Abstract
Embryonal rhabdomyosarcoma is a soft tissue sarcoma whose resistance to chemotherapies is associated with defective apoptosis or/and cell cycle. Given that we have reported that the liposoluble extract from the leaves of Nicotiana glauca induces apoptosis in embryonal rhabdomyosarcoma cells, this research aims to know the molecular mechanism involved, to elucidate the pharmacological potential of the Nicotiana glauca.
The liposoluble extract from Nicotiana glauca induces apoptosis in rhabdomyosarcoma cells by upregulation PERP gene expression. In addition, the extract triggers a cellular defense response involving Akt phosphorylation, increasing gene expression of antioxidant enzymes and possibly increasing mRNA levels of the mitochondrial protein Opa 1, which results in mitochondrial performance improvement.
The results suggest that the liposoluble extract from Nicotiana glauca triggers defense mechanisms involving Akt activation and antioxidant enzymes in rhabdomyosarcoma cells. However, this response is not sufficient, since the cells die by apoptosis after treatment. These findings highlight the pharmacological potential of Nicotiana glauca by revealing different molecular targets.
Keywords
Rhabdomyosarcoma, Nicotiana glauca, apoptosis, PERP, Akt, Gpx 1, Opa 1
Introduction
Rhabdomyosarcoma is the most common soft tissue sarcoma in childhood [1]. Soft tissue sarcomas refer to a group of cancers that arise in connective and supporting tissues such as muscles, nerves and blood vessels [2]. Most of these tumors are located in the head and neck region, genitourinary tract, and extremities [3]. Childhood rhabdomyosarcoma accounts for approximately 3.5% of cancer cases in children aged 0-14 years, and 2% of cases among adolescents and young adults aged 15-19 years. The incidence of this malignancy is 4.5 per million children [4].
Rhabdomyosarcoma could arise from an alteration in the regulation of the growth and proliferation of skeletal muscle precursor cells [5]. Embryonal rhabdomyosarcoma, the tumor studied here, is the most common subtype [6]. Embryonal rhabdomyosarcoma presents resistance to chemotherapeutic drugs, which may be caused by alterations in the ability to detect or repair DNA damage, or by defects in the induction of growth arrest in the cell cycle or defects in apoptosis [7].
Apoptosis occurs during development and aging and as a homeostatic mechanism to maintain cell populations in the organism. It also acts as a defense mechanism in immune responses or when cells are damaged by a harmful agent or disease [8]. PERP (p53 apoptosis effector related to PMP-22) is a transcriptional target of the p53 protein whose transcription is induced during apoptosis and not during cell cycle arrest [9]. The negative regulation of this protein is related to the development of different types of cancer [10,11]. Here we investigate the possible role of PERP in embryonal rhabdomyosarcoma origin and development.
Since ancient times, plants products have been used for the treatment of diseases [12,13]. In agreement, investigations have shown that plant extracts can be used in numerous cancer treatments. Specifically, the use of plant extracts has shown effectiveness by abrogating cancer initiation, development, and progression [14,15]. The use of these natural compounds instead of synthetic chemotherapeutic drugs is an alternative since they are environmentally sustainable and lack adverse side effects [16].
The Solanaceae Nicotiana glauca (N.g.) has been used to treat swellings, bruises, cuts, boils, inflamed throats, swollen glands, and jaundice [17]. Components obtained from the leaves of the plant N.g., exert an apoptotic effect on embryonal rhabdomyosarcoma cells [16]. However, the full molecular mechanism involved is not known.
Normally, cells respond to stressful and damaging effects by activating survival signaling pathways as a rapid response to reverse this situation. However, if stressful conditions persist, such a survival response cannot be sustained, and the cells eventually die [18].
The PI3K/Akt/mTOR (phosphatidylinositol 3-kinase/ protein kinase B/ mammalian Target of Rapamycin) pathway is a potent mediator of cell survival signals. Constitutive activation of PI3K/Akt-mediated signaling is observed in a variety of human cancers including rhabdomyosarcoma and is found to play a central role in the tumor formation and progression [19]. The phosphorylated expression of Akt protein varies as a cellular response to apoptotic agents such as H2O2. An increase in Akt phosphorylation/activation has been seen when different malignant cell lines are exposed to H2O2, it confers protection against apoptosis induced by oxidative stress [20].
In addition to the basal activation of the Akt pathway, rhabdomyosarcoma has other mechanisms that favor its survival and progression. Overexpression of anti-aging genes such as Sirtuin 1 (Sirt 1) has been observed in soft tissue sarcoma cell lines, including RD, and its expression is crucial in the survival of sarcoma cells [21]. In relation to this work, a relation between Sirtuins and Akt signaling [22] has been demonstrated. Because of this, it is necessary to find alternatives to stop the development and progression of rhabdomyosarcoma, probably involving both signaling systems.
We observed morphological changes in the embryonal rhabdomyosarcoma cell line during the apoptosis process as a consequence of treatment with extracts from N.g. [16]. It is known that, during apoptosis, mitochondria undergo major structural changes including fragmentation and remodeling of their cristae [23], which fuse together and their junctions widen allowing the release of cytochrome c. Mitochondrial morphological changes are normally controlled by a precise balance of fusion and fission, regulated by a family of mitochondrial proteins such as Mitofusin 1 and 2 (Mfn 1 and Mfn 2) and optic atrophy protein 1 (Opa 1) [24]. Furthermore, dysfunction of Opa 1 has been linked to the overproduction of reactive oxygen species and imbalanced redox homeostasis [25,26]. In view of the above lines of evidence, here we investigate the possible role of Opa 1 in the apoptosis of rhabdomyosarcoma cells.
Based on our previous experience with the C2C12 cell line and N.g. extracts and their subfractions, the intention of the present work is to determine if the N.g. extracts exert any effect on RD cells. As a first analysis, we investigate the effects of the liposoluble extract from N.g. on rhabdomyosarcoma cells in relation to apoptosis; going deeper on the molecular mechanism involved. Focusing on protein and gene expression, as well as defense mechanisms against the apoptosis induced by N.g. extracts. In addition, possible alterations in the rhabdomyosarcoma cell cycle caused by the Solanaceae were evaluated. This information will allow us to know the pharmacological potential from N.g. extracts for future therapeutic strategies against rhabdomyosarcoma.
Materials and Methods
Materials
Anti phospho-Akt (Ser 473) rabbit polyclonal antibody was purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA), Anti GAPDH (G9) mouse monoclonal antibody was purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA).
Alexa Fluor 488-conjugated anti-mouse secondary antibody was purchased from Thermo Fisher Scientific (Rockford, IL, USA). High Pure RNA Isolation kit (11828665001) was from Roche Diagnostics (Mannheim, Germany). High Capacity cDNA Reverse Transcription Kit (4368814) and SYBR® Select Master Mix were purchased from Applied Biosystems and primer sets were from Invitrogen (Carlsbad, CA, USA). All other reagents used were of analytical grade.
Cell culture
RD cell line (ATCC-CCL-136), American Type Culture Collection. Primary embryonal rhabdomyosarcoma cell line from a seven-year-old female individual. RD were cultured in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin at 37°C with 5% CO2. Cultures are maintained on fresh medium, replicated at 80% confluence or, if replacing is not necessary, supplemented with fresh medium every 72hs.
Treatment
The treatments were performed with 70–80% confluent cultures in medium without serum for 20 min. Next, cells were exposed to the liposoluble extract from N. g. (1:1000 dilution in DMEM without serum) or were treated with the vehicle [0.001 % isopropanol (IPA, control)] at the times indicated.
Extraction of lipid extract from Nicotiana glauca
Nicotiana glauca plant specimens were collected from their natural habitat in Bahía Blanca, Buenos Aires, Argentina and were grown under greenhouse conditions. Plant specimens were identified by comparison with the Carlos Villamil herbarium specimen (N° 9348). It is found in the Herbarium of the Department of Biology, Biochemistry, and Pharmacy (BBB), Universidad Nacional del Sur, (8,000) Bahía Blanca, Argentina. Plant name was checked with the online website of The International Plant Name Index (IPNI), accessed in May 2019. An herbarium voucher specimen was kept at the Herbarium of the Department of Biology, Biochemistry and Pharmacy (BBB), Universidad Nacional del Sur.
The lipid extracts from N.g. leaves were obtained following essentially the method of Bligh and Dyer [27]. Briefly, the leaves (10 g) were washed with double distilled water and then frozen with liquid nitrogen to grind the tissue to a powder. Then, the powder was homogenized with chloroform-methanol (1:2, v/v; 3.6 ml/g). The samples were further homogenized twice for 30 seconds: the first for 30 seconds with chloroform and the second for the same time with the addition of double distilled water (1.2 ml/g of tissue in each case). The final homogenate was centrifuged for 20 min, at 10,000 rpm (4,300 x g) in a refrigerated Sorvall centrifuge with SS-34 rotor (Du Pont, Delawere, USA). The lipid-soluble lower phase was collected and evaporated under a constant stream of N2 at 35°C. For subsequent assays, this fraction was resuspended in isopropanol 2.5 mg/5 mL or stored at -70°C. This methodology is developed in our laboratory and is for routine use. Moreover, in our previous work we analyzed the composition of the N.g. extract and the effects of the compounds more relevant on mammalian cells, such C2C12 cell line [18,28].
Western blotting analysis
Cell cultures were scrapped and resuspended in lysis buffer (50 mM Tris–HCl, pH 7.4, 150 mM, NaCl, 0.2 mM Na2VO4, 2 mM EDTA, 25 mM NaF, 1 mM PMSF, 20 mg/mL leupeptin, and 20 mg/mL aprotinin). The protein content was quantified by the Bradford procedure [29]. Protein samples (25 μg) were mixed with sample buffer (400 mM Tris-HCl (pH 6.8), 10% SDS, 50% glycerol, 500 mM dithiothreitol (DTT), and 2 mg/ml Bromophenol Blue), boiled for 5 min and resolved by 10% SDS-PAGE according to the method of Laemmli [30]. Fractionated proteins were electrotransferred to polyvinylidene fluoride membranes (PVDF) (Immobilon-P; Millipore, Darmstadt, Germany), using a semi-dry system. Non-specific sites were blocked with 5% non-fat dry milk in PBS containing 0.1% Tween-20 (PBS–T). Blots were incubated overnight with the appropriate dilution of the primary antibodies. The membranes were repeatedly washed with PBS–T before incubation with horseradish peroxidase-conjugated secondary antibodies. The enhanced chemiluminescence (ECL) blot detection kit (Amersham, Buckinghamshire, England) was used as described by the manufacturer to visualize reactive products. Relative migration of unknown proteins was determined by comparison with molecular weight markers. When needed, membranes were stripped with stripping buffer (62.5 mM Tris–HCl (pH 6.7), 2% SDS, 50 mM b-mercaptoethanol), washed with PBS containing 1% Tween-20 and then blocked for 1 hour with 5% non-fat dry milk in PBS-T. The blots were then incubated with the corresponding primary antibody. After several washings with PBS-T, membranes were incubated with secondary antibodies. The corresponding immunoreactive bands were developed as before. Relative quantification of Western blot signals was performed using ImageJ software (NIH, USA) [31].
Quantitative real time RT-PCR (RT-qPCR)
After treatments, total RNA was extracted using the High Pure RNA Isolation kit (Roche Diagnostics, Mannheim, Germany) and approximately 2 μg of total RNA was reverse transcribed with the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems Inc., CA, USA) according to the manufacturer’s instructions. The RNA and cDNA purity has been checked by the measurement of 260/280 nm ratio. Quantitative measurement of real-time PCR was done using SYBR Select Master Mix under the standard conditions recommended by the manufacturer. Primer sets to amplify human cDNAs used in the analysis were as follows: glycer- aldehyde 3-phosphate dehydrogenase (GAPDH) set: forward 5’- TGC ACC ACC AAC TGC TTA GC -3’ , reverse 5’- GGC ATG GACTGT GGT CAT GAG -3’ ; p53 apoptosis effector related to PMP-22 (PERP) set: forward 5’- GTC CTC GCT GTG GTG GAA AT-3’, reverse 5’- TCT ACC CCA CGC GTA CTC C-3, ; manganese-dependent superoxide dismutase (SOD-2) set: forward 5’- TGG AGA ACC CAA AGG GGA GT-3’, reverse 5’- TGA GCC TTG GAC ACC AAC AG-3’; isoform 1 glutathione peroxidase (Gpx 1) set: forward 5’- AGT TTG GGC ATC AGG AGA ACG-3’, reverse 5’- AGC ATG AAG TTG GGC TCG AA-3’; catalase (Cat) set: forward 5’- GTT CAG TGA TCG GGG GAT TC-3’, reverse 5’- TGA TGC CCT GGT CAG TCT TA-3’, optic atrophy protein 1 (Opa 1) set: forward 5’- AGC CAG TCC AAG CAG GAT TC-3’, reverse 5’- TGC TTT CAG AGC TGT TCC CT-3’. The specificity of PCR products was confirmed by melting curve analysis. Relative quantification of gene expression was determined by the comparative CT method 2-ΔΔCt [32,33].
Cell cycle analysis
Cells were harvested after treatments and then centrifuged at 1000 rpm for 10 minutes. Cells were fixed by adding 70% ethanol into the cell pellets and incubated at -20°C for two hours minimum. The pellets were washed with 1X PBS to remove ethanol. Finally, after centrifuging the cells for 10 minutes at 1000 rpm, BD Pharmingen™ PI/RNase staining buffer was added, according to the instructions of the manufacturer, and cell cycle analysis by flow cytometry was performed.
Immunocytochemistry
After treatments, semi-confluent (60-70%) monolayers were washed with serum-free DMEM, and then fixed and permeabilized for 20 minutes at -20°C with methanol. After fixation, cells were rinsed three times with PBS. Non-specific sites were blocked for 60 minutes in PBS that contained 5% bovine serum albumin. Cells were incubated with appropriate primary antibodies (1:100 dilution) overnight at 4°C. The primary antibodies were recognized by fluorophore-conjugated secondary antibodies. Finally, the coverslips were analyzed by conventional fluorescence microscopy.
Statistical analysis
Data analysis was performed using standard statistical packages (InfoStat System, Córdoba, Argentina) [34]. Results are shown as means ± standard deviation (S.D.) of not less than three independent experiments. The data were considered statistically significant when p<0.05 or p<0.01.
Results
The liposoluble extract from N.g. upregulates PERP expression
Apoptosis is a type of cell death highly regulated at the genetic level [35]. In our previous works, we have shown that the extract from N.g. induces an increase in PUMA (p53 upregulated modulator of apoptosis) mRNA levels, a gene associated with apoptosis, in myoblast cells [18]. Since PUMA is upregulated by p53, here we investigated PERP expression, another target of p53 [9].
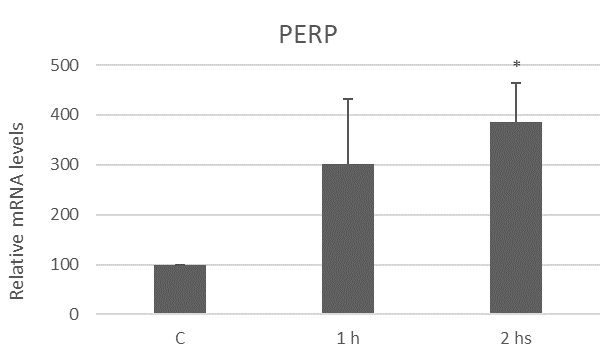
We studied the effect at the gene level of the liposoluble extract from N.g. in RD cells at different times. A significant increase in PERP mRNA levels with respect to the control after two hours of treatment (286,7 % ± 78.1), was observed (Figure 1).
Effects of liposoluble extract from N.g. in cell cycle of RD cells
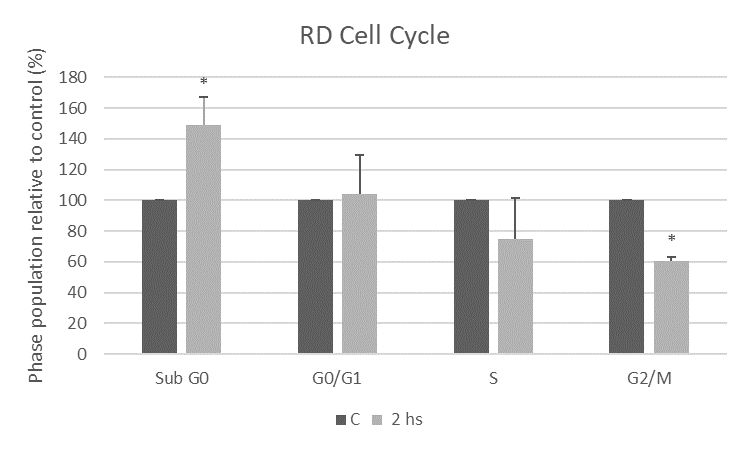
Propidium iodide is one of the dyes that can intercalate into DNA and thus produce a fluorescence signal proportional to the amount of DNA, detected by flow cytometry. Consequently, the measurement of fluorescence intensity per cell allows the discrimination of cells in the various phases of the cell cycle (G0 /G1, S, G /M) [36]. Apoptotic cells show a broad hypodiploid (Sub-G0) peak, which can be easily discriminated from the narrow peak of cells with normal DNA content (diploid) [37]. Our results showed that, after two hours of treatment with the liposoluble extract from N.g., the number of cells in the sub-G0 phase increased by 49.7 % ± 18.3, and the percentage of cell population in the mitotic G2/M phase decreased by 39.7% ± 2.9, with respect to the control in RD cells (Figure 2).
Liposoluble extract from N.g. induces Akt phosphorylation
As mentioned above, the PI3K/Akt/mTOR signaling pathway is a potent mediator of survival signals. This pathway has been suggested to be involved in the regulation of cancer cell proliferation and apoptosis [38,39].
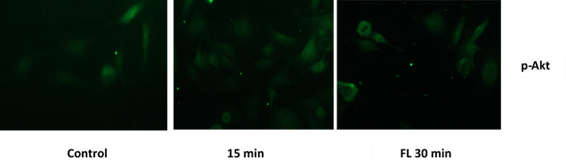
We first evaluated, by immunocytochemistry assay, the rapid phosphorylation/activation of Akt kinase in response to exposure to N.g. extracts for 15 and 30 minutes. Figure 3 shows a phosphorylation/activation of Akt in response to the treatment.
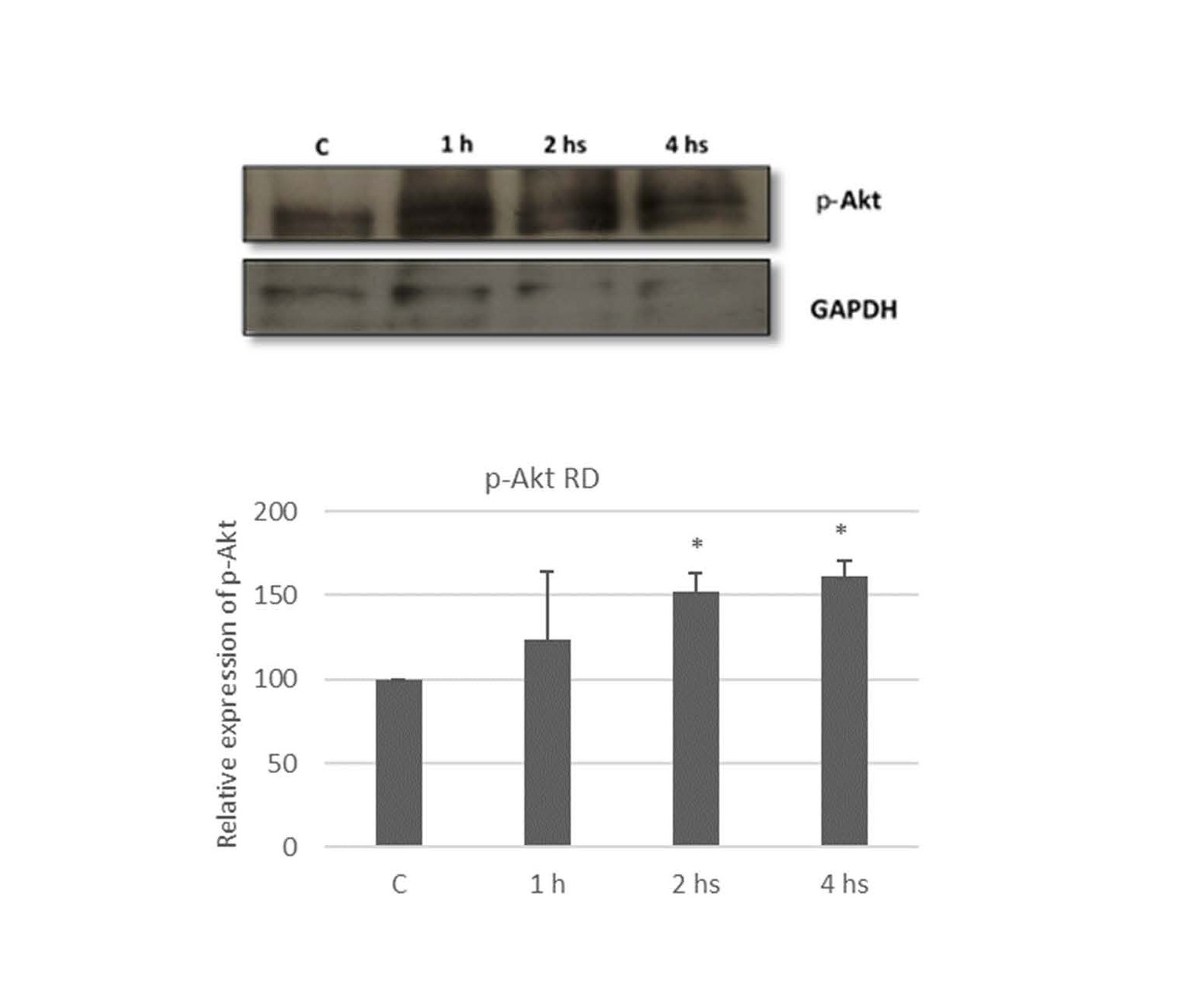
In order to confirm the immunocytochemistry, using western blotting we evaluated the phosphorylation of Akt when exposed the RD cells to liposoluble extract from N.g. for 1, 2, and 4 hours. The treatment induced a significant increase in Akt phosphorylation after 2 and 4 hours with respect to the control (52.13% ± 11.1 and 61% ± 9.9 respectively (Figure 4).
The liposoluble extract from N.g. modulates the gene expression of antioxidant enzymes in RD cells
In view of that, the liposoluble extract from N.g. induces apoptosis and that we have shown activation of Akt as a possible defense response to the cellular stress generated by the extract, now we evaluated the gene expression of antioxidant enzymes in response to the extract as another defense way of the tumoral cell.
The cell line under study was subjected to RT-qPCR assays, previously described, to evaluate possible changes in the levels of mRNA expression of antioxidant enzymes.
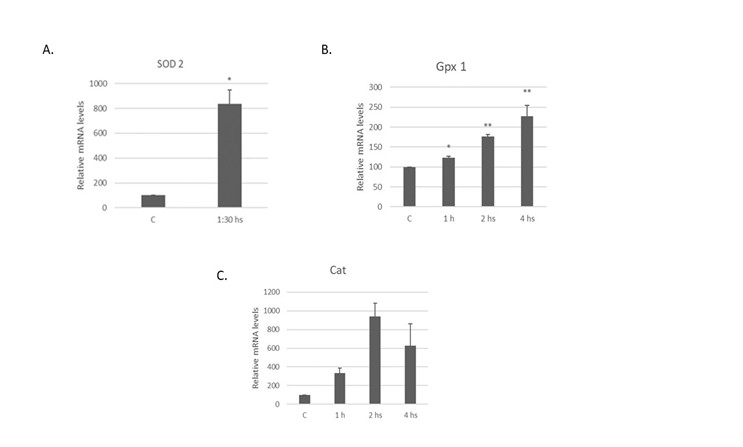
Manganese superoxide dismutase (Mn-SOD or SOD 2) is part of the three isoforms found in mammals. Superoxide dismutase catalyzes the conversion of the superoxide radical to hydrogen peroxide, preventing oxidative damage in the cell [40]. An increase with respect to the control in the expression level of SOD 2 mRNA was observed in RD cells in response to the liposoluble extract from N.g. The mRNA expression increased by 738.2% ± 109.4 after 1:30 hours of treatment (Figure 5A).
Catalase (Cat) and Glutathione peroxidase (Gpx) are also studied here. SOD (superoxide dismutase) is the first line of defense against these reactive oxygen species and hydrogen peroxide generated is further reduced to H2O by Cat or Gpx [41]. Gpx 1 is the isoform studied in this research work, which is found ubiquitously in the cytosol and mitochondria. Figure 5B shows a significant increase with respect to the control in Gpx 1 mRNA levels. The mRNA expression increased by 23.4% ± 3.6, 76.2% ± 5.3, and 127.8 % ± 26.3 after the RD cell line was treated as before with the extract from N.g. for 1, 2, and 4 hours respectively.
Figure 5C shows the gene expression levels of catalase. The behavior of this enzyme after treatments is similar to that of the other antioxidant enzymes studied here, tending to increase its expression.
The liposoluble extract from N.g. upregulates Opa 1 expression
As was described, in previous work we observed morphological changes, involving mitochondria, as a consequence of treatment with extracts from N.g. in the embryonal rhabdomyosarcoma cell line [16].
In mammalian cells, mitochondrial fusion requires the activity of three dynamin-related GTPases: Mfn1, Mfn2, and Opa 1 [42]. Opa 1 is a protein present in the mitochondrial intermembrane space, closely associated with the inner mitochondrial membrane [43].
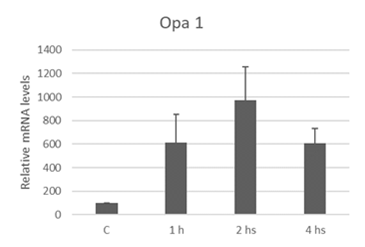
Opa 1 gene expression was determined by RT-qPCR in RD cells treated with the liposoluble extract from N.g. The preliminary results obtained showed a trend towards increased expression of the gene after 1, 2, and 4 hours of treatment (Figure 6).
Discussion
Cancer is considered as a major cause of death worldwide [44]. There are new antineoplastic therapies that increase survival rates, at the same time they are also associated with the appearance of unwanted effects [45]. The search for alternative therapeutic agents with better patient outcomes is necessary. The evidence shows that natural products from plants have been used in cancer treatments [14]. The use of N.g. in traditional medicine is already known [17]. The liposoluble extract from N.g. exerts an apoptotic effect on the RD cell line [16].
PERP is a target gene of the transcription factor p53, which acts in a signaling cascade regulating cellular responses to DNA damage by playing a tumor suppressor role by inducing cell cycle arrest or inducing cell death by apoptosis [9]. It is noted that down-regulation of PERP gene expression is associated with the development of several types of cancer and increased aggressiveness of these cancers [10,11,46]. There is evidence that PERP is a p53-dependent mediator of apoptosis in cells such as thymocytes, neurons, and uveal melanoma cells [47]. Studies in murine muscle cells showed an increase in PERP mRNA levels after induction of apoptosis with H2O2 [48]. Here, we demonstrated an increase in PERP gene expression, specifically after two hours of treatment with the extract from N.g., demonstrating the involvement of the p53-dependent apoptotic pathway in the effects observed.
To further evaluate the molecular mechanism involved in apoptotic action of the liposoluble extract from N.g., the RD cell cycle was analyzed by flow cytometry. Extracts from various medicinal plants can modulate the sub-G0/G1 and G2/M checkpoints of the cell cycle causing an antiproliferative cellular effect [49]. After two hours of treatment with the extract, we observed an increase in the percentage of cells in the sub-G0 phase, compatible with cells in the process of cell death, confirming the apoptotic effect of extract from N.g. We showed a significant decrease with respect to the control in the G2/M phase corresponding to actively dividing cells, under the treatment with the extract. Thus, the extract from N.g. alters the RD cell cycle by increasing the population undergoing cell death and causing a decrease in the proportion of cells that can divide during mitosis.
One of the features of rhabdomyosarcoma is its evasion of apoptosis. There is wide evidence that the PI3K/Akt/mTOR pathway is aberrantly activated in rhabdomyosarcoma and a variety of human cancers, playing a central role in tumor formation and progression [19]. When the C2C12 cell line, a non-tumor myogenic cell line, is exposed to an apoptosis-inducing agent such as H2O2, activation of mitogenic signaling pathways such as PI3K/Akt occurs during early exposure times to defend against the apoptotic stimulus. Eventually, this activation declines and C2C12 cells undergo apoptosis [48]. Our findings suggest a similar behavior in RD cells by demonstrating phosphorylation of the Akt after two hours of treatment with the extract from N.g. As mentioned, Akt is involved in survival events and the apoptosis regulation. As a consequence, different molecular targets related to cell survival are activated, and pro-apoptotic factors are inactivated [48]. The PI3K/Akt pathway has been reported to be involved in the phosphorylation of Nrf2 (nuclear factor erythroid-related factor 2), a transcription factor that promotes the transcription of several antioxidant genes and is activated in response to cellular attack and/or phosphorylation of protein kinases [50-52], thus promoting cell survival. It has been reported that Cat, SOD, and Gpx 1 enzymes are regulated by Nrf-2 [53-55]. Under conditions of cellular oxidative stress, this transcription factor is directed towards the nucleus where it initiates the transcription of antioxidant genes, an action facilitated by the phosphorylation of Nrf-2 by several kinases sensitive to oxidative species. The results presented here suggest the involvement of Akt in the regulation of antioxidant enzymes in rhabdomyosarcoma cells. Akt is phosphorylated and activated after two hours of exposure to the apoptotic stimulus, directing the gene transcription of Gpx-1, SOD 2 and Cat probably through the phosphorylation of the nuclear transcription factor Nrf-2. Further studies are necessary to confirm this mechanism.
Opa 1 is a protein found in the intermembrane space closely associated with the inner mitochondrial membrane [43] and is involved in the mitochondrial fusion process. Scientific studies demonstrate that negative regulation of this protein causes mitochondrial fragmentation and alters the shape of the cristae [56]. Also, Frezza et al. showed that expression of Opa 1 in murine embryonic fibroblasts protects these cells from death induced by apoptotic stimuli, delaying the release of cytochrome C, without the participation of mitochondrial mitofusins [57]. We demonstrated an increase in Opa 1 mRNA levels after treatment with the extract from N.g. It is suggested that embryonal rhabdomyosarcoma cells attempt to defend themselves against the apoptotic stimulus by elevating Opa 1 levels to maintain mitochondrial integrity.
Conclusions
The data presented here reveal part of the molecular mechanism, inducing apoptosis, triggered by the extract from N.g. in RD cells. The results show that, as early response, RD cells attempt to defend against the N.g. effects, by activating the PI3K/Akt signaling pathway and increasing gene expression of antioxidant enzymes and, possibly, mitochondrial protein Opa 1. However, these events are not sufficient to rescue embryonal rhabdomyosarcoma cells from cell death, since we observed an increase in the levels of mRNA of the proapoptotic protein PERP, as well as an increase in the percentage of cells in the RD cell cycle with low DNA content corresponding to cells in apoptosis, after treatments.
The information provided in this research yields possible molecular targets as a therapeutic strategy and highlights the pharmacological potential of N.g. Further studies are needed to fully elucidate the mechanism of action by which N.g. produces apoptosis in RD cells. We will aim to deep in these studies by searching for new molecular targets, for example, the participation of the anti-aging gene Sirt 1. This belongs to a family of deacetylase enzymes, acting on p53, thus interfering in the apoptosis process [58]. It has been seen that the inhibition of Sirt 1 has an antiproliferative effect in rhabdomyosarcoma cells and the expression of this gene is crucial for the survival of this neoplasia [21], so it is interesting to investigate the effect of N.g. on Sirt 1 in RD cells. In addition, Sirt 1 regulates other anti-aging genes, and its inactivation is related to metabolic diseases [59]. The positive regulation of this gene can occur simply by consuming a low-calorie diet [60], a behavior that could be complemented with treatment from N.g. In this way, in the future, we could have an effective therapeutic strategy against rhabdomyosarcoma, based on healthy habits and natural compounds.
Conflicts of Interest
The authors have no conflict of interest to declare relevant to this article’s content.
Funding Statement
This research was supported by a grant from CONICET PIP 11220210100761CO and by a grant from the Universidad Nacional Del Sur; Grant number: PGI24/ZB90. LP, AV, and LM are researcher members of CONICET; NF and NB thank the CONICET for a doctoral fellowship.
Acknowledgments
Cell Signaling Technology Inc. kindly provided us with the secondary antibodies.
Visuar S.A, Benito Quinquela Martín 1924, (1296), CABA, Buenos Aires, Argentina, and Fuegoservi S.R.L, Estomba 1702, B8003 AAF, de Buenos Aires for supporting our Research.
References
2. Dangoor A, Seddon B, Gerrand C, Grimer R, Whelan J, Judson I. UK guidelines for the management of soft tissue sarcomas. Clin Sarcoma Res. 2016 Nov 15;6:20.
3. Cen L, Arnoczky KJ, Hsieh FC, Lin HJ, Qualman SJ, Yu S, et al. Phosphorylation profiles of protein kinases in alveolar and embryonal rhabdomyosarcoma. Mod Pathol. 2007 Sep;20(9):936-46.
4. Mollineda Mederos K, Tabares Cruz YB, Vásquez Espinosa GDJ, Echevarría Caicedo KP. Rabdomiosarcoma embrionario en un paciente pediátrico. Revista Dilemas Contemporáneos: Educación, Política y Valores. 2020; pp. 1–16.
5. Merlino G, Helman LJ. Rhabdomyosarcoma--working out the pathways. Oncogene. 1999 Sep 20;18(38):5340-8.
6. Ferrari A, Dileo P, Casanova M, Bertulli R, Meazza C, Gandola L, et al. Rhabdomyosarcoma in adults. A retrospective analysis of 171 patients treated at a single institution. Cancer. 2003 Aug 1;98(3):571-80.
7. Hedenfalk IA, Baldetorp B, Borg A, Oredsson SM. Activated cell cycle checkpoints in epirubicin-treated breast cancer cells studied by BrdUrd-flow cytometry. Cytometry. 1997 Dec 1;29(4):321-7.
8. Norbury CJ, Hickson ID. Cellular responses to DNA damage. Annu Rev Pharmacol Toxicol. 2001;41:367-401.
9. Attardi LD, Reczek EE, Cosmas C, Demicco EG, McCurrach ME, Lowe SW, et al. PERP, an apoptosis-associated target of p53, is a novel member of the PMP-22/gas3 family. Genes Dev. 2000 Mar 15;14(6):704-18.
10. Kong CS, Cao H, Kwok S, Nguyen CM, Jordan RC, Beaudry VG, et al. Loss of the p53/p63 target PERP is an early event in oral carcinogenesis and correlates with higher rate of local relapse. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013 Jan;115(1):95-103.
11. Khan IA, Yoo BH, Masson O, Baron S, Corkery D, Dellaire G, et al. ErbB2-dependent downregulation of a pro-apoptotic protein Perp is required for oncogenic transformation of breast epithelial cells. Oncogene. 2016 Nov 3;35(44):5759-69.
12. Dutta S, Mahalanobish S, Saha S, Ghosh S, Sil PC. Natural products: An upcoming therapeutic approach to cancer. Food Chem Toxicol. 2019 Jun;128:240-55.
13. Mansoori B, Mohammadi A, Amin Doustvandi M, Mohammadnejad F, Kamari F, Gjerstorff MF, et al. Photodynamic therapy for cancer: Role of natural products. Photodiagnosis Photodyn Ther. 2019 Jun;26:395-404.
14. Mukherjee AK, Basu S, Sarkar N, Ghosh AC. Advances in cancer therapy with plant based natural products. Curr Med Chem. 2001 Oct;8(12):1467-86.
15. Ouyang L, Luo Y, Tian M, Zhang SY, Lu R, Wang JH, et al. Plant natural products: from traditional compounds to new emerging drugs in cancer therapy. Cell Prolif. 2014 Dec;47(6):506-15.
16. Musso F, Pronsato L, Milanesi L, Vasconsuelo A, Faraoni MB. Pharmacognosy non-polar extracts of nicotiana glauca (solanaceae) induce apoptosis in human rhabdomyosarcoma cells. Rodriguesia. 2020;7.
17. Janakat S, Al-Merie H. Evaluation of hepatoprotective effect of Pistacia lentiscus, Phillyrea latifolia and Nicotiana glauca. Journal of ethnopharmacology. 2002 Nov 1;83(1-2):135-8.
18. Musso F, Lincor D, Vasconsuelo A, Pronsato L, Faraoni B, Milanesi L. Adverse Effects in Skeletal Muscle Following the Medicinal Use of Nicotiana glauca. Biol Pharm Bull. 2019;42(5):671-9.
19. Cully M, You H, Levine AJ, Mak TW. Beyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat Rev Cancer. 2006 Mar;6(3):184-92.
20. Wang X, McCullough KD, Franke TF, Holbrook NJ. Epidermal growth factor receptor-dependent Akt activation by oxidative stress enhances cell survival. J Biol Chem. 2000 May 12;275(19):14624-31.
21. Ma L, Maruwge W, Strambi A, D'Arcy P, Pellegrini P, Kis L, et al. SIRT1 and SIRT2 inhibition impairs pediatric soft tissue sarcoma growth. Cell Death Dis. 2014 Oct 23;5(10):e1483.
22. Pillai VB, Sundaresan NR, Gupta MP. Regulation of Akt signaling by sirtuins: its implication in cardiac hypertrophy and aging. Circ Res. 2014 Jan 17;114(2):368-78.
23. Scorrano L, Ashiya M, Buttle K, Weiler S, Oakes SA, Mannella CA, et al. A distinct pathway remodels mitochondrial cristae and mobilizes cytochrome c during apoptosis. Dev Cell. 2002 Jan;2(1):55-67.
24. Alaimo A, Gorojod RM, Beauquis J, Muñoz MJ, Saravia F, Kotler ML. Deregulation of mitochondria-shaping proteins Opa-1 and Drp-1 in manganese-induced apoptosis. PLoS One. 2014 Mar 14;9(3):e91848.
25. Yarosh W, Monserrate J, Tong JJ, Tse S, Le PK, Nguyen K, et al. The molecular mechanisms of OPA1-mediated optic atrophy in Drosophila model and prospects for antioxidant treatment. PLoS Genet. 2008 Jan;4(1):e6.
26. Chen L, Liu T, Tran A, Lu X, Tomilov AA, Davies V, et al. OPA1 mutation and late-onset cardiomyopathy: mitochondrial dysfunction and mtDNA instability. J Am Heart Assoc. 2012 Oct;1(5):e003012.
27. Bligh EG, Dyer WJ. A RAPID METHOD OF TOTAL LIPID EXTRACTION AND PURIFICATION. Can J Biochem Physiol. 1959;37(8):911-7.
28. Darío L, Florencia M, Andrea V, María Belén F, Lorena M. Extracts from Nicotiana glauca induce apoptosis through caspases in skeletal muscle cells. 2017.
29. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248-54.
30. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680-5.
31. Abràmoff MD. Image Processing with ImageJ. 2004. Accessed: Jul. 21, 2024.
32. Giulietti A, Overbergh L, Valckx D, Decallonne B, Bouillon R, Mathieu C. An overview of real-time quantitative PCR: applications to quantify cytokine gene expression. Methods. 2001 Dec;25(4):386-401.
33. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001 Dec;25(4):402-8.
34. Balzarini M, Gonzalez LA, Tablada M, Casanoves F. Infostat: manual del usuario. 2008.
35. Fuchs Y, Steller H. Programmed cell death in animal development and disease. Cell. 2011 Nov 11;147(4):742-58.
36. Heinlein C, Deppert W, Braithwaite AW, Speidel D. A rapid and optimization-free procedure allows the in vivo detection of subtle cell cycle and ploidy alterations in tissues by flow cytometry. Cell Cycle. 2010 Sep 1;9(17):3584-90.
37. Riccardi C, Nicoletti I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat Protoc. 2006;1(3):1458-61.
38. Wu J, Li L, Wang Y, Ren X, Lin K, He Y. The HSP90/Akt pathway may mediate artemether-induced apoptosis of Cal27 cells. FEBS Open Bio. 2019 Oct;9(10):1726-33.
39. Fulda S. Targeting apoptosis resistance in rhabdomyosarcoma. Curr Cancer Drug Targets. 2008 Sep;8(6):536-44.
40. Bresciani G, da Cruz IB, González-Gallego J. Manganese superoxide dismutase and oxidative stress modulation. Adv Clin Chem. 2015;68:87-130.
41. Zalewska-Ziob M, Adamek B, Kasperczyk J, Romuk E, Hudziec E, Chwalińska E, et al. Activity of Antioxidant Enzymes in the Tumor and Adjacent Noncancerous Tissues of Non-Small-Cell Lung Cancer. Oxid Med Cell Longev. 2019 Oct 31;2019:2901840.
42. Bossy-Wetzel E, Barsoum MJ, Godzik A, Schwarzenbacher R, Lipton SA. Mitochondrial fission in apoptosis, neurodegeneration and aging. Curr Opin Cell Biol. 2003 Dec;15(6):706-16.
43. Griparic L, van der Wel NN, Orozco IJ, Peters PJ, van der Bliek AM. Loss of the intermembrane space protein Mgm1/OPA1 induces swelling and localized constrictions along the lengths of mitochondria. J Biol Chem. 2004 Apr 30;279(18):18792-8.
44. Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur Urol. 2017 Jan;71(1):96-108.
45. Basak D, Arrighi S, Darwiche Y, Deb S. Comparison of Anticancer Drug Toxicities: Paradigm Shift in Adverse Effect Profile. Life (Basel). 2021 Dec 29;12(1):48.
46. Paraoan L, Gray D, Hiscott P, Ebrahimi B, Damato B, Grierson I. Expression of p53-induced apoptosis effector PERP in primary uveal melanomas: downregulation is associated with aggressive type. Exp Eye Res. 2006 Oct;83(4):911-9.
47. Ihrie RA, Reczek E, Horner JS, Khachatrian L, Sage J, Jacks T, et al. Perp is a mediator of p53-dependent apoptosis in diverse cell types. Curr Biol. 2003 Nov 11;13(22):1985-90.
48. Pronsato L. Rol de la testosterona y su receptor frente a la apoptosis en células musculares esqueléticas murinas. 2013.
49. Vijayarathna S, Oon CE, Chen Y, Kanwar JR, Sasidharan S. Polyalthia longifolia Methanolic Leaf Extracts (PLME) induce apoptosis, cell cycle arrest and mitochondrial potential depolarization by possibly modulating the redox status in hela cells. Biomed Pharmacother. 2017 May;89:499-514.
50. Li MH, Cha YN, Surh YJ. Peroxynitrite induces HO-1 expression via PI3K/Akt-dependent activation of NF-E2-related factor 2 in PC12 cells. Free Radic Biol Med. 2006 Oct 1;41(7):1079-91.
51. Nakaso K, Yano H, Fukuhara Y, Takeshima T, Wada-Isoe K, Nakashima K. PI3K is a key molecule in the Nrf2-mediated regulation of antioxidative proteins by hemin in human neuroblastoma cells. FEBS Lett. 2003 Jul 10;546(2-3):181-4.
52. Chen B, Lu Y, Chen Y, Cheng J. The role of Nrf2 in oxidative stress-induced endothelial injuries. J Endocrinol. 2015 Jun;225(3):R83-99.
53. Wang P, Li CG, Qi Z, Cui D, Ding S. Acute exercise stress promotes Ref1/Nrf2 signalling and increases mitochondrial antioxidant activity in skeletal muscle. Exp Physiol. 2016 Mar;101(3):410-20.
54. Crilly MJ, Tryon LD, Erlich AT, Hood DA. The role of Nrf2 in skeletal muscle contractile and mitochondrial function. J Appl Physiol (1985). 2016 Sep 1;121(3):730-40.
55. Li T, He S, Liu S, Kong Z, Wang J, Zhang Y. Effects of different exercise durations on Keap1-Nrf2-ARE pathway activation in mouse skeletal muscle. Free Radic Res. 2015 Oct;49(10):1269-74.
56. Arnoult D, Grodet A, Lee YJ, Estaquier J, Blackstone C. Release of OPA1 during apoptosis participates in the rapid and complete release of cytochrome c and subsequent mitochondrial fragmentation. J Biol Chem. 2005 Oct 21;280(42):35742-50.
57. Frezza C, Cipolat S, Martins de Brito O, Micaroni M, Beznoussenko GV, Rudka T, et al. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell. 2006 Jul 14;126(1):177-89.
58. Martins IJ. Anti-Aging Genes Improve Appetite Regulation and Reverse Cell Senescence and Apoptosis in Global Populations. Adv Aging Res. 2016;5(1):9-26.
59. Martins IJ. Single gene inactivation with implications to diabetes and multiple organ dysfunction syndrome. Journal of Clinical Epigenetics. 2017 Aug 1;3(3):1-8.
60. Martins IJ. Nutrition therapy regulates caffeine metabolism with relevance to NAFLD and induction of type 3 diabetes. J Diabetes Metab Disord. 2017;4(1):1-9.
