Abstract
Intensive treatments for acute myeloid leukemia (AML) have traditionally been administered on an inpatient basis due in part to chemotherapy regimen infusion requirements, transfusion support, and the need for close monitoring for infectious complications and adverse events. However, hospitalization is a major component of burgeoning healthcare costs and may contribute to impaired quality of life in patients with AML. To help inform the ongoing discussion regarding the merits and challenges of outpatient administration of AML therapy, a multidisciplinary panel of experts were engaged to identify areas of consensus, explore ongoing uncertainties, and develop an algorithm that may help inform discussions on outpatient treatment between healthcare providers and patients. Based on available evidence and clinical experience, inpatient treatment remains appropriate for majority of patients with AML undergoing conventional intensive induction chemotherapy. The more recently introduced liposomal formulation of cytarabine and daunorubicin (CPX-351) has an infusion schedule that is more amenable to outpatient administration. Outpatient administration of CPX-351 for select patients with close daily monitoring has been implemented via a multidisciplinary team-based model. The feasibility of safely managing AML patients receiving outpatient CPX-351 is being prospectively evaluated in an ongoing phase 4 study. Panelists generally agreed that lower-intensity regimens including venetoclax combined with hypomethylating agents or lowdose cytarabine (LDAC), glasdegib plus LDAC, enasidenib, ivosidenib, and gilteritinib can be administered safely in the outpatient setting for most newly diagnosed AML patients. Venetoclax-based combinations are also promising for outpatient administration but may require risk stratification due to the potential for tumor lysis syndrome (TLS). The proposed algorithm developed to inform consideration of outpatient treatment is focused on consideration of patient fitness, the treatment regimen selected, infrastructure in place to support outpatient administration, and patient/caregiver agreement with the outpatient approach. Educational needs for clinicians and recommendations to overcome knowledge gaps regarding outpatient therapy were also formulated. Outpatient administration of AML therapy is feasible in the appropriate clinical setting and patient. However, further research is needed regarding feasibility, logistics, safety, and patient outcomes including quality of life.
Abbreviations
AML: Acute Myeloid Leukemia; CIVI: Continuous Intravenous Infusion; CLL: Chronic Lymphocytic Leukemia; COVID-19: Coronavirus Disease 2019; HCT: Hematopoietic Cell Transplantation; HDAC: High-Dose Cytarabine; HMA: Hypomethylating Agent; IPOP: Inpatient/Outpatient; LDAC: Low-Dose Cytarabine; MDS: Myelodysplastic Syndrome; NCCN: National Comprehensive Cancer Network; QoL: Quality of Life; TLS: Tumor Lysis Syndrome; WBC: White Blood Cell
Introduction
In patients with newly diagnosed acute myeloid leukemia (AML), the initial treatment decision is often predicated on the individual’s candidacy for intensive chemotherapy. For those patients considered eligible for intensive treatment, the standard approach historically has been induction with a combination chemotherapy regimen such as cytarabine for 7 days and an anthracycline for 3 days (“7+3” therapy) [1]. Those patients who achieve remission will typically go on to receive consolidation chemotherapy and/or allogeneic hematopoietic cell transplantation (HCT) [2,3].
Intensive AML treatments have traditionally been administered on an inpatient basis due in part to chemotherapy regimen infusion requirements, transfusion support, and to allow for close monitoring for infectious and other complications. However, prolonged hospitalization is a major contributor to burgeoning healthcare costs and may contribute to the severely impaired quality of life (QoL) observed among patients with AML [4].
Interest in moving toward outpatient management has increased as healthcare providers have become more comfortable with the prevention and treatment of complications associated with the intensive treatment of AML [5]. The trend toward outpatient management is driven not only by the potential to reduce the substantial financial costs of inpatient treatment, but also the desire to improve quality of life and family/caregiver support for patients, some of whom may find prolonged inpatient stay inconvenient or extremely challenging [5,6].
The AML treatment landscape has changed considerably since the advent of 7+3 therapy decades ago (Figure 1). Outpatient administration is a standard practice for lower intensity approaches to treatment of newly diagnosed AML. This is true not only for traditional treatment options such as low-dose cytarabine (LDAC) or hypomethylating agents (HMAs) including azacitidine or decitabine, but also for more recently introduced regimens including the combination of glasdegib and LDAC, and for the targeted agents enasidenib, ivosidenib, and gilteritinib, which are indicated for the treatment of mutation-defined patient subgroups.
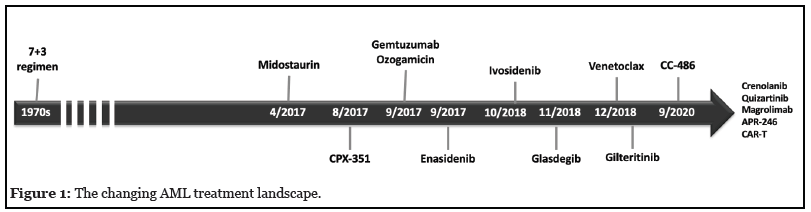
The introduction of a liposomal formulation of cytarabine and daunorubicin (CPX-351) and venetoclax-based combination therapy has further increased interest in outpatient administration of AML therapy. The published manuscript cited the 2019 US prescribing information for the liposomal combination of daunorubicin and cytarabine. The US prescribing information updated and released in March 2021 indicates that the liposomal daunorubicin and cytarabine is indicated for the treatment of newlydiagnosed therapy-related acute myeloid leukemia (t-AML) or AML with myelodysplasia-related changes (AML-MRC) in adults and pediatric patients 1 year and older [7]. In contrast to 7+3, the induction regimen is administered via intravenous infusion over 90 minutes on days 1, 3, and 5 of induction and days 1 and 3 of consolidation, and thus is more amenable to administration in an outpatient setting with careful monitoring [8]. In addition, venetoclax combined with azacitidine has demonstrated substantially improved outcomes in older, unfit AML patients, leading to rapid and widespread uptake of venetoclax-based combinations in both academic and community-based settings [9]. The risk of tumor lysis syndrome (TLS) is associated with venetoclax, prompting the need for risk assessment and prophylaxis, with more intensive measures (including hospitalization) recommended as risk increases. An initial venetoclax ramp-up dosing schedule designed to decrease TLS risk is indicated, during which hospitalization has been recommended [10,11].
Despite ongoing interest and exploration of outpatient approaches to AML treatment and monitoring, gaps in evidence and a lack of clear consensus or guidelines represent key challenges to implementation. Relatively few studies have prospectively evaluated the feasibility of outpatient approaches, though available evidence suggests that outpatient management can be feasible, well tolerated and potentially cost effective [4]. Likewise, there remains relatively little guidance from experts and persistent uncertainties regarding best practices. The recent American Society of Hematology guidelines for treating newly diagnosed AML in older adults acknowledge that in-hospital administration of intensive antileukemic therapy is “a burden to the patients and the system” when compared to less intensive approaches [12].
To better inform the ongoing discussion regarding the merits and challenges of outpatient administration of AML therapy, a panel of experts were engaged to identify areas of consensus, explore ongoing uncertainties, and develop an algorithm that may help inform discussions of inpatient versus outpatient treatment between community healthcare providers and patients. This article provides a discussion of current evidence regarding outpatient AML therapy, expert perspectives, and a summary of needs for patient communication and education for clinicians who provide care for patients with newly diagnosed AML.
Determining AML Patient Fitness
Assessment of patient fitness is important to determine the optimal treatment regimen as well as feasibility for treatment in an outpatient setting. Historically, the treatment choice for newly diagnosed AML patient has been dichotomous between intensive induction chemotherapy and non-intensive approaches and hinged on overall fitness of patient to be able to tolerate such therapy. Clinical metrics to assess the fitness have been developed and have focused on chronological age, performance status, and comorbidities. However, with newer approved therapies that include HMA and venetoclax as well as other targeted approaches, the traditional model of fitness has been questioned. Moreover, emphasis on disease-specific features such as genomic profile as well as cytogenetics is becoming increasingly imperative. For example, patients with mutations in TP53 gene have significantly inferior outcomes with traditional intensive chemotherapy regimens used in AML compared to the patients with wildtype TP53 gene. Newer data is also questioning the benefit of adding venetoclax to HMA therapy for such patients [13]. Therefore, careful evaluation of the disease biology and expected outcomes need to be considered in addition to fitness to individualize treatment selection.
Intensive Chemotherapy Regimens
Based on available evidence and clinical experience, participants in the expert panel emphasized that inpatient treatment is appropriate for most AML patients undergoing conventional intensive induction chemotherapy to monitor treatment related adverse events including TLS, infectious complications and the anticipated need for blood product transfusions.
Conventional intensive chemotherapy
A limited body of evidence suggests that conventional intensive induction chemotherapy can be safely administered in the outpatient setting. For example, the feasibility and potential benefits of an early hospital discharge strategy (i.e., within several days after completion of intensive induction chemotherapy) have been evaluated, with results suggesting feasibility, safety, and lower costs of care versus inpatient management [14,15]. By contrast, there is less evidence to support outpatient administration of intensive induction chemotherapy in patients with AML, although some does exist. In a recent pilot study including patients with AML or high-risk myelodysplastic syndrome (MDS), 14 of 17 subjects (82.4%) completed administration of intensive induction chemotherapy (7+3 and high-dose cytarabine based regimens) on an outpatient basis with no deaths within 14 days of induction initiation. The traditional 7+3 regimen includes continuous intravenous infusion (CIVI) of cytarabine over 24 hours for 7 days; which can pose a challenge for outpatient administration; in this study, outpatient infusion pumps were available to allow for continuous intravenous infusion of cytarabine. Based on results of the study, investigators concluded that outpatient induction chemotherapy was feasible in select patients who were treated by a multidisciplinary team including medical providers, nurses, pharmacists, and social workers [16].
Liposomal cytarabine and daunorubicin (CPX- 351)
Outpatient administration of the liposomal formulation of cytarabine and daunorubicin (CPX-351) has been evaluated in both the consolidation and induction settings with results that suggest the feasibility and safety of this approach in specialized settings.
In select patients, there is the potential to administer CPX-351 as outpatient therapy on days 1, 3 and 5, with close daily monitoring (including evaluation of serum uric acid, phosphate, complete blood count, and comprehensive metabolic panel), and with planned admission to the hospital on day 6 for continued care throughout the nadir period until count recovery [17]. At Moffitt Cancer Center, this approach has been implemented via the Inpatient/ Outpatient (IPOP) program, a multidisciplinary teambased model that has successfully transitioned multiple traditionally inpatient chemotherapy regimens into the outpatient setting [18]. Specifically, the feasibility of outpatient CPX-351 administration was evaluated with a planned admission on day 6 until adequate count recovery, or approximately 30 days. Pilot data from this program suggest that the IPOP approach is safe and significantly reduces the length of hospitalization in patients with secondary AML [17]. Of 22 patients receiving a full course of induction with CPX-351, 14 (64%) received induction in IPOP, while 8 had inpatient induction. One patient was admitted due to hypotension and a fall, while the other 13 outpatients (93%) tolerated the induction regimen and, as planned, were admitted on day 6 to receive continued care. The mean duration of hospitalization was 28.3 days for patients who received outpatient induction and 30.5 days for inpatient administration. These results warrant larger studies to evaluate the patient outcomes, safety, and financial implications of decreased hospital utilization [17,19].
In a randomized phase 2 clinical trial that led to Food and Drug Administration (FDA) fast track approval of CPX-351, 37 of 85 patients randomized to this treatment received consolidation as outpatients, and following treatment, CPX-351 improved the duration and proportion of time spent as an outpatient as compared to 7+3 treatment [20]. In addition, a substantial proportion of subjects received CPX-351 as consolidation in a randomized phase 3 trial of CPX-351 versus 7+3 in older patients with newly diagnosed secondary AML [21]. In primary results of that study, CPX-351 had a similar safety profile as compared to 7+3, but significantly prolonged overall survival (9.56 vs 5.95 months, respectively; 1-sided P = .003) and resulted in a higher overall remission rate (47.7% vs 33.3%, respectively; 2-sided P = .016) [21]. In a subsequent analysis of consolidation outcomes, CPX-351 was administered completely in the outpatient setting for 51% of patients during the first consolidation cycle, and in 61% during the second cycle; by contrast, 7+3 consolidation was completely outpatient in just 6% of patients during the first cycle and in no patients in the second cycle. Moreover, outpatient administration did not diminish the overall survival benefit, and safety was consistent with what was previously observed and reported in the primary analysis. Based on these findings, investigators said CPX- 351 could be administered successfully on an outpatient basis, with careful monitoring [8]. The feasibility of safely managing AML patients receiving this treatment in the outpatient setting is being prospectively evaluated in an ongoing phase 4 study that will include an estimated 120 participants (NCT03988205) [22].
Consolidation Regimens
Consolidation treatment for AML is increasingly administered in the outpatient setting, with a number of studies supporting the feasibility, safety, and costeffectiveness of this approach [23-26]. However, administration of conventional post-remission therapy (e.g., high-dose cytarabine (HDAC) can be challenging in the outpatient setting due to the required infusion times. By contrast, CPX-351 consolidation is given over 90 minutes on days 1 and 3, facilitating administration in the inpatient or outpatient setting [7]. From the perspective of minimizing resource utilization, it is notable that the HDAC-123 regimen (i.e., delivery condensed to days 1, 2, and 3) induces faster hematological recovery and considerably reduces length of hospital stay as compared to the day 1, 3, and 5 delivery of HDAC that is standard in younger adult AML patients [27,28], and may also be viewed favorably by the patients by avoiding “off days” during consolidation.
Lower Intensity Treatments
Panelists generally agreed that lower intensity regimens, including venetoclax plus HMA, venetoclax plus LDAC, glasdegib plus LDAC, and targeted agents including enasidenib, ivosidenib, and gilteritinib, can be administered safely in the outpatient setting for the majority of newly diagnosed AML patients. With the targeted agents such as enasidenib, ivosidenib and gilteritinib, appropriate cytoreduction with hyroxyurea along with stringent clinical monitoring should be considered at the time of therapy initiation for a patient with proliferative AML. As such, outpatient initiation of such therapy can safely commence without an upfront hospitalization in an otherwise clinically stable patient. Venetoclax-based combinations (venetoclax + HMA, venetoclax + LDAC), which may be viewed as more of an intermediate-intensity option, and may require risk stratification for consideration of upfront hospitalization due to the potential for TLS.
The potent apoptotic effect of BCL-2 inhibition with venetoclax makes TLS a significant risk of treatment [29]. However, incidence of this adverse effect appears to be higher in studies of chronic lymphocytic leukemia (CLL) as compared to the pivotal studies in AML [9]. TLS incidence was noted to be up to 13% with initial phase 1 studies in CLL, which had shorter ramp-up phase and higher starting doses. This was mitigated with slower weekly ramp-up as well as lower starting doses and careful assessments of the disease for the risk of TLS [10]. In AML, the risk of TLS was noted to be 1% in VIALE-A study (azacitidine + venetoclax) [30] and 5.6% in the VIALE-C study (venetoclax + LDAC) [31]. Thus, 3-5 day ramp-up of venetoclax in AML is acceptable compared to slower weekly ramp up in patients with CLL. Potential risk factors for TLS in AML include bulky disease, leukocytosis, high levels of lactate dehydrogenase, hyperuricemia, and concomitant kidney disease, indicating that uric acidlowering therapy with allopurinol or rasburicase may be important. Caution also may be warranted in AML patients with NPM1 and IDH1/2 mutations, who may also have increased risk of TLS due to an increased sensitivity to venetoclax in these mutation-defined subgroups of AML [9]. Furthermore, Esparza and co-authors reported on 2 patients with venetoclax-induced TLS that had similar genomic profiles (ASXL1, RUNX1, and TET2 mutations), suggesting the potential of this mutational spectrum as a predictor of venetoclax sensitivity [32].
For AML patients starting on venetoclax plus HMA or LDAC, hospitalization has been recommended during the initial venetoclax ramp-up dosing schedule that is designed to decrease TLS risk [10,33]. Current clinical practice guidelines from the National Comprehensive Cancer Network (NCCN) strongly recommend inpatient treatment, particularly through dose escalation, and also indicate that hospitalization may be needed beyond the first cycle and that outpatient treatment may be considered based on institutional practice or treatment preference [11]. In addition, with infection prevention in mind, DiNardo and Wei recently recommended considering hospitalization until hematologic recovery for some patients with high complication risk or poor social support networks [34].
The recommendation for inpatient management during venetoclax ramp-up is based in part on the protocols of pivotal AML trials, which required initial hospitalization for TLS assessment and prophylaxis [30,3]. However, recently reported clinical experience indicates that venetoclax ramp-up may be safe with monitoring in the outpatient setting. The safety of outpatient venetoclax ramp-up when given along with HMAs for AML was evaluated in a recent retrospective study including 43 patients with AML, including 22 who received the combination as frontline treatment; there was no clinical TLS in this cohort, and only one episode of laboratory TLS in a patient with a pretreatment WBC count greater than 25 x 109/l, which subsequently resolved following hospital admission for intravenous fluids and rasburicase. The results suggested outpatient venetoclax ramp-up can be safe with careful monitoring [35]. Furthermore, it has been noted that the low TLS incidence seen in the pivotal AML venetoclax combination trials were achieved with careful attention to preventive measures; based on those reassuring data, treatment might be started in an appropriate outpatient setting that includes monitoring and preventive measures such as proper hydration, potential use of uric-acidlowering agents, and adjusted ramp-up dosing in patients on CYP3A4 inhibitors, along with use of hydroxyurea to lower white blood cell (WBC) count below 25 x 109/l before starting venetoclax [36].
Ultimately, panelists reported varying approaches to venetoclax ramp-up (i.e., inpatient or outpatient) due to institutional preferences or potential challenges in obtaining the medication in a timely manner. Several of the panelists’ institutions stock venetoclax in their inpatient pharmacy allowing for rapid inpatient initiation while awaiting drug procurement via outpatient specialty pharmacy. However, many institutions do not carry venetoclax on their formulary; before venetoclax can be initiated in this situation the drug must be processed via the patient’s insurance and then dispensed by the outpatient pharmacy. While some institutions favor starting and escalating venetoclax in the inpatient setting, others consistently and routinely administer venetoclax entirely on an outpatient basis. Regardless, panelists agreed starting venetoclax in the outpatient setting is not unreasonable provided vigilant monitoring for TLS and appropriate institution of uricosuric agents.
Treatment Decision Algorithm
A proposed algorithm to inform the potential feasibility of AML outpatient treatment in community practice is shown in Figure 2. Although not intended to be comprehensive or prescriptive, this algorithm illustrates key factors that are typically considered in the treatment decision making process. The first step is determining patient fitness for outpatient treatment, and may be based on a variety of factors including clinical presentation, performance status, disease characteristics, leukemia subtype, and assessment of underlying comorbidities.
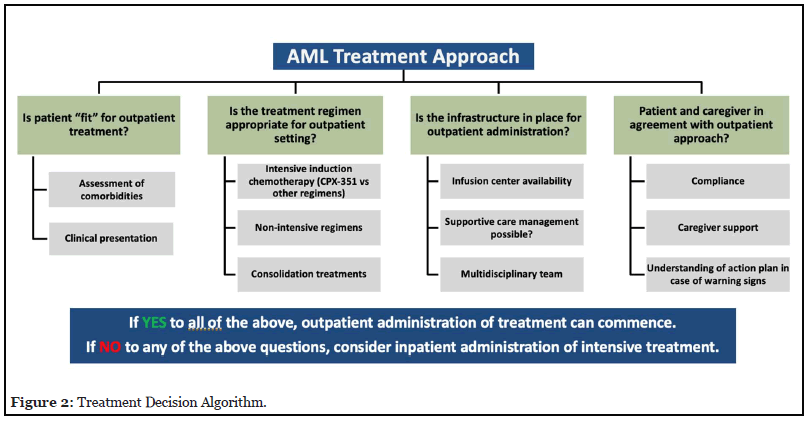
Type of treatment regimen is also an important consideration in deciding whether outpatient administration of intensive chemotherapy is feasible. While most intensive induction chemotherapy regimens should be administered in the hospital, CPX-351 potentially can be given on an outpatient basis; in the Moffitt Cancer Center experience, this is done in a selected subgroup of patients with careful monitoring and with planned admission during nadir period until count recovery [17,18]. Lower-intensity regimens are generally acceptable to administer on an outpatient basis; however, for venetoclax combination regimens (venetoclax + HMA or LDAC), inpatient administration is generally recommended though dose escalation [11].
Outpatient administration may not always be feasible from the standpoint of the treating facility, which must have adequate infrastructure in place, including infusion center availability, and a multidisciplinary team (e.g., physicians, nursing, pharmacy, advanced practice providers, and others) to manage the patient. In particular, panelists noted that CPX-351 outpatient administration can be cumbersome without proper infrastructure in place for daily multidisciplinary patient support and monitoring.
Finally, and perhaps most importantly, it is critical that patients and caregivers are comfortable with the outpatient approach and understand the need for ongoing assessment and monitoring. They should be willing and able to comply with the follow-up plan (including consideration of potential logistical barriers such as travel) and should be ready to proactively report symptoms that may require prompt evaluation. With all of the preceding factors in place, outpatient treatment may be considered; otherwise, an inpatient approach to treatment may be warranted.
Patient Communication and Shared Decision Making
A diagnosis of AML is particularly challenging and overwhelming for most patients and caregivers due to sudden changes in health, the complexity of disease and treatment information, and urgency to make quick decisions that have serious consequences [37]. Due to growing complexity of AML risk stratification and the approval of multiple new treatment options, it is increasingly important that clinicians help patients make informed decisions that balance risk of relapse with patient preferences and goals of treatment, including desired quality of life [38]. Considering the perspective of the AML patient and caregiver is vitally important when weighing inpatient versus outpatient treatment approaches. While some patients will perceive the value in avoiding hospitalization, others may view outpatient therapy as inconvenient due to distance from the treatment center, need to coordinate travel, or other logistical factors. Additionally, caregivers may be overwhelmed by the expectations for patient support during treatment nadirs.
Shared decision making can be useful when more than one treatment choice would be medically appropriate for a patient with newly diagnosed AML (e.g., an intensive versus a less intensive option) [38,39]. Although treatment recommendations for an individual patient should be based on what members of the care team feel is most medically appropriate, panelists agreed that patients and caregivers should be actively involved in the treatment decision making process to the extent they feel comfortable doing so. When multiple initial treatment choices may be appropriate, guiding patients and caregivers through the relative advantages and disadvantages of each option, along with setting realistic expectations for treatment outcomes and potential adverse events, may help lead the patient/caregiver to an option that aligns with their preferences, goals, and values.
In general, panelists recommended a metered approach to providing treatment and disease information (i.e., over the course of multiple discussions) to avoid overwhelming the patient or caregiver, and to improve digestion and retention of information that will be useful in making an informed treatment decision. All members of the multidisciplinary team can be encouraged to contribute meaningfully to the communication effort. Communicating in simple, easyto- understand language may enhance patient/caregiver comprehension of critical information; toward that end, patient education materials from trusted sources such as the MDS Foundation, the Leukemia & Lymphoma Society, or the National Comprehensive Cancer Network can be useful if in-house resources are lacking, and when healthcare provider time is too limited to provide extensive background information.
Educational Gaps for Healthcare Providers
The expert panel identified substantial educational needs for clinicians that provide care for patients with AML and provided recommendations to overcome knowledge gaps regarding outpatient therapy. These issues ranged from management of cytopenias in the outpatient setting to the need for partnerships between academia and community practice to educate on the nuances of optimal outpatient AML therapy administration. Other key practice gaps and challenges include more clearly defining the potential benefits and drawbacks of outpatient therapy in relation to inpatient therapy, defining the ideal approach to outpatient therapy for both intensive and less intensive regimens. The COVID-19 pandemic has introduced unique challenges and considerations in the care of AML, including the need to weigh management of an acute and potentially lethal illness that may need aggressive treatment against the barriers introduced by inpatient stays and clinic visits [40]. The ideal setting for therapy remains unclear, particularly with issues surrounding inpatient and outpatient facility guidelines for navigating the pandemic (e.g. visitor policies).
Case-based education on specific patient scenarios in which outpatient therapy was suggested as a useful tool to address these challenges. In addition, practical strategies for incorporating shared decision making into clinical practice are needed, particularly when multiple AML treatment options are medically appropriate. Although shared decision making is vitally important to the optimal management of acute leukemias, many clinicians misunderstand this patient-centered process or may lack the resources to apply it for patients with AML. The aforementioned unique considerations in AML further complicate and confound decision making [37,39]. Based on these identified needs, continuing education focused on outpatient AML therapy is warranted and planned.
Conclusion
The outpatient administration of AML treatment is a therapeutic strategy with the potential to curb costs, ensure judicious use of healthcare resources, and improve patient quality of life. Historically, hospitalization has been the standard of care for most intensive induction chemotherapy regimens. Newer AML regimens such as CPX-351 and venetoclax combination regimens are logistically feasible for outpatient administration due to their dosing schedules. Reported clinical experience, albeit based on small studies to date, demonstrates that outpatient administration may be safely administered without compromise in outcomes when appropriate patient factors are taken into consideration and multidisciplinary team support and careful monitoring are provided. Although recommendations for inpatient administration remain in place, outpatient administration and/or monitoring may allow for improved patient quality of life and reduced healthcare expense in the right clinical setting and patient. Further research is needed to ensure safety and to provide specific recommendations regarding logistics of successful outpatient treatment.
Acknowledgements
Supported by an educational grant from Jazz Pharmaceuticals to MediCom Worldwide, Inc.
The authors gratefully acknowledge writing and editorial assistance from Andrew D. Bowser, ELS, CHCP, of IconCME, Narberth, Pa. (abowser@iconcme.com)
Author Contributions Statement
The initial draft of this manuscript was created by Andrew D. Bowser of IconCME, based in part on proceedings of two online panel meetings organized by MediCom Worldwide, Inc. All authors attended and participated in the panel meetings. All authors subsequently contributed to the review, editing, and revision of the manuscript.
Conflict of Interest Disclosures
Dr. Chetasi Talati has received honoraria related to formal advisory activities from Bristol-Myers Squibb Company, speakers’ bureau activities from Astellas Pharma US, Inc. and Jazz Pharmaceuticals plc, as well as consultant fees from Daiichi Sankyo, Inc. and Pfizer Inc.
Dr. Harry Erba has received consultant fees from AbbVie Inc., Agios, Astellas Pharma US, Inc., Celgene Corporation - A Bristol-Myers Squibb Company, Daiichi Sankyo, Inc., GlycoMimetics, Inc., Incyte Corporation, Jazz Pharmaceuticals plc, and Novartis AG, as well as honoraria related to speakers’ bureau activities from AbbVie, Agios, Celgene Corporation - A Bristol-Myers Squibb Company, Incyte, Jazz, and Novartis. He has received grant support related to research activities from AbbVie, Agios, Amgen Inc., Daiichi Sankyo, FORMA Therapeutics, Inc., Forty- Seven Inc., GlycoMimetics, ImmunoGen, Inc., Jazz, MacroGenics, Inc., Novartis, and PTC Therapeutics.
Dr. Daniel Pollyea has received honoraria as a consultant from AbbVie Inc, Celgene Corporation - A Bristol-Myers Squibb Company, Genentech, Inc., Kiadis Pharma, Karyopharm Therapeutics, Novartis AG, Syndax Pharmaceuticals, Inc., and Syros Pharmaceuticals, Inc. He has received grant support related to research activities from AbbVie.
Dr. Kendra Sweet has received honoraria related to formal advisory activities from Astellas Pharma US, Inc., Bristol-Myers Squibb Company, Novartis AG, and Takeda Oncology, and consultant fees from Stemline Therapeutics, Inc. She has received grant support related to research activities from Incyte Corporation
Dr. Jennifer Eatrides has received honoraria related to formal advisory activities from Amgen Inc.
Dr. Sandra Kurtin has received honoraria related to formal advisory activities and as a consultant from Amgen Inc., AstraZeneca, Bristol-Myers Squibb Company, Celgene Corporation - A Bristol-Myers Squibb Company, Incyte Corporation, Jazz Pharmaceuticals plc, Novartis AG, Pharmacyclics, Inc., and Takeda Oncology.
Dr. Jeff Klaus has received honoraria related to speakers’ bureau activities from Astellas Pharma US, Inc., Merck & Co., and Jazz Pharmaceuticals, plc.
References
2. Mayer RJ, Davis RB, Schiffer CA, Berg DT, Powell BL, Schulman P, et al. Intensive postremission chemotherapy in adults with acute myeloid leukemia. New England Journal of Medicine. 1994 Oct 6;331(14):896-903.
3. Appelbaum FR. Indications for allogeneic hematopoietic cell transplantation for acute myeloid leukemia in the genomic era. American Society of Clinical Oncology Educational Book. 2014 Jan 1;34(1):e327-33.
4. Halpern AB, Walter RB, Estey EH. Outpatient induction and consolidation care strategies in acute myeloid leukemia. Current Opinion in Hematology. 2019 Mar 1;26(2):65-70.
5. Vaughn JE, Buckley SA, Walter RB. Outpatient care of patients with acute myeloid leukemia: benefits, barriers, and future considerations. Leukemia Research. 2016 Jun 1;45:53-8.
6. Halpern AB, Walter RB. Practice patterns and outcomes for adults with acute myeloid leukemia receiving care in community vs academic settings. Hematology 2014, The American Society of Hematology Education Program Book. 2020 Dec 4;2020(1):129-34.
7. Daunorubicin and cytarabine liposome for injection, for intravenous use [prescribing information]. Palo Alto, CA: Jazz Pharmaceuticals, Inc.; March, 2021.
8. Kolitz JE, Strickland SA, Cortes JE, Hogge D, Lancet JE, Goldberg SL, et al. Consolidation outcomes in CPX- 351 versus cytarabine/daunorubicin-treated older patients with high-risk/secondary acute myeloid leukemia. Leukemia & Lymphoma. 2020 Feb 23;61(3):631-40.
9. Samra B, Konopleva M, Isidori A, Daver N, DiNardo C. Venetoclax-Based Combinations in Acute Myeloid Leukemia: Current Evidence and Future Directions. Frontiers in Oncology. 2020 Nov 5;10: 562558.
10. Venetoclax tablets, for oral use [prescribing information]. Noorth Chicago, IL: AbbVie Inc.; 2020.
11. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Acute Myeloid Leukemia. Version 2.2021 - November 12, 2020. Accessed February 10, 2021. https:// www.nccn.org/professionals/physician_gls/pdf/aml.pdf
12. Sekeres MA, Guyatt G, Abel G, Alibhai S, Altman JK, Buckstein R, et al. American Society of Hematology 2020 guidelines for treating newly diagnosed acute myeloid leukemia in older adults. Blood Advances. 2020 Aug 11;4(15):3528-49.
13. Short NJ, Montalban-Bravo G, Hwang H, Ning J, Franquiz MJ, Kanagal-Shamanna R, et al. Prognostic and therapeutic impacts of mutant TP53 variant allelic frequency in newly diagnosed acute myeloid leukemia. Blood Advances. 2020 Nov 24;4(22):5681-9.
14. Halpern AB, Howard NP, Othus M, Hendrie PC, Baclig NV, Buckley SA, et al. Early hospital discharge after intensive induction chemotherapy for adults with acute myeloid leukemia or other high-grade myeloid neoplasm. Leukemia. 2020 Feb;34(2):635-9.
15. Halpern AB, Othus M, Howard NP, Hendrie PC, Percival ME, Scott BL, et al. Comparison of outpatient care following intensive induction versus post-remission chemotherapy for adults with acute myeloid leukemia and other high-grade myeloid neoplasms. Leukemia & Lymphoma. 2021 Jan 2;62(1):234-8.
16. Mabrey FL, Gardner KM, Shannon Dorcy K, Perdue A, Smith HA, Davis AM, et al. Outpatient intensive induction chemotherapy for acute myeloid leukemia and high-risk myelodysplastic syndrome. Blood Advances. 2020 Feb 25;4(4):611-6.
17. Kubal TE, Salamanca C, Komrokji RS, Sallman DA, Sweet KL, Padron E, et al. Safety and feasibility of outpatient induction chemotherapy with CPX-351 in selected older adult patients with newly diagnosed AML. Journal of Clinical Oncology. 2018;36(15_suppl):e19013-e19013.
18. Kubal TE, Salamanca C, Tobon K, Lubas A, Frantz DK, Nelson R, et al. Successful creation of an outpatient teambased model for traditionally inpatient chemotherapy. Journal of Clinical Oncology. 2019;37(27_suppl):86-86.
19. Deutsch YE, Presutto JT, Brahim A, Raychaudhuri J, Ruiz MA, Sandoval-Sus J, et al. Safety and feasibility of outpatient liposomal daunorubicin and cytarabine (Vyxeos) induction and management in patients with secondary AML. Blood. 2018 Nov 29;132(Supplement 1):3559.
20. Lancet JE, Cyr P, Sacks N, Chiarella MT, Louie AC, Cortes JE. CPX-351 enables administration of consolidation treatment in the outpatient setting and increases the time spent out of the hospital after completion of AML treatment compared with 7+ 3. Blood. 2015;126(23):4507.
21. Lancet JE, Uy GL, Cortes JE, Newell LF, Lin TL, Ritchie EK, et al. CPX-351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia. Journal of Clinical Oncology. 2018 Sep 10;36(26):2684-92.
22. Michaelis L. The Feasibility of Safely Managing Patients Receiving Induction with Liposomal Daunorubicin and Cytarabine (CPX-351) for Acute Myeloid Leukemia (AML) in an Outpatient Environment. clinicaltrials.gov; 2019. Accessed April 19, 2021. https://clinicaltrials.gov/ ct2/show/NCT03988205
23. Allan DS, Buckstein R, Imrie KR. Outpatient supportive care following chemotherapy for acute myeloblastic leukemia. Leukemia & Lymphoma. 2001 Jan 1;42(3):339-46.
24. Saini L, Minden MD, Schuh AC, Yee KW, Schimmer AD, Gupta V, et al. Feasibility of outpatient consolidation chemotherapy in older versus younger patients with acute myeloid leukemia. American Journal of Hematology. 2012 Mar;87(3):323-6.
25. Eisele L, Guenther F, Ebeling P, Nabring J, Duehrsen U, Duerig J. Outpatient management of acute myeloid leukemia after intensive consolidation chemotherapy is feasible and reduces hospital treatment costs. Oncology Research and Treatment. 2010;33(12):658-64.
26. Møller T, Nielsen OJ, Welinder P, Dünweber A, Hjerming M, Moser C, et al. Safe and feasible outpatient treatment following induction and consolidation chemotherapy for patients with acute leukaemia. European Journal of Haematology. 2010 Apr;84(4):316-22.
27. Jaramillo S, Benner A, Krauter J, Martin H, Kindler T, Bentz M, et al. Condensed versus standard schedule of highdose cytarabine consolidation therapy with pegfilgrastim growth factor support in acute myeloid leukemia. Blood Cancer Journal. 2017 May;7(5):e564.
28. Dumas PY, Bertoli S, Bérard E, Leguay T, Tavitian S, Galtier J, et al. Delivering HDAC over 3 or 5 days as consolidation in AML impacts health care resource consumption but not outcome. Blood Advances. 2020 Aug 25;4(16):3840-9.
29. McBride A, Houtmann S, Wilde L, Vigil C, Eischen CM, Kasner M, et al. The role of inhibition of apoptosis in acute leukemias and myelodysplastic syndrome. Frontiers in Oncology. 2019 Mar 27;9:192.
30. DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. New England Journal of Medicine. 2020 Aug 13;383(7):617-29.
31. Wei AH, Montesinos P, Ivanov V, DiNardo CD, Novak J, Laribi K, et al. Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: a phase 3 randomized placebo-controlled trial. Blood, The Journal of the American Society of Hematology. 2020 Jun 11;135(24):2137-45.
32. Esparza S, Muluneh B, Galeotti J, Matson M, Richardson DR, Montgomery ND, et al. Venetoclax-induced tumour lysis syndrome in acute myeloid leukaemia. British Journal of Haematology. 2020 Jan;188(1):173-7.
33. Jonas BA, Pollyea DA. How we use venetoclax with hypomethylating agents for the treatment of newly diagnosed patients with acute myeloid leukemia.Leukemia. 2019 Dec;33(12):2795-804.
34. DiNardo CD, Wei AH. How I treat acute myeloid leukemia in the era of new drugs. Blood, The Journal of the American Society of Hematology. 2020 Jan 9;135(2):85- 96.
35. Pelcovits A, Bakow B, Waroich J, Egan PC, Niroula R, Olszewski AJ, et al. Tumor Lysis Syndrome Risk in Outpatient Versus Inpatient Administration of Venetoclax and Hypomethlators for Acute Myeloid Leukemia. Blood. 2020;136(Supplement 1):6-7. doi:10.1182/ blood-2020-133880
36. Richard-Carpentier G, DiNardo CD. Venetoclax for the treatment of newly diagnosed acute myeloid leukemia in patients who are ineligible for intensive chemotherapy. Therapeutic Advances in Hematology. 2019 Oct;10:2040620719882822.
37. LeBlanc TW, Fish LJ, Bloom CT, El-Jawahri A, Davis DM, Locke SC, et al. Patient experiences of acute myeloid leukemia: A qualitative study about diagnosis, illness understanding, and treatment decision-making. Psycho- Oncology. 2017 Dec;26(12):2063-8.
38. Walker AR. How to approach shared decision making when determining consolidation, maintenance therapy, and transplantation in acute myeloid leukemia. Hematology 2014, the American Society of Hematology Education Program Book. 2020 Dec 4;2020(1):51-6.
39. LeBlanc TW. Shared decision-making in acute myeloid leukemia. InSeminars in oncology nursing 2019 Dec 1 (Vol. 35, No. 6, p. 150958). WB Saunders.
40. Paul S, Rausch CR, Jain N, Kadia T, Ravandi F, DiNardo CD, et al. Treating leukemia in the time of COVID-19. Acta Haematologica. 2021;144(2):132-45.
