hitting the golden TORget: curcumin's effects on mTOR signaling. Anticancer Agents in Medicinal Chemistry. 2013;13(7):988-94.
155. Nishimuro H, Ohnishi H, Sato M, Ohnishi- Kameyama M, Matsunaga I, Naito S, et al. Estimated daily intake and seasonal food sources of quercetin in Japan. Nutrients. 2015;7(4):2345-58.
156. Li Y, Yao J, Han C, Yang J, Chaudhry MT, Wang S, et al. Quercetin, Inflammation and Immunity. Nutrients. 2016;8(3):167.
157. Vafadar A, Shabaninejad Z, Movahedpour A, Fallahi F, Taghavipour M, Ghasemi Y, et al. Quercetin and cancer:new insights into its therapeutic effects on ovarian cancer cells. Cell & Bioscience. 2020;10:32.
158. Kumar P, Sharma S, Khanna M, Raj HG. Effect of Quercetin on lipid peroxidation and changes in lung morphology in experimental influenza virus infection. International Journal of Experimental Pathology. 2003;84(3):127-33.
159. Hubbard GP, Wolffram S, Lovegrove JA, Gibbins JM. Ingestion of quercetin inhibits platelet aggregation and essential components of the collagen-stimulated platelet activation pathway in humans. Journal of Thrombosis and Haemostasis : JTH. 2004;2(12):2138-45.
160. Moon J-H, Eo SK, Lee JH, Park S-Y. Quercetininduced autophagy flux enhances TRAIL-mediated tumor cell death. Oncology Reports. 2015;34(1):375-81.
161. Rivera Rivera A, Castillo-Pichardo L, Gerena Y, Dharmawardhane S. Anti-Breast Cancer Potential of Quercetin via the Akt/AMPK/Mammalian Target of Rapamycin (mTOR) Signaling Cascade. PLoS One. 2016;11(6):e0157251.
162. Bruning A. Inhibition of mTOR signaling by quercetin in cancer treatment and prevention. Anti-cancer Agents in Medicinal Chemistry. 2013;13(7):1025-31.
163. Se Hee L, In-Seop K, Song Yi P, Ock Jin P. Quercetin Induces Apoptosis via Regulation of mTOR-VASP Signaling Pathway in HT-29 Colon Cancer Cells. Journal of Cancer Prevention. 2011;16(4):340-7.
164. Lalani S, Poh CL. Flavonoids as antiviral agents for Enterovirus A71 (EV-A71). Viruses. 2020;12(2):184.
165. Rojas Á, Del Campo JA, Clement S, Lemasson M, García-Valdecasas M, Gil-Gómez A, et al. Effect of Quercetin on Hepatitis C Virus Life Cycle: From Viral to Host Targets. Scientific Reports. 2016;6(1):31777.
166. Zakaryan H, Arabyan E, Oo A, Zandi K. Flavonoids: promising natural compounds against viral infections. Archives of Virology. 2017;162(9):2539-51.
167. Lin L-T, Hsu W-C, Lin C-C. Antiviral Natural Products and Herbal Medicines. Journal of Traditional and Complementary Medicine. 2014;4(1):24-35.
168. Colunga Biancatelli RML, Berrill M, Catravas JD, Marik PE. Quercetin and Vitamin C: An Experimental, Synergistic Therapy for the Prevention and Treatment of SARS-CoV-2 Related Disease (COVID-19). Frontiers in Immunology. 2020;11:1451-.
169. Venugopala KN, Rashmi V, Odhav B. Review on natural coumarin lead compounds for their pharmacological activity. BioMed Research International.2013;2013:963248-.
170. Murray RDH, Méndez J, Brown SA. The natural coumarins. Wiley; Chichester, New York, 1982.
171. Lončar M, Jakovljević M, Šubarić D, Pavlić M, Buzjak Služek V, Cindrić I, et al. Coumarins in Food and Methods of Their Determination. Foods (Basel, Switzerland). 2020;9(5).
172. Menezes JC, Diederich M. Translational role of natural coumarins and their derivatives as anticancer agents. Future Medicinal Chemistry. 2019;11(9):1057-82.
173. Hassanein EHM, Sayed AM, Hussein OE, Mahmoud AM. Coumarins as Modulators of the Keap1/Nrf2/ARE Signaling Pathway. Oxidative Medicine and Cellular Longevity. 2020;2020:1675957-.
174. Basile A, Sorbo S, Spadaro V, Bruno M, Maggio A, Faraone N, et al. Antimicrobial and antioxidant activities of coumarins from the roots of Ferulago campestris (Apiaceae). Molecules (Basel, Switzerland). 2009;14(3):939-52.
175. Mishra S, Pandey A, Manvati S. Coumarin: An emerging antiviral agent. Heliyon. 2020;6(1):e03217-e.
176. Wang Q, Guo Y, Jiang S, Dong M, Kuerban K, Li J, et al. A hybrid of coumarin and phenylsulfonylfuroxan induces caspase-dependent apoptosis and cytoprotective autophagy in lung adenocarcinoma cells. Phytomedicine. 2017;39.
177. Liu H, Wang Y, Sharma A, Mao R, Jiang N, Dun B, et al. Derivatives containing both coumarin and benzimidazole potently induce caspase-dependent apoptosis of cancer cells through inhibition of PI3K-AKT-mTOR signaling. Anti-cancer Drugs. 2015;26(6):667-77.
178. Majnooni MB, Fakhri S, Smeriglio A, Trombetta D, Croley CR, Bhattacharyya P, et al. Antiangiogenic Effects of Coumarins against Cancer: From Chemistry to Medicine. Molecules (Basel, Switzerland). 2019;24(23).
179. Sona L, Sharat S, Sourav D, Atanu SR. In Silico Screening of Naturally Occurring Coumarin Derivatives for the Inhibition of the Main Protease of SARS-CoV-22020.
180. Milenković DA, Dimić DS, Avdović EH, Marković ZS. Several coumarin derivatives and their Pd(ii) complexes as potential inhibitors of the main protease of SARS-CoV-2, an in silico approach. RSC Advances. 2020;10(58):35099- 108.
181. Du G-J, Zhang Z, Wen X-D, Yu C, Calway T, Yuan C-S, et al. Epigallocatechin Gallate (EGCG) is the most effective cancer chemopreventive polyphenol in green tea. Nutrients. 2012;4(11):1679-91.
182. Forester SC, Lambert JD. The role of antioxidant versus pro-oxidant effects of green tea polyphenols in cancer prevention. Molecular Nutrition & Food Research. 2011;55(6):844-54.
183. Min S-Y, Yan M, Kim SB, Ravikumar S, Kwon S-R, Vanarsa K, et al. Green Tea Epigallocatechin-3-Gallate Suppresses Autoimmune Arthritis Through Indoleamine- 2,3-Dioxygenase Expressing Dendritic Cells and the Nuclear Factor, Erythroid 2-Like 2 Antioxidant Pathway. Journal of Inflammation. 2015;12(1):53.
184. Wu D, Wang J. The ability of green tea to alleviate autoimmune diseases: fact or fiction? Expert Review of Clinical Immunology. 2011;7(6):711-3.
185. Ohishi T, Goto S, Monira P, Isemura M, Nakamura Y. Anti-inflammatory Action of Green Tea. Antiinflammatory & Anti-allergy Agents in Medicinal Chemistry. 2016;15(2):74-90.
186. Menegazzi M, Campagnari R, Bertoldi M, Crupi R, Di Paola R, Cuzzocrea S. Protective Effect of Epigallocatechin- 3-Gallate (EGCG) in Diseases with Uncontrolled Immune Activation: Could Such a Scenario Be Helpful to Counteract COVID-19? International Journal of Molecular Sciences. 2020;21(14):5171.
187. Joo S-Y, Song Y-A, Park Y-L, Myung E, Chung C-Y, Park K-J, et al. Epigallocatechin-3-gallate Inhibits LPSInduced NF-κB and MAPK Signaling Pathways in Bone Marrow-Derived Macrophages. Gut Liver. 2012;6(2):188- 96.
188. Yang F, Oz HS, Barve S, de Villiers WJ, McClain CJ, Varilek GW. The green tea polyphenol (-)-epigallocatechin- 3-gallate blocks nuclear factor-kappa B activation by inhibiting I kappa B kinase activity in the intestinal epithelial cell line IEC-6. Molecular Pharmacology. 2001;60(3):528-33.
189. Menegazzi M, Tedeschi E, Dussin D, de Prati AC, Cavalieri E, Mariotto S, et al. Anti-interferon-γ action of epigallocatechin-3-gallate mediated by specific inhibition of STAT1 activation. The FASEB Journal. 2001;15(7):1309- 11.
190. Wang Y, Ren X, Deng C, Yang L, Yan E, Guo T, et al. Mechanism of the inhibition of the STAT3 signaling pathway by EGCG. Oncology Reports. 2013;30(6):2691-6.
191. Huang CH, Tsai SJ, Wang YJ, Pan MH, Kao JY, Way TD. EGCG inhibits protein synthesis, lipogenesis, and cell cycle progression through activation of AMPK in p53 positive and negative human hepatoma cells. Molecular Nutrition & Food Research. 2009;53(9):1156-65.
192. Van Aller GS, Carson JD, Tang W, Peng H, Zhao L, Copeland RA, et al. Epigallocatechin gallate (EGCG), a major component of green tea, is a dual phosphoinositide- 3-kinase/mTOR inhibitor. Biochemical and Biophysical Research Communications. 2011;406(2):194-9.
193. Kim H-S, Montana V, Jang H-J, Parpura V, Kim J-a. Epigallocatechin Gallate (EGCG) Stimulates Autophagy in Vascular Endothelial Cells: A POTENTIAL ROLE FOR REDUCING LIPID ACCUMULATION. Journal of Biological Chemistry. 2013;288(31):22693-705.
194. Holczer M, Besze B, Zámbó V, Csala M, Bánhegyi G, Kapuy O. Epigallocatechin-3-Gallate (EGCG) Promotes Autophagy-Dependent Survival via Influencing the Balance of mTOR-AMPK Pathways upon Endoplasmic Reticulum Stress. Oxidative Medicine and Cellular Longevity. 2018;2018:6721530.
195. Kondo A, Takeda T, Li B, Tsuiji K, Kitamura M, Wong TF, et al. Epigallocatechin-3-gallate potentiates curcumin's ability to suppress uterine leiomyosarcoma cell growth and induce apoptosis. International Journal of Clinical Oncology. 2013;18(3):380-8.
196. Jadeja R, Devkar R. Polyphenols in Human Health and Disease. Polyphenols in Chronic Diseases and their Mechanisms of Action. 2014:615-23.
197. Soltana H, De Rosso M, Lazreg H, Vedova AD, Hammami M, Flamini R. LC-QTOF characterization of non-anthocyanic flavonoids in four Tunisian fig varieties. Journal of Mass Spectrometry : JMS. 2018;53(9):817-23.
198. Venkateswararao P, Kiran S, Rohini P, Bhagyasree P. Flavonoid: A review on Naringenin. Journal of Pharmacognosy and Phytochemistry. 2017;6:2778-83.
199. Salehi B, Fokou PVT, Sharifi-Rad M, Zucca P, Pezzani R, Martins N, et al. The Therapeutic Potential of Naringenin: A Review of Clinical Trials. Pharmaceuticals (Basel). 2019;12(1):11.
200. Jin L, Zeng W, Zhang F, Zhang C, Liang W. Naringenin Ameliorates Acute Inflammation by Regulating Intracellular Cytokine Degradation. Journal of Immunology. 2017;199(10):3466-77.
201. Alam MA, Subhan N, Rahman MM, Uddin SJ, Reza HM, Sarker SD. Effect of citrus flavonoids, naringin and naringenin, on metabolic syndrome and their mechanisms of action. Advances in Nutrition. 2014;5(4):404-17.
202. Mohammad SA-h. HEPATOPROTECTIVE EFFECT AND ANTIOXIDANT CAPACITY OF NARINGENIN ON ARSENIC-INDUCED LIVER INJURY IN RATS. International Journal of Pharmacy and Pharmaceutical Sciences. 2016;8(4).
203. Song HM, Park GH, Eo HJ, Lee JW, Kim MK, Lee JR, et al. Anti-Proliferative Effect of Naringenin through p38-Dependent Downregulation of Cyclin D1 in Human Colorectal Cancer Cells. Biomolecules & Therapeutics (Seoul). 2015;23(4):339-44.
204. Krishnakumar N, Sulfikkarali N, RajendraPrasad N, Karthikeyan S. Enhanced anticancer activity of naringeninloaded nanoparticles in human cervical (HeLa) cancer cells. Biomedicine & Preventive Nutrition. 2011;1(4):223- 31.
205. Nouri Z, Fakhri S, El-Senduny FF, Sanadgol N, Abd- ElGhani GE, Farzaei MH, et al. On the Neuroprotective Effects of Naringenin: Pharmacological Targets, Signaling Pathways, Molecular Mechanisms, and Clinical Perspective. Biomolecules. 2019;9(11):690.
206. Wang B, Shen J, Zhou Q, Meng D, He Y, Chen F, et al. Effects of naringenin on the pharmacokinetics of tofacitinib in rats. Pharmaceutical Biology. 2020;58(1):225-30.
207. Mahmoud AM, Hernández Bautista RJ, Sandhu MA, Hussein OE. Beneficial Effects of Citrus Flavonoids on Cardiovascular and Metabolic Health. Oxidative Medicine and Cellular Longevity. 2019;2019:5484138.
208. Benkerrou D, Minicozzi V, Gradogna A, Milenkovic S, Bodrenko IV, Festa M, et al. A perspective on the modulation of plant and animal two pore channels (TPCs) by the flavonoid naringenin. Biophysical Chemistry. 2019;254:106246.
209. Pafumi I, Festa M, Papacci F, Lagostena L, Giunta C, Gutla V, et al. Naringenin Impairs Two-Pore Channel 2 Activity And Inhibits VEGF-Induced Angiogenesis. Scientifc Reports. 2017;7(1):5121.
210. Tsai Y-J, Tsai T-H. Mesenteric Lymphatic Absorption and the Pharmacokinetics of Naringin and Naringenin in the Rat. Journal of Agricultural and Food Chemistry. 2012;60(51):12435-42.
211. Ou X, Liu Y, Lei X, Li P, Mi D, Ren L, et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARSCoV. Nature Communications. 2020;11(1):1620.
212. Kang Y-L, Chou Y-Y, Rothlauf PW, Liu Z, Piccinotti S, Soh TK, et al. Inhibition of PIKfyve kinase prevents infection by EBOV and SARS-CoV-2. bioRxiv. 2020:2020.04.21.053058.
213. Sakurai Y, Kolokoltsov AA, Chen C-C, Tidwell MW, Bauta WE, Klugbauer N, et al. Ebola virus. Two-pore channels control Ebola virus host cell entry and are drug targets for disease treatment. Science (New York, NY). 2015;347(6225):995-8.
214. Khan N, Halcrow PW, Lakpa KL, Afghah Z, Miller NM, Dowdy SF, et al. Two-pore channels regulate Tat endolysosome escape and Tat-mediated HIV-1 LTR transactivation. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2020;34(3):4147-62.
215. Raha S, Yumnam S, Hong GE, Lee HJ, Saralamma VV, Park HS, et al. Naringin induces autophagy-mediated growth inhibition by downregulating the PI3K/Akt/mTOR cascade via activation of MAPK pathways in AGS cancer cells. International Journal of Oncology. 2015;47(3):1061- 9.
216. Cheng H, Jiang X, Zhang Q, Ma J, Cheng R, Yong H, et al. Naringin inhibits colorectal cancer cell growth by repressing the PI3K/AKT/mTOR signaling pathway. Experimental and Therapeutic Medicine. 2020;19(6):3798-804.
217. Kumar V, Verma A, Bhatt PC. 42P - Dual inhibitory effects of novel naringenin analogue in tobacco-carcinogen induced lung cancer via inhibition of PI3K/Akt/mTOR pathway. Annals of Oncology. 2017;28:ii12.
218. Cao W, Feng S-J, Kan M-C. Naringin Targets NFKB1 to Alleviate Oxygen-Glucose Deprivation/Reoxygenation– Induced Injury in PC12 Cells Via Modulating HIF-1α/ AKT/mTOR-Signaling Pathway. Journal of Molecular Neuroscience. 2020.
219. Ahsan AU, Sharma VL, Wani A, Chopra M. Naringenin Upregulates AMPK-Mediated Autophagy to Rescue Neuronal Cells From β-Amyloid (1–42) Evoked Neurotoxicity. Molecular Neurobiology. 2020;57(8):3589- 602.
220. Tutunchi H, Naeini F, Ostadrahimi A, Hosseinzadeh- Attar MJ. Naringenin, a flavanone with antiviral and antiinflammatory effects: A promising treatment strategy against COVID-19. Phytotherapy Research.n/a(n/a).
221. Clementi N, Scagnolari C, D’Amore A, Palombi F, Criscuolo E, Frasca F, et al. Naringenin is a powerful inhibitor of SARS-CoV-2 infection in vitro. Pharmacological Research. 2020:105255.
222. Alberca RW, Teixeira FME, Beserra DR, de Oliveira EA, Andrade MMdS, Pietrobon AJ, et al. Perspective: The Potential Effects of Naringenin in COVID-19. Frontiers in Immunology. 2020;11:570919.
223. Mani JS, Johnson JB, Steel JC, Broszczak DA, Neilsen PM, Walsh KB, et al. Natural product-derived phytochemicals as potential agents against coronaviruses: A review. Virus Research. 2020;284:197989.
224. Elfiky AA. Natural products may interfere with SARSCoV- 2 attachment to the host cell. Journal of Biomolecular Structure and Dynamics. 2020:1-10.
225. Huang S. Inhibition of PI3K/Akt/mTOR signaling by natural products. Anti-cancer Agents in Medicinal Chemistry. 2013;13(7):967-70.
226. Kousar K, Majeed A, Yasmin F, Hussain W, Rasool N. Phytochemicals from Selective Plants Have Promising Potential against SARS-CoV-2: Investigation and Corroboration through Molecular Docking, MD Simulations, and Quantum Computations. BioMed Research International. 2020;2020:6237160.
227. Antonio AdS, Wiedemann LSM, Veiga-Junior VF. Natural products' role against COVID-19. RSC Advances. 2020;10(39):23379-93.
228. Attia YA, Alagawany MM, Farag MR, Alkhatib FM, Khafaga AF, Abdel-Moneim A-ME, et al. Phytogenic Products and Phytochemicals as a Candidate Strategy to Improve Tolerance to Coronavirus. Frontiers in Veterinary Science. 2020;7(783).
229. Idrees M, Khan S, Memon NH, Zhang Z. Effect of the Phytochemical Agents Against the SARS-CoV and Selected Some of them for Application to COVID-19: A Mini- Review. Current Pharmaceutical Biotechnology. 2020.
230. Zhou X, Yang J, Zhou M, Zhang Y, Liu Y, Hou P, et al. Resveratrol attenuates endothelial oxidative injury by inducing autophagy via the activation of transcription factor EB. Nutrition & Metabolism. 2019;16(1):42.
231. Zhang Y, Cao X, Zhu W, Liu Z, Liu H, Zhou Y, et al. Resveratrol Enhances Autophagic Flux and Promotes Ox- LDL Degradation in HUVECs via Upregulation of SIRT1. Oxidative Medicine and Cellular Longevity. 2016;2016.
232. de Sá Coutinho D, Pacheco MT, Frozza RL, Bernardi A. Anti-Inflammatory Effects of Resveratrol: Mechanistic Insights. International Journal of Molecular Sciences. 2018;19(6):1812.
233. Chiang S-S, Pan T-M. Beneficial effects of phytoestrogens and their metabolites produced by intestinal microflora on bone health. Applied Microbiology and Biotechnology. 2013;97(4):1489-500.
234. Costeira R, Lee KA, Murray B, Christiansen C, Castillo- Fernandez J, Ni Lochlainn M, et al. Estrogen and COVID-19 symptoms: associations in women from the COVID Symptom Study. medRxiv. 2020:2020.07.30.20164921.
235. Di Stadio A, Della Volpe A, Ralli M, Ricci G. Gender differences in COVID-19 infection. The estrogen effect on upper and lower airways. Can it help to figure out a treatment? European Review for Medical and Pharmacological Sciences. 2020;24(10):5195-6.
236. Groban L, Wang H, Sun X, Ahmad S, Ferrario CM. Is Sex a Determinant of COVID-19 Infection? Truth or Myth? Current Hypertension Reports. 2020;22(9):62.
237. Echigo R, Shimohata N, Karatsu K, Yano F, Kayasuga-Kariya Y, Fujisawa A, et al. Trehalose treatment suppresses inflammation, oxidative stress, and vasospasm induced by experimental subarachnoid hemorrhage. Journal of Translational Medicine. 2012;10:80.
238. Martinon D, Borges VF, Gomez AC, Shimada K. Potential Fast COVID-19 Containment With Trehalose. Frontiers in Immunology. 2020;11(1623).
239. Li BQ, Fu T, Gong WH, Dunlop N, Kung H, Yan Y, et al. The flavonoid baicalin exhibits anti-inflammatory activity by binding to chemokines. Immunopharmacology. 2000;49(3):295-306.
240. Li Y, Liu T, Li Y, Han D, Hong J, Yang N, et al. Baicalin Ameliorates Cognitive Impairment and Protects Microglia from LPS-Induced Neuroinflammation via the SIRT1/HMGB1 Pathway. Oxidative Medicine and Cellular Longevity. 2020;2020:4751349.
241. Chou T-C, Chang L-P, Li C-Y, Wong C-S, Yang S-P. The Antiinflammatory and Analgesic Effects of Baicalin in Carrageenan-Evoked Thermal Hyperalgesia. Anesthesia & Analgesia. 2003;97(6):1724-9.
242. Song J, Zhang L, Xu Y, Yang D, Zhang L, Yang S, et al. The comprehensive study on the therapeutic effects of baicalein for the treatment of COVID-19 in vivo and in vitro. Biochemical Pharmacology. 2021;183:114302.
243. Su H-X, Yao S, Zhao W-F, Li M-J, Liu J, Shang W-J, et al. Anti-SARS-CoV-2 activities in vitro of Shuanghuanglian preparations and bioactive ingredients. Acta pharmacologica Sinica. 2020;41(9):1167-77.
244. Jo S, Kim S, Kim DY, Kim M-S, Shin DH. Flavonoids with inhibitory activity against SARS-CoV-2 3CLpro. Journal of Enzyme Inhibition and Medicinal Chemistry. 2020;35(1):1539-44.
245. Liu Z, Ying Y. The Inhibitory Effect of Curcumin on Virus-Induced Cytokine Storm and Its Potential Use in the Associated Severe Pneumonia. Frontiers in Cell and Developmental Biology. 2020;8(479).
246. Manoharan Y, Haridas V, Vasanthakumar KC, Muthu S, Thavoorullah FF, Shetty P. Curcumin: a Wonder Drug as a Preventive Measure for COVID19 Management. Indian Journal of Clinical Biochemistry. 2020;35(3):373- 5.
247. Soni VK, Mehta A, Ratre YK, Tiwari AK, Amit A, Singh RP, et al. Curcumin, a traditional spice component, can hold the promise against COVID-19? European Journal of Pharmacology. 2020;886:173551.
248. Babaei F, Nassiri-Asl M, Hosseinzadeh H. Curcumin (a constituent of turmeric): New treatment option against COVID-19. Food Science & Nutrition. 2020;8(10):5215-27.
249. Al-Rasheed NM, Fadda L, Attia HA, Sharaf IA, Mohamed AM, Al-Rasheed NM. Pulmonary prophylactic impact of melatonin and/or quercetin: A novel therapy for inflammatory hypoxic stress in rats. Acta Pharmaceutica. 2017;67(1):125-35.
250. Derosa G, Maffioli P, D'Angelo A, Di Pierro F. A role for quercetin in coronavirus disease 2019 (COVID-19). Phytotherapy Research.n/a(n/a).
251. Aucoin M, Cooley K, Saunders PR, Cardozo V, Remy D, Cramer H, et al. The effect of quercetin on the prevention or treatment of COVID-19 and other respiratory tract infections in humans: A rapid review. Advances in Integrative Medicine. 2020;7(4):247-51.
252. Ahmed A, Albalawi Y, Shora HA. Effects of Quadruple Therapy: Zinc, Quercetin, Bromelain and Vitamin C on the Clinical Outcomes of Patients Infected with COVID-19. 2020.
253. Di Pierro F, Khan A, Bertuccioli A, Maffioli P, Derosa G, Khan S, et al. Quercetin Phytosome® as a potential candidate for managing COVID-19. Minerva gastroenterologica e dietologica. 2020.
254. Hadjipavlou-Litina DJ, Litinas KE, Kontogiorgis C. The Anti-inflammatory Effect of Coumarin and its Derivatives. Anti-inflammatory & Anti-allergy Agents in Medicinal Chemistry. 2007;6(4):293-306.
255. Wang L, Sun X, Zhu M, Du J, Xu J, Qin X, et al. Epigallocatechin-3-gallate stimulates autophagy and reduces apoptosis levels in retinal Müller cells under high-glucose conditions. Experimental Cell Research. 2019;380(2):149-58.
256. Jang M, Park Y-I, Cha Y-E, Park R, Namkoong S, Lee JI, et al. Tea Polyphenols EGCG and Theaflavin Inhibit the Activity of SARS-CoV-2 3CL-Protease In Vitro. EvidenceBased Complementary and Alternative Medicine. 2020;2020:5630838.
257. Mhatre S, Srivastava T, Naik S, Patravale V. Antiviral activity of green tea and black tea polyphenols in prophylaxis and treatment of COVID-19: A review. Phytomedicine : International Journal of Phytotherapy and Phytopharmacology. 2020:153286.
258. Storozhuk M. COVID-19: could green tea catechins reduce the risks? medRxiv. 2020:2020.10.23.20218479.
259. Zhao Y, Fan D, Ru B, Cheng K-W, Hu S, Zhang J, et al. 6-C-(E-phenylethenyl)naringenin induces cell growth inhibition and cytoprotective autophagy in colon cancer cells. European Journal of Cancer. 2016;68:38-50.
260. Riva L, Yuan S, Yin X, Martin-Sancho L, Matsunaga N, Pache L, et al. Discovery of SARS-CoV-2 antiviral drugs through large-scale compound repurposing. Nature. 2020.
261. Zeng W, Jin L, Zhang F, Zhang C, Liang W. Naringenin as a potential immunomodulator in therapeutics. Pharmacological Research. 2018;135:122-6.
262. Zhang C, Zeng W, Yao Y, Xu B, Wei X, Wang L, et al. Naringenin Ameliorates Radiation-Induced Lung Injury by Lowering IL-1β Level. The Journal of Pharmacology and Experimental Therapeutics. 2018;366(2):341-8.
263. Tutunchi H, Naeini F, Ostadrahimi A, HosseinzadehAttar MJ. Naringenin, a flavanone with antiviral and antiinflammatory effects: A promising treatment strategy against COVID-19. Phytotherapy Research : PTR. 2020:10.1002/ptr.6781.
