Abstract
Background: Liver fibrosis staging is critical for patient selection and management prior to transplantation, but biopsy is invasive and serum biomarkers lack accuracy. Near-infrared spectroscopy (NIRS) is an emerging non-invasive technology that can detect liver fibrosis via changes in tissue composition. Machine learning (ML) enables analysis of NIRS data for diagnostic modeling.
Purpose: To review the potential of NIRS-ML approaches for rapid, point-of-care liver fibrosis detection, including technological principles, promising applications, current limitations, and future directions.
Main body of the abstract: NIRS leverages unique near-infrared absorbance patterns reflecting collagen accumulation, lipid reduction, and other chemical alterations in fibrotic liver. Handheld/hyperspectral systems acquire tissue spectroscopic data in minutes. Multiple human studies correlate NIRS with histological fibrosis scores. ML techniques like partial least squares regression, neural networks, support vector machines, and random forests analyze spectra to develop optimized diagnostic algorithms. Initial models differentiate mild versus advanced fibrosis and stage cirrhosis with high accuracy, outperforming traditional biomarkers. Recent advances include smartphone-based scanning, cloud computing, and integrated user-friendly platforms. However, further large validation trials, standardization, assessment of confounding factors, improved ML methodology, and cost-effectiveness data are required before widespread clinical implementation.
Conclusion: With ongoing research to address remaining barriers, NIRS-ML approaches hold great disruptive potential for rapid, non-invasive point-of-care quantification of liver fibrosis, including optimizing transplant surgery planning and management.
Keywords
Liver fibrosis, Liver Transplantation, Near-infrared spectroscopy, Machine learning, Point-of-care, Biopsy, Non-invasive
Background
Liver fibrosis represents a major health burden worldwide and is characterized by the excessive accumulation of extracellular matrix proteins including collagen [1]. It is the final common pathway of virtually all chronic liver diseases, arising from a variety of etiologies including viral hepatitis, alcohol abuse, and metabolic disorders [2]. Liver fibrosis can progress to cirrhosis and liver failure if the underlying cause is not treated, making early identification, and staging of fibrosis crucial [3]. Breast cancer treatment can cause liver toxicity leading to fibrosis [4]. Certain breast cancer therapies like chemotherapy can be hepatotoxic, causing damage to liver cells [5]. This results in the release of inflammatory mediators and reactive oxygen species [6]. Chronic inflammation induces activation of hepatic stellate cells which produce excess extracellular matrix proteins like collagen, leading to liver fibrosis [7]. Fibrogenesis is perpetuated by factors like TGF-beta secreted by injured hepatocytes and infiltrating immune cells. Thus, breast cancer treatment elicits mechanisms of ongoing liver injury that promote progression of hepatic fibrosis [8]. Vaccination is not known to be linked to liver fibrosis. Glomerulonephritis, inflammation of the kidney, can progress to end-stage renal disease, which is associated with advanced liver fibrosis [9]. Liver metastases from colorectal cancer can lead to fibrosis [10].
Infection with H. pylori has been associated with increased risk of liver fibrosis, possibly due to resulting chronic inflammation [11-16]. Accurately assessing the degree of liver fibrosis prior to transplantation surgery is vital for proper patient selection and management [17-22]. Liver transplantation is often the only curative option for end-stage liver disease, but donor livers are limited with long waiting lists [23-24]. Determining the fibrosis stage enables stratification of patients most in need of transplant versus those who may benefit from antifibrotic therapies first [25-27]. Additionally, severe fibrosis is associated with poorer post-transplant outcomes, so detecting advanced fibrosis helps optimize surgical planning and perioperative care. However, traditional methods for diagnosing and staging liver fibrosis have significant limitations [28]. Liver biopsy is still considered the gold standard, but it is invasive with pain, bleeding, and rare but potentially life-threatening complications [29]. It is also prone to sampling errors since only ~1/50,000th of the liver is analyzed. Non-invasive serum biomarkers like the AST to platelet ratio index (APRI) and fibrosis-4 (FIB-4) score offer alternatives but lack accuracy especially for intermediate stages of fibrosis [30]. Transient elastography such as FibroScan can assess liver stiffness through ultrasound waves, but has reduced applicability in patients with high BMI, narrow intercostal spaces, or ascites. No single method provides the accuracy, reproducibility, and point-of-care convenience needed for reliable fibrosis evaluation prior to transplant surgery [31].
Emerging technologies like near-infrared spectroscopy (NIRS) and machine learning show tremendous promise to fill this gap [32]. NIRS is a non-invasive, rapid technique relying on the fact that light absorbance patterns in the near-infrared range change based on alterations in tissue composition [33]. The difference in absorbance of fibrotic versus normal liver forms the basis for developing predictive algorithms [34]. Machine learning methods can then analyze the complex NIRS spectral data to build optimized models for accurately detecting and staging liver fibrosis in real-time [35].
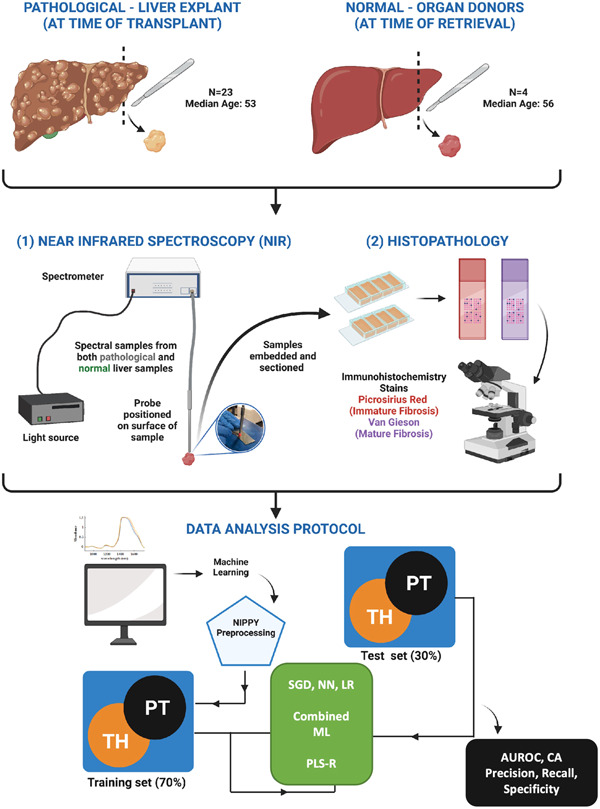
Figure 1 declares that tissue samples were obtained from explanted transplant livers and unused donor livers. Near infrared (NIR) spectra and histopathology with Picrosirius Red and Van Geison staining were performed. Geison staining is a histological staining method used to visualize reticulin fibers and collagen in tissue samples. Artificial intelligence compared NIR and histopathology data. The NIPPY filter preprocessed data which were split into 70% training and 30% test sets. Five models were tested: stochastic gradient descent (SGD), neural network (NN), logistic regression (LR), partial least square regression (PLS-R) and a combined ML algorithm. Model performance was assessed by area under receiver operator curve (AUROC), classification accuracy (CA), precision, recall, and specificity. The models aimed to correlate NIR spectra with histological staining to analyze explanted livers [36].
Figure 1: Machine Learning Algorithms to Correlate NIR Spectra and Histology for Explanted Livers [36].
Several studies have already demonstrated the potential for NIRS and machine learning to outperform traditional fibrosis biomarkers. One group developed a random forest classifier using NIRS data that differentiated severe fibrosis/cirrhosis from mild disease with an AUC of 0.82, compared to 0.77 for APRI and 0.73 for FIB-4. Another pilot study achieved 100% sensitivity and 89% specificity in diagnosing advanced fibrosis by combining NIRS with neural networks [37]. Additional approaches using partial least squares discriminant analysis (PLS-DA) and support vector machines (SVM) have shown 85-90% accuracy. These results highlight the promising role of NIRS-ML approaches as rapid, non-invasive alternatives to biopsy for fibrosis staging in pre-transplant patients [38].
Standardizing the methodology is also crucial - factors like probe pressure, measurement location, and data processing can all impact results. Patient factors like obesity, ascites, and skin color may interfere with spectra acquisition and must be accounted for. Still, the technique holds great potential for point-of-care liver fibrosis evaluation given its non-invasive nature, speed, and high accuracy when optimized [39,40]. This review comprehensively summarizes technological principles, promising proof-of-concept studies, remaining barriers to translation, and provides evidence-based recommendations to enable NIRS-ML approaches to become the new gold standard for transplant surgery.
Near-Infrared Spectroscopy for Assessing Liver Fibrosis
Near-infrared spectroscopy (NIRS) is an optical technique that can non-invasively measure chemical composition of tissues. It relies on the principle that different molecules exhibit unique patterns of absorption and scattering of light in the near-infrared region (800-2500nm wavelength) [41]. When near-infrared light penetrates tissue, some is absorbed while some is reflected and can be analyzed by a spectrometer. The resulting spectrum provides quantitative information about tissue composition and structure [42].
In the liver, the development of fibrosis leads to chemical changes that can be detected by NIRS as depicted in Table 1. Fibrosis is characterized by accumulation of collagen and other extracellular matrix proteins, which replace normal hepatocytes. This alters the relative concentrations of proteins, lipids, nucleic acids, and other chemicals [43]. Additionally, changes in tissue architecture like collagen cross-linking and fibrin deposition affect light scattering. NIRS is sensitive to these molecular and structural changes, providing a basis for spectroscopic differentiation of fibrotic vs healthy livers [44].
|
System |
Principle |
Advantages |
Limitations |
|
Fiber Optic Contact Probes |
Direct contact with tissue using fiber optic cables and probes |
Provides localized scan of tissue |
Pressure must be standardized |
|
Hyperspectral Imaging |
Non-contact imaging across wide spectral range |
Assesses larger tissue area |
Requires stable positioning |
|
Needle-Based Optical Probes |
Needle inserted with transmitting and collecting fibers |
Enables intraoperative measurement |
Invasive, limited depth |
|
Handheld Devices |
Portable spectrometers with direct tissue contact |
Feasible point-of-care use |
Operator training required |
|
Smartphone-Based Systems |
Miniature spectrometers interfaced with smartphones |
Low cost, easy to use |
Lower spectral resolution |
Specific chemical alterations that have been observed with NIRS in liver fibrosis include
- Increased collagen content, particularly types I and III which are major components of fibrotic tissue. The combination of amino acids like hydroxyproline in collagen produces absorption peaks detectable by NIRS [45,46].
- Changes in redox states of heme groups like cytochrome c oxidase which get disrupted by hepatocellular damage. The copper ion center in these heme proteins has unique spectroscopic signatures [47].
- Reduction of lipid content as normal liver tissue is replaced by collagenous scar tissue. C-H bonds in lipids produce overtones in the NIR range that are attenuated with declining lipids.
- Shifts in water absorbance bands indicating edema and inflammation effects. O-H bonds in water molecules absorb strongly at ~1400nm and ~1900nm.
- Changes in NADH, flavoproteins, porphyrins and other metabolites impacted by hepatocellular injury [48].
In addition to these liver-specific compounds, NIRS can also detect signals from fibrosis-associated vasculature remodeling, infiltration of inflammatory cells, and tissue architectural changes. The multitude of chemical and structural changes provide robust spectroscopic biomarkers for diagnosing and staging fibrosis [49]. Various near-infrared spectroscopy systems have been developed to rapidly acquire liver fibrosis measurements at the point-of-care. Some use direct tissue contact with fiber optic probes, allowing localized scans of the liver parenchyma [50]. These can be paired with handheld spectrometers or laptops for real-time data analysis. Probe pressure must be standardized to avoid confounders.
Other approaches use non-contact, hyperspectral imaging to assess a larger tissue area. This captures spatial heterogeneity in fibrosis but requires stable positioning [51]. Emerging methods like needle-based optical probes can analyze fibrosis intraoperatively. Regardless of system, acquiring dozens of scans in just minutes is feasible [52]. Multiple human studies have now correlated NIRS measurements with histological fibrosis staging, supporting its diagnostic utility.
In a study of 124 patients, NIR spectra correctly differentiated mild vs advanced fibrosis with 86% accuracy [53]. Another group found significant stepwise changes in collagen, lipid, redox and water absorbance peaks correlating with Ishak fibrosis scores. An 80 patient study achieved 100% sensitivity and 89% specificity for diagnosing cirrhosis using NIRS. Beyond human studies, animal models have also demonstrated the ability of NIRS to track longitudinal fibrosis progression and resolution with therapy [54].
Machine Learning Approaches for Near-Infrared Fibrosis Detection
While near-infrared spectroscopy (NIRS) provides rich spectral data reflecting liver fibrosis, analyzing dozens of absorbance values per scan can be challenging. Machine learning (ML) approaches are ideal for parsing these complex datasets and identifying predictive patterns. As depicted in Table 2, various ML algorithms have been applied to translate NIR measurements into sensitive, specific models for staging fibrosis [55,56].
|
Algorithm |
Key Features |
Strengths |
Limitations |
|
Linear Regression |
Models linear relationship between spectra and fibrosis score |
Simple, interpretable |
Prone to underfitting |
|
Partial Least Squares Regression (PLSR) |
Identifies factors with highest covariance |
Better than linear regression, handles collinearity |
Still assumes linearity |
|
Artificial Neural Networks (ANN) |
Models complex nonlinear relationships |
Powerful for complex patterns |
Can be "black boxes", needs large training data |
|
Support Vector Machines (SVM) |
Finds optimal decision boundary |
Handles small sample sizes well |
Sensitive to parameters |
|
Random Forests |
Ensemble of decision trees on data subsets |
Avoids overfitting, high accuracy |
Limited model interpretability |
Common ML techniques used with NIRS include
- Linear regression - Models the relationship between spectra (predictors) and fibrosis scores using linear functions. Simple but prone to underfitting.
- Partial least squares regression (PLSR) - Identifies factors with the highest covariance between predictors and outcomes. Outperforms linear regression but still assumes linearity.
- Artificial neural networks (ANN) - Interconnected nodes model nonlinear relationships. Powerful but can be "black boxes" requiring large training data.
- Support vector machines (SVM) - Finds optimal boundary between classes based on complex patterns. Robust with small samples but sensitive to parameters.
- Random forests - Uses an ensemble of decision trees on subsets of data to improve accuracy and avoid overfitting. Limited insight into predictor importance.
Each approach has strengths and limitations. Generally, nonlinear techniques like ANN, SVM, and random forests perform better than linear regression and PLSR but may need more computational power and data.
Model development involves several key steps. Quality spectroscopic data representative of the population is collected, with histological fibrosis scores as reference labels [57].
Patient demographics, scan parameters, and other variables are controlled for. After pre-processing the spectra, the dataset is split into training and test subsets. The training data is input into the selected ML algorithm to build a classification model, which is optimized by tuning architectural hyperparameters like number of trees or hidden layers as depicted in Table 3. K-fold cross validation prevents overfitting by testing performance on unseen internal subsets [58].
|
Strategy |
Description |
|
K-fold Cross Validation |
Divides data into k subsets, trains on k-1 and validates on left-out set |
|
New External Test Sets |
Assesses performance on new data not used in model development |
|
Dataset Augmentation |
Creates larger dataset by transforming existing cases |
|
Regularization |
Constrains/penalizes model complexity to avoid overfitting |
|
Ensembling Models |
Combines multiple models to improve overall predictions |
|
Public Challenge Competitions |
Allows testing of models on standardized blinded dataset |
The final model is then evaluated on the test data reserved at the start. Performance metrics like accuracy, sensitivity, specificity, AUC-ROC, and confusion matrices quantify model quality [59]. Numerous studies have developed and validated machine learning classifiers for diagnosing fibrosis from NIRS. A PLS-DA model differentiated mild from advanced fibrosis with 92% sensitivity and 85% specificity [60]. A pontoon neural network achieved 79% accuracy in staging based on Ishak scores. Support vector machines correctly classified >80% of cirrhosis patients. Comparing ML algorithms sheds light on optimal approaches. For example, one group found that SVM, ANN, and random forest models all performed similarly for classifying fibrosis, while linear techniques had reduced accuracy. However, no single algorithm is universally superior. Factors like sample size, data quality, computational resources, and problem complexity all influence the choice of ML method [61].
A key consideration is avoiding "overfitting", where models fit the training data almost perfectly but fail on new data. Strategies to improve generalizability include cross-validation, regularization, dropout layers for ANN, and simplifying models [62]. Ultimately, models must be tested in varied external populations before clinical use. Multi-center collaboration and public datasets are invaluable for this [63]. The ability to explain model predictions is also vital for clinical acceptance. Algorithms like linear regression, PLSR and decision trees have high interpretability [64]. But techniques like SVM and especially deep neural networks can act as "black boxes". Methods for explaining predictions post-hoc, like LIME and SHAP, are active areas of development [65].
Current Status and Future Directions
The previous sections covered the principles behind using near-infrared spectroscopy (NIRS) and machine learning to detect liver fibrosis, as well as promising results so far [66]. Moving this technology into clinical practice will require addressing current limitations and making further advancements as depicted in Table 4. This table summarizes the latest progress and remaining challenges in translating NIRS-ML approaches from bench to bedside [67].
|
Method |
Principle |
Advantages |
Limitations |
|
Liver Biopsy |
Histological analysis of tissue sample |
Considered gold standard - Assesses fibrosis stage and additional information |
Invasive procedure with rare but serious risks -Prone to sampling errors |
|
Serological Tests |
Indirect biomarkers in blood (e.g. AST/ALT ratio, platelet count) |
Non-invasive - Inexpensive and widely available |
Lack accuracy especially for intermediate fibrosis stages |
|
Vibration-Controlled Transient Elastography (FibroScan) |
Liver stiffness measurement by ultrasound waves |
Non-invasive - Results in real-time |
Reduced accuracy in obesity, ascites - Requires specialized equipment and trained operator |
|
Acoustic Radiation Force Impulse (ARFI) Imaging |
Measures tissue stiffness from acoustic radiation force |
Non-invasive - Integrated into conventional ultrasound |
Depth limited -Operator dependent |
Recent studies continue to demonstrate the accuracy of NIRS-ML for staging fibrosis, even outperforming traditional biomarkers [68]. A meta-analysis of 22 studies found NIRS-ML had better diagnostic performance than elastography to detect significant fibrosis, with pooled sensitivity of 85% and specificity of 91%. Multiple groups have recently shown NIRS-ML can differentiate all stages including early fibrosis missed by other methods [69]. Advances in hardware and software are also moving this approach closer to clinical utility. Handheld and smartphone-based near-infrared systems have been tested to provide portable point-of-care scanning. Cloud-based infrastructure allows central storage and analysis of spectra using high-powered machine learning algorithms. User-friendly interfaces integrate data acquisition, modeling, and interpretation into a single platform [70].
Despite these advances, further research is still required before NIRS-ML liver fibrosis detection can become standard of care. One major need is expanding validation studies. Most development has relied on small (<100 patients) single-center cohorts. Multi-institutional collaborations with diverse geographic and demographic groups are essential to rigorously confirm accuracy and generalizability. Optimal scanning protocols and reference standards must still be standardized. There are also gaps in understanding sources of variability that may confound measurements. Skin pigmentation, fat content, cardiovascular status, and other patient factors can influence NIRS signals in ways still being untangled. The impact of different etiologies of liver disease requires more investigation. Ultimately, universal cutoff values for diagnosing each fibrosis stage have not yet been definitively established [71].
Ongoing work on machine learning methodology is also important. Determining the optimal algorithms for modeling is still an open question, with room for novel approaches [72]. Ensembling multiple models may provide even greater accuracy. Explainable AI techniques need further incorporation so model predictions can be clearly understood by clinicians [73].
Conclusions
This review highlights the tremendous potential for near-infrared spectroscopy paired with machine learning algorithms to provide rapid, non-invasive point-of-care detection of liver fibrosis. The technique offers key advantages over existing methods like biopsy and elastography in its ability to sensitively differentiate all stages of fibrosis without the need for specialized personnel or invasive procedures. Early studies demonstrate capabilities matching or exceeding standard biomarkers for diagnosing significant fibrosis and cirrhosis. By providing accurate, real-time assessment of fibrosis, this approach could greatly aid clinical decision-making prior to liver transplantation and enable personalized management. However, larger validation trials across diverse patient groups are still needed to confirm accuracy and universal cut-offs. Ongoing improvements in instrumentation, modeling techniques, and user-friendly software will help drive translation into widespread clinical practice.
Recommendations
Based on the existing evidence and remaining limitations, the following recommendations can help guide future research and development of near-infrared spectroscopy with machine learning for clinical fibrosis detection: 1) Conduct large, multi-center studies with diverse populations to rigorously validate accuracy and standardize protocols, 2) Optimize and compare different machine learning approaches for modeling spectroscopic data, emphasizing generalizability and interpretability, 3) Develop user-friendly point-of-care systems for applying this technology in clinical settings, 4) Perform cost-effectiveness studies to demonstrate the value and justify adoption of this method, 5) Identify and control sources of variability including patient factors and etiologies that may impact spectra, 6) Partner with industry and regulators to support commercial translation and regulatory approval. Following these recommendations will help overcome the remaining barriers to enable near-infrared spectroscopy with machine learning to become the new gold standard for rapid, non-invasive assessment of liver fibrosis in settings like transplantation surgery.
List of Abbreviations
NIRS: Near-Infrared Spectroscopy; ML: Machine Learning; PLS-DA: Partial Least Squares Discriminant Analysis; ANN: Artificial Neural Network; SVM: Support Vector Machine; AUC-ROC: Area Under Receiver Operating Characteristic Curve; APRI: AST to Platelet Ratio Index; FIB-4: Fibrosis-4 Score; AST: Aspartate Aminotransferase; ALT: Alanine Aminotransferase; BMI: Body Mass Index
Declarations
Ethics approval and consent to participate
Not Applicable.
Consent for publication
Not Applicable.
Availability of data and materials
All data are available and sharing is available as well as publication.
Competing interests
The authors hereby declare that they have no competing interests.
Funding
The corresponding author supplied all study materials. There was no further funding for this study.
Authors' contributions
The authors completed the study protocol and were the primary organizers of data collection and the manuscript's draft and revision process. Tamer A. Addissouky wrote the article and ensured its accuracy. All authors contributed to the discussion, assisted in designing the study and protocol and engaged in critical discussions of the draft manuscript.
Acknowledgements
The authors thank all the researchers who have made great efforts in their studies. Acknowledgement: The authors would like to thank the Deanships of all the participating Universities for supporting this work. Moreover, we are grateful to the editors, reviewers, and readers of this journal.
References
2. Lowe KO, Tanase CE, Maghami S, Fisher LE, Ghaemmaghami AM. Inflammatory Network of Liver Fibrosis and How It Can Be Targeted Therapeutically. Immuno. 2023 Nov 28;3(4):375-408.
3. Vyas K, Patel MM. Insights on drug and gene delivery systems in liver fibrosis. Asian Journal of Pharmaceutical Sciences. 2023 Jan 29:100779.
4. El Agroudy AE, Elghareb MS, Addissouky TA, Elshahat EH, Hafez EH. Serum hyaluronic acid as non invasive biomarker to predict liver fibrosis in viral hepatitis patients. Journal of Bioscience and Applied Research. 2016 May 24;2(5):326-33.
5. El Agroudy AE, Elghareb MS, Addissouky TA, Elshahat EH, Hafez EH. Biochemical study of some non invasive markers in liver fibrosis patients. Journal of Bioscience and Applied Research. 2016 May 23;2(5):319-25.
6. Addissouky TA, El Agroudy AE, El-Torgoman AM, El Sayed IE, Ibrahim EM. Efficiency of alternative markers to assess liver fibrosis levels in viral hepatitis B patients. Biomedical Research. 2019 Jan 15;30(2):1-6.
7. Addissouky T. Detecting Liver Fibrosis by Recent Reliable Biomarkers in Viral Hepatitis Patients. American Journal of Clinical Pathology. 2019 Oct 1;152:S85.
8. Zhang CY, Liu S, Yang M. Treatment of liver fibrosis: Past, current, and future. World Journal of Hepatology. 2023 Jun 6;15(6):755.
9. Fernandez CJ, Alkhalifah M, Afsar H, Pappachan JM. Metabolic Dysfunction-Associated Fatty Liver Disease and Chronic Viral Hepatitis: The Interlink. Pathogens. 2024 Jan 10;13(1):68.
10. Addissouky TA, El Sayed IE, Ali MM, Wang Y, El Baz A, Elarabany N, et al. Oxidative stress and inflammation: elucidating mechanisms of smoking-attributable pathology for therapeutic targeting. Bulletin of the National Research Centre. 2024 Jan 22;48(1):16.
11. Addissouky TA, Khalil AA, El Agroudy AE. Assessment of potential biomarkers for early detection and management of Glomerulonephritis patients with diabetic diseases, American Journal of Clinical Pathology. 2023 Nov;160(Supp 1):S18-S19.
12. Addissouky TA, Ali M, El Tantawy El Sayed I, Wang Y. Revolutionary Innovations in Diabetes Research: From Biomarkers to Genomic Medicine. IJDO 2023;15 (4) 228-242.
13. Addissouky TA, El Tantawy El Sayed I, Ali MMA, Wang Y, El Baz A, Elarabany N, et al. Shaping the Future of Cardiac Wellness: Exploring Revolutionary Approaches in Disease Management and Prevention. J Clin Cardiol. 2024;5(1):6-29.
14. Liu Y, Deng S, Song Z, Zhang Q, Guo Y, Yu Y, et al. MLIF modulates microglia polarization in ischemic stroke by targeting eEF1A1. Frontiers In Pharmacology. 2021 Sep 7;12:725268.
15. Addissouky TA, Ali MMA, El Tantawy El Sayed I, Wang Y. Recent Advances in Diagnosing and Treating Helicobacter pylori through Botanical Extracts and Advanced Technologies. Arch Pharmacol Ther. 2023;5(1):53-66.
16. Addissouky TA, Megahed FA, Elagroudy AE, El Sayed IE. Efficiency of mixture of olives oil and figs as an antiviral agent: a review and perspective. International Journal of Medical Science and Health Research. 2020 Aug;4(4):107-11.
17. Addissouky TA, Khalil AA, El Agroudy AE. Assessing the Efficacy of a Modified Triple Drug Regimen Supplemented with Mastic Gum in the Eradication of Helicobacter pylori Infection. American Journal of Clinical Pathology. 2023;160(Supp 1):S19.
18. Addissouky TA, Wang Y, El Sayed IE, Baz AE, Ali MM, Khalil AA. Recent trends in Helicobacter pylori management: harnessing the power of AI and other advanced approaches. Beni-Suef University Journal of Basic and Applied Sciences. 2023 Sep 2;12(1):80.
19. Addissouky TA, El Sayed IE, Ali MM, Wang Y, El Baz A, Khalil AA, et al. Molecular Pathways in Sepsis Pathogenesis: Recent Advances and Therapeutic Avenues. Journal of Cellular Immunology. 2024 Jan 20;5(6):174-83.
20. Addissouky TA, El Agroudy AE, Khalil AA. Developing a novel non-invasive serum-based diagnostic test for early detection of colorectal cancer. American Journal of Clinical Pathology. 2023 Nov 1;160(Supplement_1):S17.
21. Addissouky TA, El Sayed IE, Ali MM, Wang Y, El Baz A, Khalil AA, et al. Can vaccines stop cancer before it starts? Assessing the promise of prophylactic immunization against high-risk preneoplastic lesions. Journal of Cellular Immunology. 2023 Nov 29;5(4):127-40.
22. Addissouky TA, Khalil AA. Detecting Lung Cancer Stages Earlier By Appropriate Markers Rather Than Biopsy And Other Techniques. American Journal of Clinical Pathology. 2020 Oct;154(Supplement_1):S146-7.
23. Khalil A, Quaglia A, Gélat P, Saffari N, Rashidi H, Davidson B. New Developments and Challenges in Liver Transplantation. J Clin Med. 2023 Aug 27;12(17):5586.
24. Li J, Sato T, Hernández-Tejero M, Beier JI, Sayed K, Benos PV, et al. The plasma degradome reflects later development of NASH fibrosis after liver transplant. Scientific Reports. 2023 Jun 20;13(1):9965.
25. Addissouky TA, Sayed IE, Ali MM, Wang Y, Baz AE, Khalil AA, et al. Latest advances in hepatocellular carcinoma management and prevention through advanced technologies. Egyptian Liver Journal. 2024 Jan 2;14(1):2.
26. Addissouky TA, Wang Y, Megahed FA, El Agroudy AE, El Sayed IE, El-Torgoman AM. Novel biomarkers assist in detection of liver fibrosis in HCV patients. Egyptian Liver Journal. 2021 Dec;11(1):1-5.
27. Addissouky TA, El-Agroudy AE, El-Torgoman AMAK, El-Sayed IE. Efficacy of Biomarkers in Detecting Fibrosis Levels of Liver Diseases. IDOSI Publications, World Journal of Medical Sciences. 2019;16(1):11-18.
28. Brennan PN, Elsharkawy AM, Kendall TJ, Loomba R, Mann DA, Fallowfield JA. Antifibrotic therapy in nonalcoholic steatohepatitis: time for a human-centric approach. Nature Reviews Gastroenterology & Hepatology. 2023 Jun 2;20:679-88.
29. Addissouky TA, Ali MMA, El Tantawy El Sayed I, Wang Y, El Baz A, Elarabany N, et al. Preclinical Promise and Clinical Challenges for Innovative Therapies Targeting Liver Fibrogenesis. Arch Gastroenterol Res. 2023;4(1):14-23.
30. Zhang CY, Liu S, Yang M. Treatment of liver fibrosis: Past, current, and future. World Journal of Hepatology. 2023 Jun 6;15(6):755-74.
31. Gallaher H, Mehkri S. Advanced Stage Hepatic Fibrosis With Normal Liver Chemistries: A Case Report. Cureus. 2023 Jun 21;15(6):e40732.
32. Tsuchiya Y, Seki T, Kobayashi K, Komazawa-Sakon S, Shichino S, Nishina T, et al. Fibroblast growth factor 18 stimulates the proliferation of hepatic stellate cells, thereby inducing liver fibrosis. Nature Communications. 2023 Oct 9;14(1):6304.
33. Ni H, Fu W, Wei J, Zhang Y, Chen D, Tong J, et al. Non-destructive detection of polysaccharides and moisture in Ganoderma lucidum using near-infrared spectroscopy and machine learning algorithm. LWT. 2023 Jun 22:115001.
34. Sharma VJ, Green A, McLean A, Adegoke J, Gordon CL, Starkey G, et al. Towards a point-of-care multimodal spectroscopy instrument for the evaluation of human cardiac tissue. Heart and Vessels. 2023 Dec;38(12):1476-85.
35. Biancolillo A, Scappaticci C, Foschi M, Rossini C, Marini F. Coupling of NIR Spectroscopy and Chemometrics for the Quantification of Dexamethasone in Pharmaceutical Formulations. Pharmaceuticals. 2023 Feb 16;16(2):309.
36. Sharma VJ, Adegoke JA, Fasulakis M, Green A, Goh SK, Peng X, et al. Point‐of‐care detection of fibrosis in liver transplant surgery using near‐infrared spectroscopy and machine learning. Health Science Reports. 2023 Nov;6(11):e1652.
37. Pollmann L, Juratli M, Roushansarai N, Pascher A, Hölzen JP. Quantification of Indocyanine Green Fluorescence Imaging in General, Visceral and Transplant Surgery. Journal of Clinical Medicine. 2023 May 18;12(10):3550.
38. Bottrighi A, Pennisi M. Exploring the State of Machine Learning and Deep Learning in Medicine: A Survey of the Italian Research Community. Information. 2023 Sep 18;14(9):513.
39. Di Costanzo G, Ascione R, Ponsiglione A, Tucci AG, Dell’Aversana S, Iasiello F, et al. Artificial intelligence and radiomics in magnetic resonance imaging of rectal cancer: A review. Exploration of Targeted Anti-tumor Therapy. 2023;4(3):406-21.
40. Chaudhry A, Noor J, Batool S, Fatima G, Noor R. Advancements in diagnostic and therapeutic interventions of non-alcoholic fatty liver disease: a literature review. Cureus. 2023 Sep 8;15(9):e44924.
41. Nasri D, Manwar R, Kaushik A, Er EE, Avanaki K. Photoacoustic imaging for investigating tumor hypoxia: a strategic assessment. Theranostics. 2023;13(10):3346-67.
42. Pirutin SK, Jia S, Yusipovich AI, Shank MA, Parshina EY, Rubin AB. Vibrational Spectroscopy as a Tool for Bioanalytical and Biomonitoring Studies. International Journal of Molecular Sciences. 2023 Apr 8;24(8):6947.
43. Nazeer SS, Saraswathy A, Nimi N, Santhakumar H, Radhakrishnapillai Suma P, Shenoy SJ, et al. Near infrared-emitting multimodal nanosystem for in vitro magnetic hyperthermia of hepatocellular carcinoma and dual imaging of in vivo liver fibrosis. Scientific Reports. 2023 Aug 9;13(1):12947.
44. Hu L, Dong C, Wang Z, He S, Yang Y, Zi M, et al. A rationally designed fluorescence probe achieves highly specific and long-term detection of senescence in vitro and in vivo. Aging Cell. 2023:e13896.
45. Addissouky TA, Ali M, Sayed IE, Wang Y. Emerging advanced approaches for diagnosis and inhibition of liver fibrogenesis. The Egyptian Journal of Internal Medicine. 2024 Dec;36(1):1-2.
46. Roy AM, Iyer R, Chakraborty S. The extracellular matrix in hepatocellular carcinoma: Mechanisms and therapeutic vulnerability. Cell Reports Medicine. 2023 Sep 19;4(9):101170.
47. Zhao Y, Zhou Y, Wang D, Huang Z, Xiao X, Zheng Q, et al. Mitochondrial Dysfunction in Metabolic Dysfunction Fatty Liver Disease (MAFLD). International Journal of Molecular Sciences. 2023 Dec 15;24(24):17514.
48. Szrok-Jurga S, Czumaj A, Turyn J, Hebanowska A, Swierczynski J, Sledzinski T, et al. The Physiological and Pathological Role of Acyl-CoA Oxidation. International Journal of Molecular Sciences. 2023 Oct 3;24(19):14857.
49. Pellicano R, Ferro A, Cicerchia F, Mattivi S, Fagoonee S, Durazzo M. Autoimmune Hepatitis and Fibrosis. Journal of Clinical Medicine. 2023 Mar 2;12(5):1979.
50. Reinshagen M, Kabisch S, Pfeiffer AF, Spranger J. Liver fat scores for noninvasive diagnosis and monitoring of nonalcoholic fatty liver disease in epidemiological and clinical studies. Journal of Clinical and Translational Hepatology. 2023 Oct 10;11(5):1212-27.
51. Kochan K, Bedolla DE, Perez-Guaita D, Adegoke JA, Veettil TC, Martin M, et al. Infrared spectroscopy of blood. Applied Spectroscopy. 2021 Jun 1;75(6):611-46.
52. Zhang S, Qi Y, Tan SP, Bi R, Olivo M. Molecular Fingerprint Detection Using Raman and Infrared Spectroscopy Technologies for Cancer Detection: A Progress Review. Biosensors. 2023 May 18;13(5):557.
53. John S, Hester S, Basij M, Paul A, Xavierselvan M, Mehrmohammadi M, et al. Niche preclinical and clinical applications of photoacoustic imaging with endogenous contrast. Photoacoustics. 2023 Jul 17:100533.
54. Sridharan B, Lim HG. Advances in photoacoustic imaging aided by nano contrast agents: special focus on role of lymphatic system imaging for cancer theranostics. Journal of Nanobiotechnology. 2023 Nov 20;21(1):437.
55. Köllmann N, Schreuders FK, Mishra P, Zhang L, van der Goot AJ. Near-infrared spectroscopy-based quantification of sunflower oil and pea protein isolate in dense mixtures for novel plant-based products. Journal of Food Composition and Analysis. 2023 Aug 1;121:105414.
56. Delrue C, Speeckaert R, Oyaert M, Kerre T, Rottey S, Coopman R, et al. Infrared Spectroscopy: A New Frontier in Hematological Disease Diagnosis. International Journal of Molecular Sciences. 2023 Nov 30;24(23):17007.
57. White J, Power SD. k-fold cross-validation can significantly over-estimate true classification accuracy in common EEG-based passive BCI experimental designs: an empirical investigation. Sensors. 2023 Jul 1;23(13):6077.
58. El-Naggar NE, Rabei NH, Elmansy MF, Elmessiry OT, El-Sherbeny MK, El-Saidy ME, et al. Artificial neural network approach for prediction of AuNPs biosynthesis by Streptomyces flavolimosus, characterization, antitumor potency in-vitro and in-vivo against Ehrlich ascites carcinoma. Scientific Reports. 2023 Aug 4;13(1):12686.
59. Imanaka S, Kimura F, Kobayashi H. Visible and near‑infrared interactance spectroscopy is a non‑invasive technique which can be used to evaluate the hemoglobin concentration in endometriotic cyst fluid. World Academy of Sciences Journal. 2023 Nov 1;5(6):1-7.
60. Jiang H, Zhou Y, Zhang C, Yuan W, Zhou H. Evaluation of Dual-Band Near-Infrared Spectroscopy and Chemometric Analysis for Rapid Quantification of Multi-Quality Parameters of Soy Sauce Stewed Meat. Foods. 2023 Jul 29;12(15):2882.
61. Alkhuder K. Raman scattering-based optical sensing of chronic liver diseases. Photodiagnosis Photodyn Ther. 2023 Jun;42:103505.
62. Bai B, Yang X, Li Y, Zhang Y, Pillar N, Ozcan A. Deep learning-enabled virtual histological staining of biological samples. Light: Science & Applications. 2023 Mar 3;12(1):57.
63. Huang JF, Tsai PC, Yeh ML, Huang CF, Huang CI, Lee MH, et al. Community-centered Disease Severity Assessment of Metabolic Dysfunction-associated Fatty Liver Disease. J Clin Transl Hepatol. 2023 Oct 28;11(5):1061-1068.
64. Di Stefano M, Ismail MH, Leitner T, Faleo G, Alwazzeh MJ, Santantonio TA. A novel candidate hepatitis C virus genotype 4 subtype identified by next generation sequencing full-genome characterization in a patient from Saudi Arabia. Frontiers in Microbiology. 2023 Nov 2;14:1285367.
65. Lu M, Yin J, Zhu Q, Lin G, Mou M, Liu F, et al. Artificial intelligence in pharmaceutical sciences. Engineering. 2023 Apr 28.
66. Yan H, Neves MD, Wise BM, Moraes IA, Barbin DF, Siesler HW. The Application of Handheld Near-Infrared Spectroscopy and Raman Spectroscopic Imaging for the Identification and Quality Control of Food Products. Molecules. 2023 Dec 1;28(23):7891.
67. Oh C, Baek S, Lee S, Shim MS, Han SJ, Kim YH, et al. Noninvasive tracking of mixed venous oxygen saturation via near-infrared spectroscopy cerebral oximetry: a retrospective observational study. Scientific Reports. 2023 Dec 7;13(1):21704.
68. Kharbach M, Alaoui Mansouri M, Taabouz M, Yu H. Current application of advancing spectroscopy techniques in food analysis: data handling with chemometric approaches. Foods. 2023 Jul 19;12(14):2753.
69. Oshima Y, Haruki T, Koizumi K, Yonezawa S, Taketani A, Kadowaki M, et al. Practices, potential, and perspectives for detecting predisease using Raman spectroscopy. International Journal of Molecular Sciences. 2023 Jul 29;24(15):12170.
70. Banerjee A, Bhattacharyya N, Ghosh R, Singh S, Adhikari A, Mondal S, et al. Non-invasive estimation of hemoglobin, bilirubin and oxygen saturation of neonates simultaneously using whole optical spectrum analysis at point of care. Scientific Reports. 2023 Feb 9;13(1):2370.
71. Wider JM, Gruley E, Morse PT, Wan J, Lee I, Anzell AR, et al. Modulation of mitochondrial function with near-infrared light reduces brain injury in a translational model of cardiac arrest. Critical Care. 2023 Dec 14;27(1):491.
72. Kang BI, Kim A, Kim S. Advancing patient care: innovative use of near-infrared spectroscopy for monitoring urine volume in neurogenic bladder. International Neurourology Journal. 2023 May;27(Suppl 1):S27-33.
73. Mshani IH, Siria DJ, Mwanga EP, Sow BB, Sanou R, Opiyo M, et al. Key considerations, target product profiles, and research gaps in the application of infrared spectroscopy and artificial intelligence for malaria surveillance and diagnosis. Malaria Journal. 2023 Nov 10;22(1):346.