Abstract
Background: Orbital lymphoproliferative disorders (OLPDs) consist of a spectrum of diseases ranging from benign to malignant lesions including reactive lymphoid hyperplasia, atypical lymphoid hyperplasia, and lymphoma. OLPDs rarely present as an orbital mass lesion in children. Accurate discrimination of OLPDs is crucial for treatment planning. We report a case to investigate the clinical and pathological features of OLPDs in children.
Case presentation: A 3-year-old female with orbital mass was admitted to the hospital and proceeded to have a CT orbit which showed an orbital mass. The orbital mass was removed after operation and pathologic diagnosis was identified. Pathological diagnosis with histopathological features and immunohistochemical markers was lymphoproliferative lesion in the left orbit. The diagnosis was consistent with OLPDs. There was no recurrence after one-year follow-up.
Conclusions: OLPDs are rare in children. The clinical manifestations and imaging have no specificity; pathological diagnosis with histopathological features and immunohistochemical markers is the main basis for the diagnosis and treatment.
Keywords
Orbital Lymphoproliferative Disorders (OLPDs), Lymphoproliferative lesion, Orbital mass, Orbital tumor
Background
Orbital lymphoproliferative disorders (OLPDs) are a group of diseases characterized by abnormal proliferation of lymphocytes. It is frequent in adults, but rare in children. It is a group spectrum of diseases from benign to malignant, mainly including reactive lymphocytic hyperplasia, atypical lymphocytic hyperplasia, and lymphoma. There is no clear distinction standard for these three types in clinical practice, and they have similar imaging features in imaging, and MR imaging is also limited in differentiation [1]. It is often difficult to distinguish by pathological diagnosis, so immunohistochemistry and molecular biology techniques are needed to improve the accuracy of diagnosis. Due to the rare incidence in children, it is more difficult to diagnosis accurately. We report a case of orbital lymphoproliferative disorders in a 3-year-old female, and analyze the clinical characteristics, diagnosis, and treatment of this disease by reviewing the current literature.
Case Presentation
A 3-year-old girl was referred to ophthalmology for a 1-month history of swelling lesions with no ocular trauma or surgery, or vascular lesions, involving the partial bulging of the lower eyelid of the left eye which was suspected conjunctivitis by treatment with levofloxacin eye drops. But the swelling continued. When the patient was referred to ophthalmology, the swelling was increased approximately 10 mm × 20 mm in the lower eyelid with clear boundary. The conjunctiva surface seemed to have been covered with fish-like nipples. The cornea was transparent. The anterior chamber and the pupil were normal (Figures 1-3). B-ultrasound showed an inflammatory hypo echoic area, and a soft tissue mass on CT. The orbital mass was excised completely during general anesthesia (Figure 4). Pathological description: a small amount of epithelial tissue was on the focal surface under the microscope, and a large amount of lymphoid tissue hyperplasia in the fibrous tissue. Immunohistochemistry monoclonal antibody and oncogene detection: CD10 (+), CD3 (+), CD15 (+), CD30 (+), CD5 (+), CD19 (+), CD20 (+), CD21 (FDC+), CD56 (+), CD79a(+), CD68(+), CD99 (+), CD4 (+), CD8 (+), T dT(-), ALK (-), Ki67 (about 10%+), IgG(+), IgG4 (-), Bcl-2 (+), c-myc (+), EBER (-), CD163 (+), CK (AE1/AE3) (+), Pax5 (+) (Figures 5-8). Orbital lymphoproliferative disorders were diagnosed and there was no recurrence after 1 year- follow-up (Figure 9).

Figure 1. Swelling eyelid.
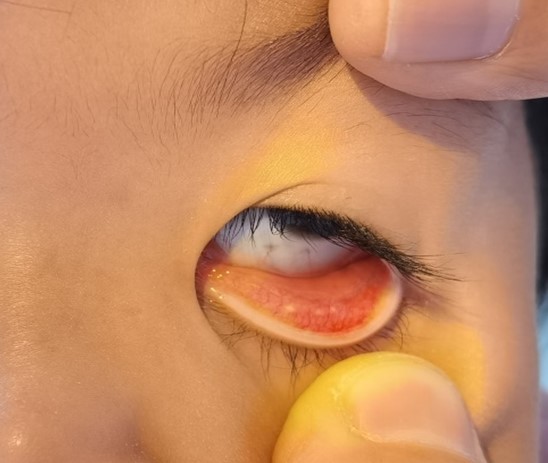
Figure 2. The hyperemia of conjunctiva.
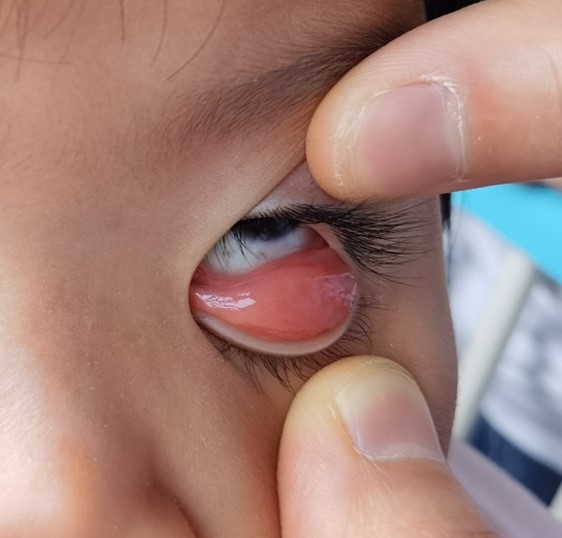
Figure 3. The fish-like conjunctiva.
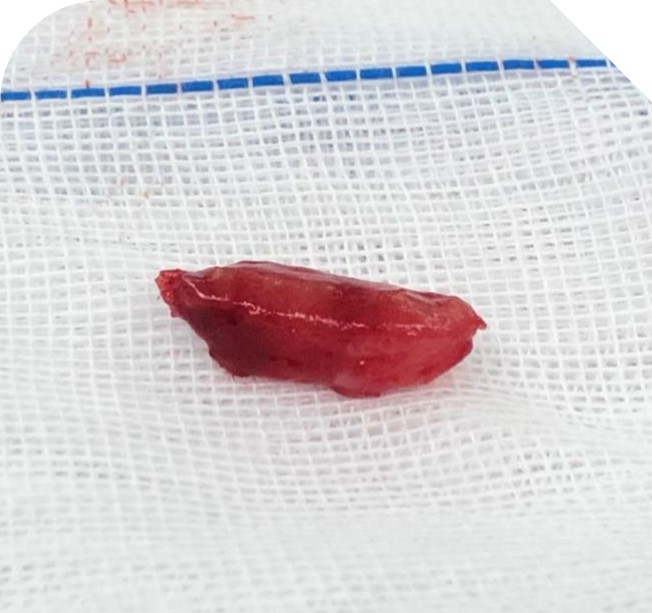
Figure 4. Excised mass.
a. 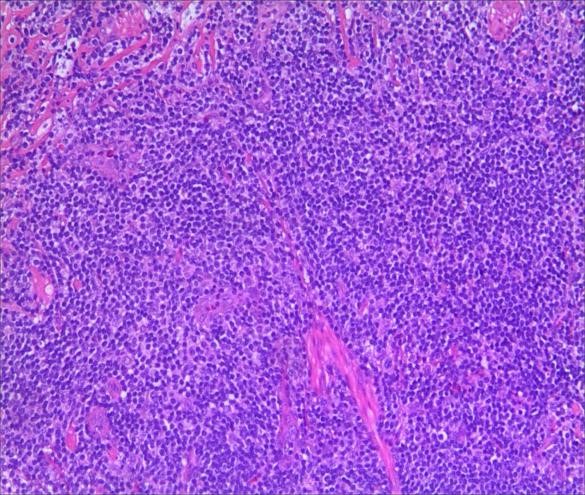 b.
b. 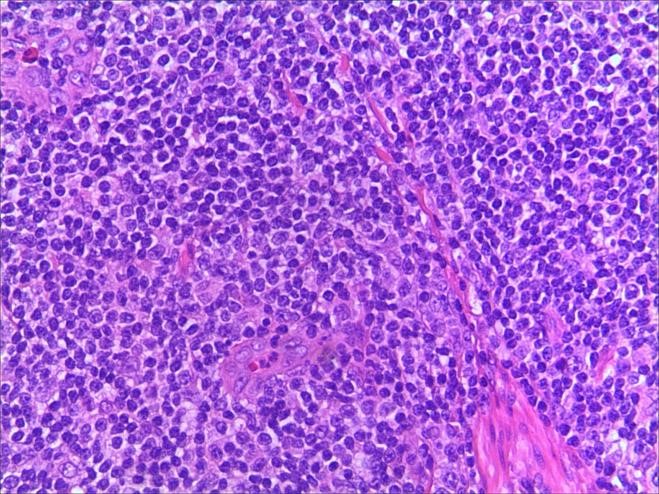
Figure 5. a. HE staining. b. HE staining (2).
a. 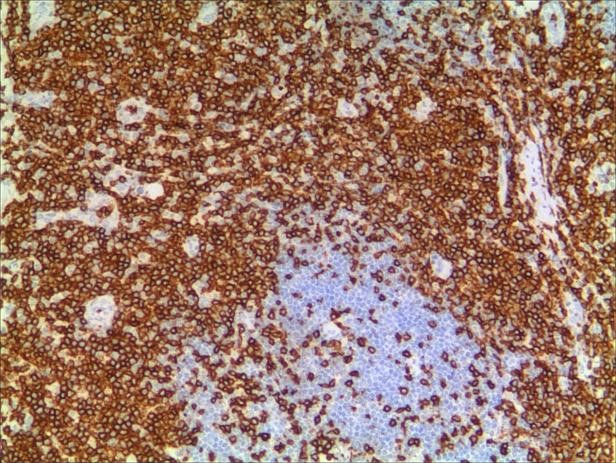 b.
b. 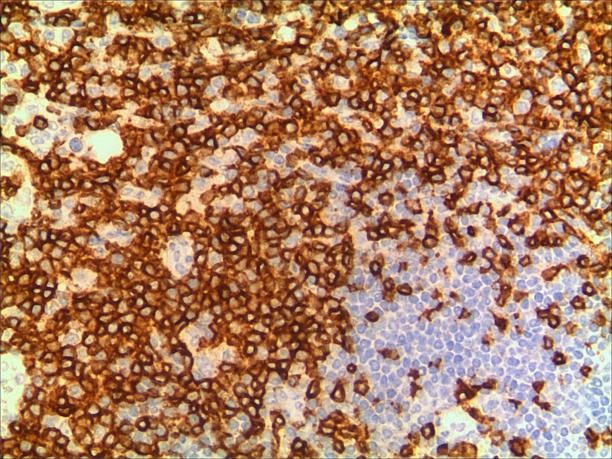
Figure 6. a. CD3. b. CD3 (2).
a. 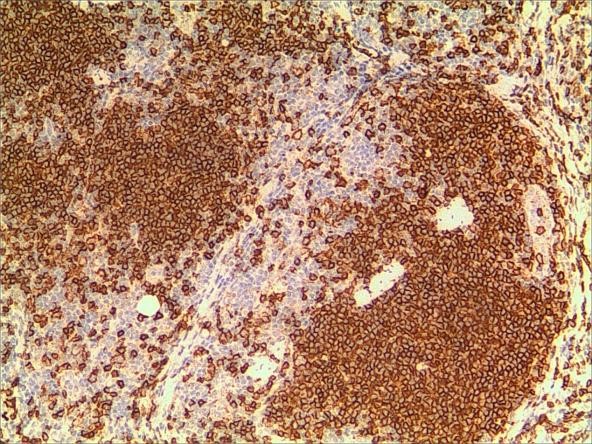 b.
b. 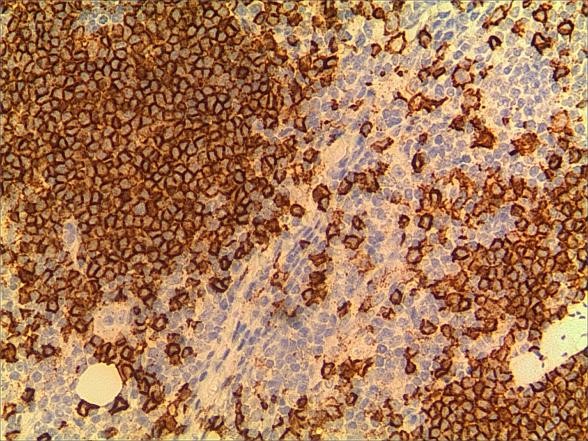
Figure 7. a. CD20. b. CD20 (2).
a. 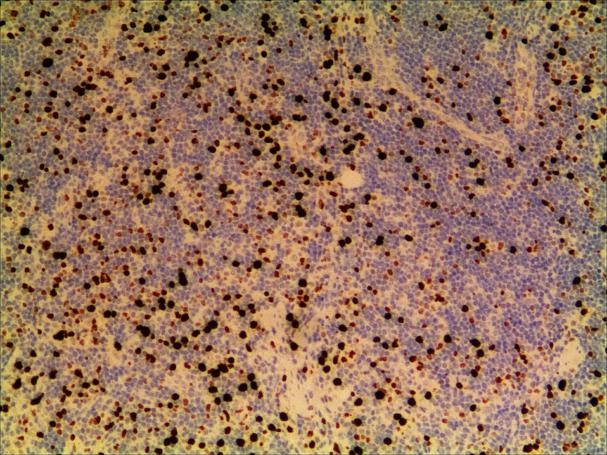 b.
b. 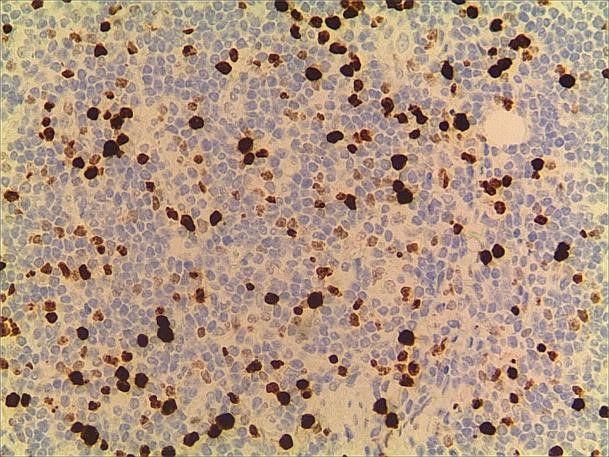
Figure 8. a. Ki-67. b. Ki-67 (2).

Figure 9. 1 month after operation.
Discussion and Conclusions
OLPDs frequently present as an orbital mass lesion (24%–49%) in adults, most of which are orbital primary lesions, especially in the lacrimal gland. Ocular adnexa include eyelid, conjunctiva, lacrimal organ is most commonly occurred because ocular lymphoid tissue is predominantly located in these areas [2-4]. About 37% of ocular adnexal lymphoma occurred in orbit, 29% in conjunctiva; 20% in the lacrimal apparatus; 14% in the eyelids. OLPDs are more common between the ages of 50 to 70 and more often in women. The pathogenesis is still unclear. And some studies reported that may be related to the infection of related pathogens, including Chlamydia psitsiti, mycoplasma, Epstein-Barr virus, etc. [5-9].
The disease usually presents as an orbital mass lesion with insidious symptoms, less painful early and more often be found until the obvious compression symptoms or enlarged masses. There are different clinical manifestations according to locations. The imaging examinations such as CT and MR can show the location, morphology, volume, and relationship with surrounding tissues, but these manifestations are not specific and have little significance in diagnosis [1,10,11]. Usually exophthalmos, blepharoptosis, strabismus, and movement disorders may occurred when the mass is located in the lacrimal gland, Light red fish flesh appearance, small pebble or multinodular appearance on conjunctiva surface, without eye movement disorder and protrusion when the mass is located in conjunctiva. Hard and tough nodules are palpable when in anterior orbitals or subconjunctival areas. Clinically, benign lesions generally have mild symptoms and signs, while malignant lesions are more severe, but they cannot be distinguished by symptoms and signs. In this case, a painless, palpable, and tough mass was located in the anterior orbit with fish-like appearance and many small pebble nodules. All these signs were consistent with lymphoproliferative lesions. However, due to the age and no systemic signs, the diagnosis should be combined with pathological basis.
OLPDs comprise of a wide spectrum of diseases ranging from benign to malignant lesions. Lymphoid hyperplasia and lymphoma are in each end of the spectrum, while atypical lymphoid hyperplasia is in the middle region. Mostly small cell non-Hodgkin B-cell lymphoma is difficult to be diagnosed in traditional histology. Reactive lymphoid hyperplasia can be diffuse distribution mainly composed of diffuse hyperplasia mature lymphocytes or follicular structure with a variety of transformed cells in the center and mature lymphocytes surrounding. Atypical lymphoproliferative lesions are mostly diffuse distribution, and there may be residual follicles. The cells may be monomorphic or polymorphic, mainly near mature or with large nucleolus-stained atypical cells scattered between lymphocytes and plasma cells. Ocular adnexal lymphomas are mostly B-cell non-Hodgkin's lymphomas, while T-cell lymphomas are rare. The traditional classification of lymphoma is complicated. WF [12] classifies tumors into low, medium, and high malignant degrees according to the morphological characteristics and the 5-year survival rate of patients. This classification is simple and easy, acceptable by clinicians, but it completely depends on the morphology of tumor cells. In 1994, REAL [13] reached a new classification of lymphoma according to the clinical process, morphology, immunophenotype, and genetic characteristics of the disease [14]. In 2001, a new classification was promulgated by WHO [15-17], which was partially modified on the basis of the REAL classification to make it more scientific. Coupland classified ocular adnexal lymphomas into 5 types according to REAL as follows: mucosa-associated lymphoid tissue lymphoma, follicular central lymphoma, diffuse large B-cell lymphoma, plasmacytoma, and Gonoctic lymphoma. Jenkins classified it into mucosa-associated lymphoid tissue lymphoma, plasmacytoid cell lymphoma, follicular central lymphoma, diffuse large B-cell lymphoma and other rare lymphomas [18]. The characteristics of ocular accessory lymphoma are that the cell morphology of lymphoma is a certain stage in the process of normal lymphocyte transformation with corresponding morphology and immunophenotype. For example, Mucous-associated lymphoid tissue (MALT) lymphoma usually shows the characteristics of memory cells or marginal zone B cells. Follicular lymphoma shows follicular central cells and central blasts; plasmacytoma shows nearly mature plasma cells; and diffuse large B-cell lymphoma shows germinal centers and post-germinal memory cells. When the pathological tissue is typical, the diagnosis is not difficult. However, some overlaps often exist on morphology, especially when the cell morphology is immature and there is no typical manifestation of lymphoma, the differentiation is more difficult. Immunohistochemistry is needed to assist diagnosis, which is to use the principle of combining specific antibodies with specific antigens in cells to detect markers of different types of cells in lymphoid tissue. It is currently an indispensable auxiliary detection technique for the pathological diagnosis, differential diagnosis, and classification of lymphoma of lymphoid tissue proliferative lesions. At present, there are 247 international CD series antibodies, and there are several non-CD series, such as CK, EMA, S100, TdT, Cyclin D1, etc., and the vast majority of antibodies can be labeled with paraffin-embedded tissue sections. Antibodies can be divided into polyclonal and monoclonal, the former has poor specificity, and the latter has strong specificity but high cost. There are also problems with insufficient sensitivity and specificity.
The morphological structure characteristics of histiocytes are the main elements of diagnosis, and immunomarkers and genetic detection play an important role in the differential diagnosis of lymphoma. Different antibody positively-expressed cell sites are different. For example, CD20, CD10, and CD45RO are positive for cell membranes, while CD3, CK, and CD68 are cytosolic positive, PAX5 and Ki-67 are nucleus-positive, and S100 is co-positive to nucleus-cytoplasmic. B-cell labeled antibody PAX5 nucleus is positive and has the strongest specificity, while T cell labeled antibody CD3 has good specificity, CD21 is used to label follicular dendritic cell network, which can play an auxiliary role in judging lymphatic follicle preservation or erosion and destruction. Ki-67 has a good effect on labeling the proliferation index of lymphohistiocyte or tumor cells, and has a good auxiliary effect on the labeling and abnormal destruction of follicular germinal centers.
Lymphoid tissue reactive hyperplasia was composed of simplex diffuse lymphocyte hyperplasia and a few lymphocytes of different stages of maturation among the mature lymphocytes, but there was no obvious atypical and mitosis. Immunohistochemistry showed polyclonal hyperplasia to help to diagnose. In atypical lymphoid hyperplasia, there is mitotic phase outside the germinal center. Immunohistochemistry shows monoclonal proliferation, but the lesion is mainly composed of mature lymphocytes, without single immature characteristics. Mucous-associated lymphoid tissue (MALT) lymphoma is a low-grade malignant small B-cell lymphoma with clinical manifestations of indolent progression and monocular onset. The lesion with gray- white or gray- red fish-like surface grows irregular mucosa-like tissue around the eye ring without obvious boundary. On the morphology, MALT lymphoma lesions for different types of cells proliferation, with marginal zone B cell or mononuclear cell is given priority to, with varying degrees of mother cell transformation and plasma cell differentiation, can have follicular structure and the implantation of germinal center, and infringement of epithelial or glands, formation of lymphatic epithelial lesions [19-21]. Follicular lymphoma is a type of B-cell lymphoma that usually occurs in children and often lacks t (14; 18) translocation and bcl-2 protein expression that is difficult to distinguish from MALT lymphoma. Typical pediatric follicular lymphoma often occurs in the cervical lymph nodes of boys and presents as large follicles with vigorous hyperplasia, mantle area is often present, and sometimes accompanied by progressive germinal center transformation. Immunohistochemistry of CD10 and bcl-6 showed that the positive tumor cells outside the follicular were more supportive of pediatric follicular lymphoma, while CD21 and CK were supportive to diagnosis of MALT lymphoma [16]. Other small B-cell lymphomas are rare in children and adolescents who usually express specific antigens, such as CD5, CD23, or cyclin D1 [16].
In this case, the monocular lesion was irregular mucosa-like tissue, and the section surface was gray-red, medium, or soft, and had a fish-like appearance. In morphology, more lymphocyte hyperplasia and CD10 and Bcl-2 are positive, which cannot exclude the indolent clinical process of MALT. At the same time, B cells and T cell immune antibodies, CD20 and CD79a are positive, PAX5 nucleus is positive, indicating that its B cell antibodies are positive, CD3 and CD5 are positive, indicating that T cell antibodies are positive, consider polyclonal hyperplasia, and reactive hyperplasia. CD21 (FDC+) shows irregular follicular dendritic cell network (FDC net), Ki67 proliferation index of about 10%, in situ hybridization EBER negative, more inclined to benign lesions of OLPDs. We cannot diagnose exactly which type of OLPDs it is.
OLPDs represent a spectrum of changes from polyclonal to monoclonal B-cell populations. Reactive hyperplasia is polyclonal, while lymphoma is monoclonal. This method is of great significance for the diagnosis. Therefore, the application of immunohistochemistry and PCR technology is helpful to further clarify the diagnosis of some cases that are difficult to be diagnosed morphologically. Histomorphological characteristics and immunohistochemical examination are the basic methods for the diagnosis of OLPDs, and the detection of IgH gene rearrangement and κ or λ restriction expression has certain clinical advantages in the diagnosis of OLPDs, and the higher positive rate of the two is of great significance for diagnosis. The failure to rearrange the IgH gene and κ or λ testing in this patient is also a deficiency of this article.
Most of the lymphoproliferative lesions showed localized with clear boundaries which can be completely resected. Only a little are invasive and extensive which is difficult to be completely resected with a long course and poor prognosis. In addition, due to the brittle and fragile swelling material of lymphoproliferative lesions, clamp in the operation often causes to break, which is difficult to completely remove, so it is necessary to fully separate the lesions in advance and avoid clamping. Most lymphomas are in lazy with slow growth and few systemic metastases. For those with a wide range of lymphomas, excessive and thorough resection should not be forced. Postoperative radiotherapy [21,22] and chemotherapy [22,23] can be combined to avoid the occurrence of serious complications. Specific treatment options depend on the location, shape, and size of the lesion, as well as the clinical stage of the lesion and the patient's general condition [24-26]. Therefore, prior to treatment, we must perform relevant examinations and pre-evaluation. Due to the lack of specific clinical features of orbital lymphoma, some patients may be misdiagnosed or missed. If orbital lymphoma has non-specific manifestations, detailed imaging studies combined with early tissue biopsy are helpful for the early diagnosis. Once the diagnosis is made and the stage of the disease is defined, treatment should be given according to the specific situation. In study, the boundary of the tumor was clear and easily resected completely. After surgical treatment, there was no sign of recurrence until now.
We should familiarize with the clinical features of OLPDs. Immunohistochemical staining should be reasonably used, combined with the clinical characteristics of patients, detailed and comprehensive analysis. And molecular biological techniques and gene rearrangement techniques [27-29] should be used to confirm the diagnosis. At the same time, the follow-up work should be strengthened. No matter if it is benign or malignant lesions, postoperative recurrence is possible. Therefore, strengthening the clinical follow-up of patients can timely grasp the patient's condition, so as to make corresponding treatment in time, and change the treatment plan if necessary.
Abbreviations
OLPDs: Orbital Lymphoproliferative Disorders; MALT: Mucous-associated Lymphoid Tissue
Declarations
Ethics approval and consent to participate
This study was approved by the Children's Hospital Zhejiang University School of Medicine Ethics Committee Board.
Consent for publication
Written informed consent was obtained from the mother of the patient for publication of this case report and any accompanying images. All authors have viewed and agreed to the submission.
Availability of data and material
All relevant data are included in this manuscript and associated figures. However, if more information is required, the datasets analysed for the current study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare that they have no competing interests. We have full access to all the data in this study; and are fully responsible for the completeness of the data and the accuracy of the data analysis.
Funding
This study received no funding support.
Authors' contributions
CPS, SJZ and YHR performed the literature search; and collection; and drafted the manuscript. JF, JZ, and WZG completed all examinations and confirmed the final diagnosis. XYZ contributed to the photograph collection. All authors read, revised, and approved the final version of the manuscript.
Acknowledgements
We would like to extend sincere thanks to the patient and her parents for their cooperation in the study.
References
2. Liesegang TJ. Ocular adnexal lymphoproliferative lesions. Mayo Clinic Proceedings. 1993 Oct 1;68(10): 1003-10).
3. Oh DE , Kim YD. Lymphoproliferative diseases of the ocular adnexa in Korea. Archives of Ophthalmology. 2007 Dec;125(12):1668-73.
4. Verdijk RM. Lymphoproliferative tumors of the ocular adnexa. The Asia-Pacific Journal of Ophthalmology. 2017 Mar 1;6(2):132-42.
5. Hoshida Y, Tomita Y, Zhiming D, Yamauchi A, Nakatsuka SI, Kurasono Y, et al. Lymphoproliferative disorders in autoimmune diseases in Japan: analysis of clinicopathological features and Epstein-Barr virus infection. International Journal of Cancer. 2004 Jan 20;108(3):443-9.
6. Isaacson PG. Update on MALT lymphomas. Best Practice & Research Clinical Haematology. 2005 Mar 1;18(1):57-68.
7. Isaacson PG, Du MQ. Gastric lymphomas: genetics and resistance to H. pylori eradication. Verhandlungen der Deutschen Gesellschaft fur Pathologie. 2003 Jan 1;87:116-22.
8. Piccaluga PP, Agostinelli C, Gazzola A, Tripodo C, Bacci F, Sabattini E, et al. Pathobiology of Hodgkin lymphoma. Advances in Hematology. 2011;2011:920898.
9. Du MQ, Isaccson PG. Gastric MALT lymphoma: from aetiology to treatment. The Lancet Oncology. 2002 Feb 1;3(2):97-104.
10. Knowles II DM, Jakobiec FA. Ocular adnexal lymphoid neoplasms: clinical, histopathologic, electron microscopic, and immunologic characteristics. Human Pathology. 1982 Feb 1;13(2):148-62.
11. Nathwani BN, Anderson JR, Armitage JO, Cavalli F, Diebold J, Drachenberg MR, et.al. Marginal zone B-cell lymphoma: a clinical comparison of nodal and mucosa-associated lymphoid tissue types. Journal of Clinical Oncology. 1999 Aug;17(8):2486.
12. Non-Hodgkin's Lymphoma Pathologic Classification Project. National Cancer Institute sponsored study of classifications of non-Hodgkin's lymphomas: summary and description of a working formulation for clinical usage. Cancer. 1982;49(10):2112-35.
13. Tonami H, Matoba M, Yokota H, Higashi K, Yamamoto I, Sugai S. Mucosa-associated lymphoid tissue lymphoma in Sjogren's syndrome: initial and follow-up imaging features. American Journal of Roentgenology. 2002 Aug;179(2):485-9.
14. Coupland SE, Hummel M, Stein MH. Ocular adnexal lymphomas: five case presentations and a review of the literature. Survey of Ophthalmology. 2002 Sep 1;47(5):470-90.
15. Hara Y, Nakamura N, Kuze T, Hashimoto Y, Sasaki Y, Shirakawa A, et.al. Immunoglobulin heavy chain gene analysis of ocular adnexal extranodal marginal zone B-cell lymphoma. Investigative Ophthalmology & Visual Science. 2001 Oct 1;42(11):2450-7.
16. Knowles DM, Jakobiec FA, Mcnally L, Burke JS. Lymphoid hyperplasia and malignant lymphoma occurring in the ocular adnexa (orbit, conjunctiva, and eyelids): a prospective multiparametric analysis of 108 cases during 1977 to 1987. Human Pathology. 1990 Sep 1;21(9):959-73.
17. Plaisier MB, Sie-Go DM, Berendschot TT, Petersen EJ, Mourits MP. Ocular adnexal lymphoma classified using the WHO classification: not only histology and stage, but also gender is a predictor of outcome. Orbit. 2007 Jan 1;26(2):83-8.
18. Schroder-Frei B, Kurrer M, Chaloupka K, Frei J, Landau K. Orbital MALT lymphomas: clinicopathological correlation. KLINISCHE MONATSBLATTER FUR AUGENHEILKUNDE. 2006;223(5):405.
19. Nola M, Lukenda A, Bollmann M, Kalauz M, Petrovecki M, Bollmann R. Outcome and prognostic factors in ocular adnexal lymphoma. Croatian Medical Journal. 2004 Jun 1;45(3):328-32.
20. Zhang JF, Zhang SX, Li J. Molecular Pathological Diagnosis of Mucosa-associated Lymphoid Tissue Lymphoma. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2015 Jun;23(3):689-92.
21. Kaushik M, Pulido JS, Schild SE, Stafford S. Risk of radiation retinopathy in patients with orbital and ocular lymphoma. International Journal of Radiation Oncology* Biology* Physics. 2012 Dec 1;84(5):1145-50.
22. Shi YY, Jia RB, Fan XQ. The progress in the diagnosis and management of orbital lymphoma. Zhonghua Yan Ke Za Zhi. 2017 Aug 11; 53(8):632-636.
23. Shome D, Esmaeli B. Targeted monoclonal antibody therapy and radioimmunotherapy for lymphoproliferative disorders of the ocular adnexa. Current Opinion in Ophthalmology. 2008 Sep 1;19(5):414-21.
24. Olsen TG, Heegaard S. Orbital lymphoma. Surv Ophthalmol. 2019 Jan-Feb; 64(1):45-66
25. Aldave AP, Jaiswal S, Davidson SL. Marginal zone mucosa associated lymphoid tissue diffuse large B cell lymphoma. North American Journal of Medical Sciences. 2014 Aug;6(8):422.
26. Tovilla-Canales JL, y Pomar JL, Ceron JR. Lymphoproliferative disorders of the ocular adnexa. Current Opinion in Ophthalmology. 2004 Oct 1;15(5):401-5.
27. Farmer JP, Lamba M, Lamba WR, Jordan DR, Gilberg S, Sengar DP, et.al. Lymphoproliferative lesions of the lacrimal gland: clinicopathological, immunohistochemical and molecular genetic analysis. Canadian Journal of Ophthalmology. 2005 Apr 1;40(2):151-60.
28. Jakobiec FA, Neri A, Knowles II DM. Genotypic monoclonality in immunophenotypically polyclonal orbital lymphoid tumors: a model of tumor progression in the lymphoid system. Ophthalmology. 1987 Aug 1;94(8):980-94.
29. Bertoni F, Zucca E.Delving deeper into MALT lymphoma biology. Journal of Clinical Investigation. 2006 Jan; 116(1):22-6.
