Commentary
Treatment of sickle cell disease (SCD) remains varied with only a minority of patients benefiting from stem cell transplant as a near cure. Others await the promise of more effective and less toxic treatments than hydroxyurea, especially children who are most susceptible to the morbidities and mortalities of SCD. The recent report of Hebert, Rakotoson et al. [1] points to an evolution of in vitro diagnostics (IVD) testing of blood cells with the emerging need for cell-by-cell measurement of red blood cell (RBC) specific hemoglobin variant levels. The study further provides clinical validation as to the utility of measuring HbF content in RBCs of SCD patients, not just as a prognostic test, but likely serving as a companion diagnostic in phase 3 clinical trials of new SCD therapies. The assay described by the French group is an “off label” adaptation of a CE-IVD flow cytometric assay kit (Fetal Cell Count Kit, IQ Products, Groningen, NL), based upon anti-HbF monoclonal antibody detection and multiparameter flow cytometry. The original intended use of the kit is to measure fetomaternal hemorrhage (FMH) as a companion diagnostic for the use of Rh immune globulin therapy to prevent hemolytic disease of the newborn, where the target analyte is fetal RBCs, rather than adult RBCs containing lower HbF levels, so-called F cells. Heretofore interest in red cell hemoglobin levels related only to total cellular hemoglobin expressed as a simple mean level across the entire blood RBC population, although the importance of HbF levels correlating with the reduction of SCD complications had been previously appreciated [2-4]. Routine blood cell counters largely provide a calculated, albeit reasonably precise, parameter of mean cell hemoglobin (MCH), that is included in most clinical algorithms for anemia differential diagnosis. Only cell-by-cell hemoglobin determinations when applied to the reticulocyte population has found niches of clinical utility, such as in the management of anemia and erythropoietin therapy associated with chronic renal disease [5]. Understanding the HbF RBC levels is poised for a clear role in the personalized care and therapeutic management of sickle cell disease (SCD) patients, long known to be a genetically and clinically heterogenous patient population [6,7]. The recent enthusiasm regarding molecular therapeutic strategies affecting the fetal to adult hemoglobin switching mechanisms [8-14] and importance of understanding the level of fetal hemoglobin (HbF) within the cell as a major moderator of hemoglobin polymerization and hence sickling conformational changes is clearly demonstrated by the work of the French group [1].
Only the clinical application of fetomaternal hemorrhage detection in certain complicated gestational conditions and the monitoring of Rh immune globulin therapy in the prevention of hemolytic disease of the newborn heretofore demanded the need for cell-by-cell measurement of HbF. Despite the IVD availability of highly precise cell by cell HbF RBC assays using semi-quantitative immunophenotypic flow cytometry, the mainstay of clinical practice largely remains the microscopic Kleihauer Betke Braun test, despite its high inter-observer imprecision in the range of 25-100% coefficient of variation (CV). Interestingly the College of American Pathologists (CAP), normally an advocate of improved laboratory performance, has advocated the over-dosing of Rh immune globulin as one approach to mitigation of the inaccuracies of the manual test, rather than recommending the implementation of the better performing flow cytometry assay [15,16]. Likely the adherence to the older manual methodologies relates to the low reimbursement for both the drug and laboratory test, along with the relatively low toxicity of Rh immune globulin therapy. Whatever the cause for retarded acceptance of the FMH HbF RBC assay into clinical practice, the methodologic technology clearly exists to allow a format would allow rapid cell-by-cell measurements of HbF levels in RBCs to support extended applications in SCD, along with improving the FMH testing needs [17]. The author’s assessment, having been among the pioneers of IVD HbF RBC assays [18-25], is that the incremental testing volume of SCD patient in developed countries, cost justified by the savings afforded by reduced hospitalization and morbidity of SCD patients when appropriately dosed by the HbF induction therapies, is sufficient to foster a second-generation IVD cell-by-cell HbF RBC assay. The purpose of this commentary is to provide an experienced perspective as to the optimal design for an IVD HbF RBC assay in an easy to perform, rapid testing format.
There remains one aspect to the optimal HbF RBC assay that remains to be determined, namely what are the clinically relevant expression units. The study of Hebert, Rakotoson et al. [1] is a strong proof-of-principal study, but from a regulatory perspective the transformation of their laboratory developed test (LDT) assay to an IVD test has issues of calibration and traceability that would be challenged both in the EU CE IVD and USA FDA regulatory processes. The authors devised an indirect calibration of the assay to MCH values from various clinical specimens exhibiting pan-cellular hereditary persistence of HbF in order to define a fluorescence threshold for those RBCs with >4 picograms of HbF per cell. While such an experimental approach is adequate for a single laboratory proof-of-principle study; this approach is not practical for a commercially viable IVD assay, as each new lot of reagents, including the antibodies with its varying fluorochrome: protein ratio would require a repeat calibration and interlot adjustments. Another variable unappreciated by many labs is the importance of cell fixation and permeabilization steps given the hemoglobin intracellular location. Fixation and permeability conditions (time, concentration, cell counts) are the biggest variables of anti-HbF binding in our experience in developing the current IVD HbF RBC kits of Quik Quant (IQ Products, Groningen, NL) and Fetal Hemoglobin Test (Thermo Fisher Scientific, USA) [Table 1, 24,25].
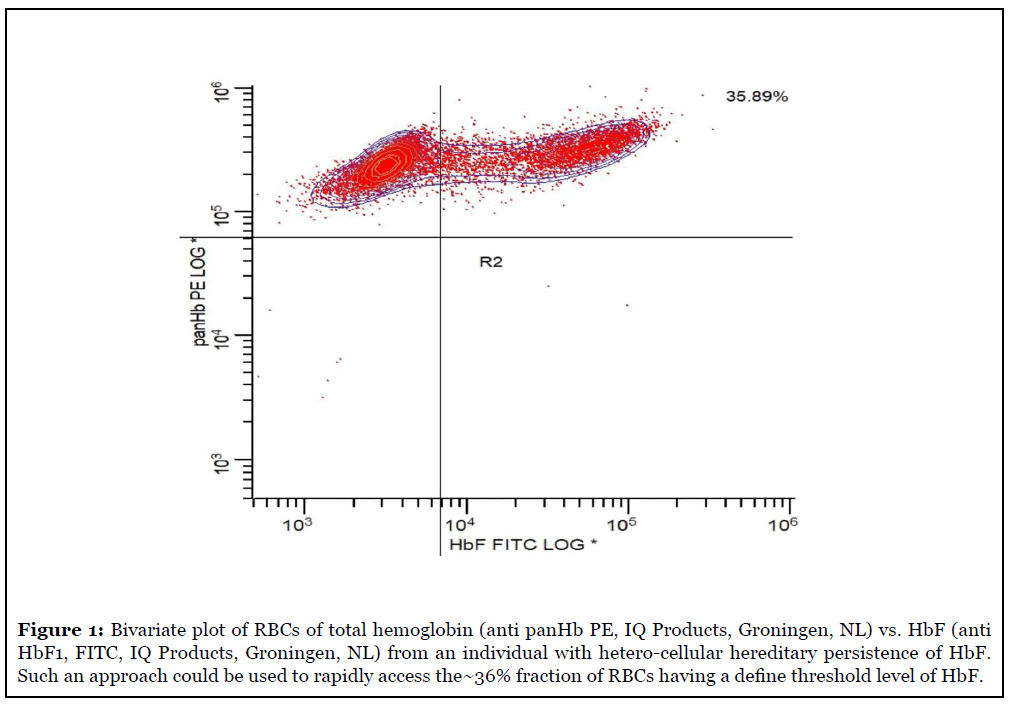
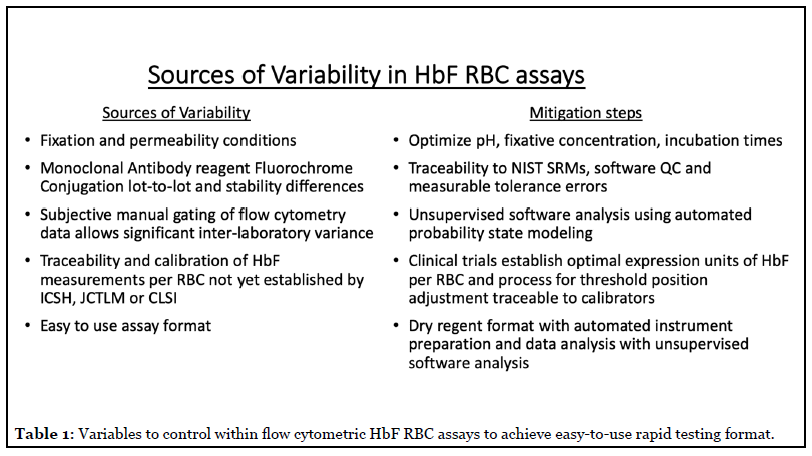
While understanding an absolute threshold for the physiologic inhibition of RBC sickling is of biologic and physiologic interest, it may be equally relevant simply to measure the proportion of the total cellular hemoglobin that is HbF. An assay design determining the proportion of hemoglobin to be HbF on a cell-by-cell basis is an easier IVD claim, as simple inter-lot regression can be used for quantitative traceability and calibration without the impractical manufacturing need for access to uncommon clinical specimens manifesting hereditary persistence of HbF on pan-cellular basis. An underlying assumption is that successful clinical acceptance is dependent upon the availability of both CE marked (under pending EU IVD directives) and US FDA clearance. This is in contrast to expecting successful broad laboratory adoption of LDT assays of HbF RBC. The IVD pathway to clinical adoption compared to hoping that LDT formats of a HbF RBC assay show minimal inter-laboratory variance, which has not been the track record of flow cytometry assays, would also be demanded in a companion diagnostic role. In order to expedite clinical practice, the ideal IVD format should be validated as a companion diagnostic so as to address issues raised by this commentary article in determining the ideal reporting units for the HbF RBC assay. Additionally, the assay must be both easy to use with a rapid time to reporting results on a 24 hour and 7 day a week, only this 24/7 rapid test format would sufficiently support the emergency treatment of pregnant women and SCD patients and the need for rapid dose adjustments of therapies of Rh immune globulin and the new molecular based therapies designed to increase cellular levels of HbF in SCD. Ideally these tests should require less than 1 hour to complete so as to support patient clinics where same encounter dose adjustments could be made.
Another requisite component for an easy to use, yet accurate, IVD assay for flow cytometry is the integration of unsupervised data analysis by automated software analysis of the datafiles. Many current flow cytometric IVD assays use so-called manual or subjective gating techniques, typically requiring extensive technical training. However, automated unsupervised data analysis using probability state modeling techniques afforded by Gemstone software (Verity Software, Topsham, ME) has been found to reduce inter-observer variance to effectively zero for two IVD assays, one being the FMH HbF RBC assay of Quik Quant [25,26]. The integration of validated probability state modeling template for data analysis and reporting is the key to both a rapid format and the ease of use.
Why am I confident that this or a similar calibrated flow cytometric (or cell by cell measurement technology) assay satisfies an emerging clinical need? The evidence for continued development of therapeutics able of inducing the increased RBC levels of HbF in sickle cell disease is convincing both from economic and risk/ benefit perspectives [8-14]. These studies and others paint an increasingly clearer understanding of the molecular regulation of hemoglobin switching and genetic heterogeneity in SCD and other hemoglobin variant disorders, such as thalassemia [6,7]. Early studies with LDT versions of HbF RBC assays make clear that patients respond with either a homo-cellular or hetero-cellular HbF distribution fashion among RBCs, as clearly reaffirmed by the current study by Hebert, Rakotoson et al. [1]. Thus, the important parameter correlating with therapeutic response is the population size of RBCs having a threshold approximating >4 picogram HbF per cell or ~20% of the total cellular hemoglobin level, seemingly where sufficient intercalating HbF prevents the polymerizing sickling phenomenon of SCD. It remains to be determined if the critical threshold for sickling prevention is a specific level of HbF in the cell or if merely achieving a certain proportion of HbF of the total cellular hemoglobin content is sufficient to predict sickling phenotype remains to be determined. The genetic heterogeneity of SCD and the proven inability of simple total blood or fractional HbF measurement levels to predict a therapeutic response to HbF-inducing SCD therapies makes it probable that rapid dose adjustments based upon cell-by-cell RBC HbF levels are requisite to achieve the most desirous clinical outcomes, promising SCD patients near normal lifetimes with significantly lower healthcare costs. All the right reasons to adopt a new laboratory test are present, provided it is designed to meet both the clinical and laboratory demands – namely rapid 24/7 results obtained with technical ease.
As best exemplified by the current COVID-19 testing crisis, the development of IVD assays and clinical acceptance has become more challenging as the regulatory climate changes, particularly in the EU with evolving CE marking and ISO guidelines for IVD testing after 2021. These regulations also impact diagnostic laboratories by requiring they utilize commercial CE marked IVD kits, unless they can demonstrate superiority through auditable and expensive validation testing, an exercise outside the scope of most diagnostic flow cytometry labs. Commercial IVD developers are already operating under the 2021 regulation due to the requisite development time for IVD devices. Additional EU IVD regulatory changes require that IVD assays provide measurement traceability for any analyte, found acceptable to the Joint Committee for Traceability in Laboratory Medicine (www.JCTLM.org). Traceability in the case of fluorescence flow cytometry HbF RBC assays it would be the hemoglobin content (or fluorescent surrogate reagent fluorochrome) per RBC. Thus, pragmatically it is clear that any HbF RBC assay must be delivered to the clinical laboratory by experienced IVD developers, meeting current regulatory requirements and contemporary clinical laboratory practice requirements. While the current proof of principle report describes a credible calibration method for the 4 picogram HbF per RBC threshold (AUC = 0.896), this LDT assay potentially has lot-to-lot variations and is susceptible to the known lotto- lot variation in cell fixative (and permeability) reagents, particularly on a lab-to-lab basis. Manual software gating in flow cytometric assays is subjective and its accuracy is experientially based, which in the case of HbF RBC assays can be fully automated to improve reproducibility and precision [11].
The second IVD device requirement is to address the actual clinical need in a format that allows both rapid testing to support SCD clinics and additionally address the Obstetrical demand for 24/7 FMH testing availability. Certainly, methods for rapid HbF RBC assays are known [12-16] and existing CE IVD kits for FMH could easily be modified as done in the current study, additionally modern software algorithms are feasible and advocated to provide for fully automated high precision analysis of flow cytometric files and controls [11]. These features would be requisite specifications for a commercial HbF RBC assay providing requisite rapid turnaround (< 60 minutes time to report), high precision, and moderate complexity ease of use within the laboratory workplace to allow for 24-hour availability, thus supportive of SCD outpatients clinics, patients in crisis and facilitate a more accurate adjustment of anti-D therapy in FMH patients.
The format of any HbF content of RBC assay could take two pathways. The current IVD assays for FMH could be “improved” to both enhance the performance in FMH assessment and provide a new SCD solution. Alternatively, an assay for the SCD intended use could be developed separately from FMH assays. Currently in the absence of development of any new IVD solution with high precision and ease of use, the FMH testing has straddled heretofore two worlds of testing precision. Surprisingly most FMH testing remains performed with the manual microscopic method of Kleihauer Betke Braun with variable assay precision in the range of 25 - >100% (more of a qualitative assay in performance), clearly not acceptable for F cell HbF content assay, which requires the <5% CV precision afforded to rare event analysis by flow cytometry [2]. Furthermore given the IVD status of the Quick Quant (IQ Products, Groningen, NL), FETAL Cell Count (IQ Products, Groningen, NL) and Fetal HEMOGLOBIN kits (Thermo Fisher Scientific, USA); these future regulations will severely restrict the use of LDT assays that do not perform in some superior fashion compared to the commercial IVD solutions, which in FMH testing has never been reported [15,16,22]. What is the mostly likely course for an IVD company, two separate assays or a single improved assay? Years past the obvious choice would be to continue with the old test and just introduce a new application, but the pending 2021 EU regulatory rules again influence that decision. Another IVD company requirement with the new regulation is to re-register IVD products given CE IVD status under previous validation and documentation requirements. Thus, from a cost-benefit perspective, one can make a cogent argument that since existing FMH IVD assays now require more validation (now including clinical studies) and the reagents and instrument platform of both assays are similar, it is strategically wiser to re-engineer an improved assay format that meets the needs of both SCD and FMH testing. A fully calibrated CE IVD flow cytometric HbF RBC assay kit is sufficient and likely required as a companion diagnostic to support the initial new generation SCD treatments intended to increase RBC HbF levels, so likely Pharma will need a fully validated assay. As with reticulocyte analysis with improved parameters of immature reticulocyte fraction and reticulocyte hemoglobin content [5], it is conceivable that HbF RBC assays could migrate to automated blood cell counters [17], but more likely this platform migration would be a second-generation iteration and the anticipated development time would be a disservice to SCD patients currently needing improved therapeutic options. While other diagnostic approaches addressing the role of RBC adhesion are altered in SCD [27,28], but the need for rapid test reporting may a find a faster IVD development pathway for a multiparameter flow cytometric HbF RBC assay using unsupervised data analysis.
Thus, the testing and clinical communities concerned with SCD and FMH testing need to prepare for both evolution of current FMH assays to more precise cytometric assays reporting both fetal RBC level and F Cell enumeration of subsets based upon HbF fractional or absolute content and plan for the changes outlined in the new IVD guidelines. The impact of a cytometric HbF content RBC assay on the prognosis of SCD patients is still being written, but for my two cents, SCD patients are truly poised to benefit from an elegant bench to bedside story.
References
2. Borba R, Lima CS, Grotto HZ. Reticulocyte parameters and hemoglobin F production in sickle cell disease patients undergoing hydroxyurea therapy. Journal of Clinical Laboratory Analysis. 2003;17(2):66-72.
3. Antwi-Boasiako C, Frimpong E, Ababio GK, Dzudzor B, Ekem I, Gyan B, et al. Sickle Cell Disease: Reappraisal of the role of Foetal Haemoglobin Levels in the frequency of Vaso-Occlusive crisis. Ghana Medical Journal. 2015;49(2):102-6.
4. Steinberg MH, Chui DH, Dover GJ, Sebastiani P, Alsultan A. Fetal hemoglobin in sickle cell anemia: a glass half full?. Blood, The Journal of the American Society of Hematology. 2014 Jan 23;123(4):481-5.
5. Piva E, Brugnara C, Spolaore F, Plebani M. Clinical utility of reticulocyte parameters. Clinics in Laboratory Medicine. 2015 Mar 1;35(1):133-63.
6. Piel FB, Steinberg MH, Rees DC. Sickle cell disease. New England Journal of Medicine. 2017 Apr 20;376(16):1561-73.
7. Habara A, Steinberg MH. Minireview: Genetic basis of heterogeneity and severity in sickle cell disease. Experimental Biology and Medicine. 2016 Apr;241(7):689-96.
8. Carden MA, Little J. Emerging disease-modifying therapies for sickle cell disease. Haematologica. 2019 Sep 1;104(9):1710-9.
9. Meier ER. Treatment options for sickle cell disease. Pediatric Clinics. 2018 Jun 1;65(3):427-43.
10. Wu Y, Zeng J, Roscoe BP, Liu P, Yao Q, Lazzarotto CR, et al. Highly efficient therapeutic gene editing of human hematopoietic stem cells. Nature Medicine. 2019 May;25(5):776-83.
11. Demirci S, Leonard A, Haro-Mora JJ, Uchida N, Tisdale JF. CRISPR/Cas9 for sickle cell disease: applications, future possibilities, and challenges. InCell Biology and Translational Medicine, Volume 5 2019; 1144:37-52.
12. Magrin E, Miccio A, Cavazzana M. Lentiviral and genome-editing strategies for the treatment of ß-hemoglobinopathies. Blood. 2019 Oct 10;134(15):1203- 13.
13. Orkin SH, Bauer DE. Emerging genetic therapy for sickle cell disease. Annual Review of Medicine. 2019 Jan 27;70:257-71.
14. Shah F, Dwivedi M. Pathophysiology and recent therapeutic insights of sickle cell disease. Annals of Hematology. 2020;99(5):925-935.
15. Ramsey G. Inaccurate doses of Rh immune globulin after Rh-incompatible fetomaternal hemorrhage: survey of laboratory practice. Archives of Pathology & Laboratory Medicine. 2009 Mar;133(3):465-9.
16. Lafferty J, Raby A, Keeney M, Flynn GJ, Crowther M. Inaccurate Doses of Rh Immune Globulin After Rh- Incompatible Fetomaternal Hemorrhage—Survey of Laboratory Practice. Archives of Pathology & Laboratory Medicine. 2009 Dec;133(12):1910-1.
17. Campbell TA, Ware RE, Mason M. Detection of hemoglobin variants in erythrocytes by flow cytometry. Cytometry: The Journal of the International Society for Analytical Cytology. 1999 Mar 1;35(3):242-8.
18. Davis BH, Olsen S, Bigelow NC, Chen JC. Detection of fetal red cells in fetomaternal hemorrhage using a fetal hemoglobin monoclonal antibody by flow cytometry. Transfusion. 1998 Aug;38(8):749-56.
19. Mundee Y, Bigelow NC, Davis BH, Porter JB. Simplified flow cytometric method for fetal hemoglobin containing red blood cells. Cytometry. 2000 Dec 15;42(6):389-93.
20. Chen JC, Bigelow N, Davis BH. Proposed flow cytometric reference method for the determination of erythroid F-cell counts. Cytometry: The Journal of the International Society for Analytical Cytology. 2000 Aug 15;42(4):239-46.
21. Davis BH. Diagnostic utility of red cell flow cytometric analysis. Clinics in Laboratory Medicine. 2001 Dec;21(4):829-40.
22. Chen JC, Davis BH, Wood B, Warzynski MJ. Multicenter clinical experience with flow cytometric method for fetomaternal hemorrhage detection. Cytometry: The Journal of the International Society for Analytical Cytology. 2002 Dec 15;50(6):285-90.
23. Davis BH, Davis KT. Laboratory assessment of fetomaternal hemorrhage is improved using flow cytometry. Laboratory Medicine. 2007 Jun 1;38(6):365- 73.
24. Davis BH. Enumeration of Fetal Red Blood Cells, Hemoglobin-Specific RBC Cells, and F Reticulocytes in Human Blood. Current Protocols in Cytometry. 2019 Sep;90(1):e56.
25. Wong L, Hunsberger BC, Bruce Bagwell C, Davis BH. Automated quantitation of fetomaternal hemorrhage by flow cytometry for HbF-containing fetal red blood cells using probability state modeling. International Journal of Laboratory Hematology. 2013 Oct;35(5):548-54.
26. Wong L, Hill BL, Hunsberger BC, Bagwell CB, Curtis AD, Davis BH. Automated analysis of flow cytometric data for measuring neutrophil CD64 expression using a multiinstrument compatible probability state model. Cytometry Part B: Clinical Cytometry. 2015 Jul 8;88(4):227-35.
27. Di Caprio G, Schonbrun E, Gonçalves BP, Valdez JM, Wood DK, Higgins JM. High-throughput assessment of hemoglobin polymer in single red blood cells from sickle cell patients under controlled oxygen tension. Proceedings of the National Academy of Sciences. 2019 Dec 10;116(50):25236-42.
28. Alapan Y, Kim C, Adhikari A, Gray KE, Gurkan- Cavusoglu E, Little JA, et al. Sickle cell disease biochip: a functional red blood cell adhesion assay for monitoring sickle cell disease. Translational Research. 2016 Jul 1;173:74-91.e8. 71
