Abstract
Microbial resistance to antibiotics has become a major area of research having caused over a million human deaths in 2019. At present, lower respiratory infection is the most burdensome disease. Antimicrobial Photodynamic Therapy (PDT) is regularly reported not to cause resistance in any pathogen, and to eradicate both microbes that are susceptible to antibiotics and those that are resistant. However, evidence now suggests that resistance to photosensitiser drugs at low concentrations is possible, and that tolerance to the reactive oxygen species (ROS) created during subsequent light exposure will occur eventually. Additionally, an increased optical fluence and the addition of medication have been necessary to destroy antibiotic resistant strains of Staphylococcus Aureus. Research has mostly focussed on bacteria, though the importance of fungi is highlighted here given the ubiquity of clinical manifestations like mycosis and urinary tract infection. Proposed next steps are the definition of terminology and methodology for experiments on microbial resistance/tolerance to PDT, varying the photosensitiser used for repeat PDT while controlling oxygen and salt levels, and alternative treatments including the interception of neuroimmunological signalling. Similar to the ESKAPE ranking of antibiotic resistant pathogens, a list summarising the degree of microbial resistance to PDT may have a place.
Short Communication
Microbial resistance to antibiotics has become a major area of research given that it caused 1.27 million human deaths in 2019. Methicillin-resistant staphylococcus aureus (MRSA) accounted for half these deaths, and lower respiratory infection is the most burdensome syndrome [1]. Photodynamic therapy is an alternative to antibiotics for treating infection and its use is supported by the following rationale: 1) The technique does not cause resistance to develop in microbes; and 2) Its action is unaffected by a microbes existing drug resistance status [2-4]. The purpose of this article is to discuss the validity of these stock phrases by considering the mechanisms by which resistance to any treatment occurs in pathogens. Th author will also discuss the extent to which antimicrobial stewardship, including the introduction of alternative methods to antibiotics (i.e. PDT), has already influenced the antibiotic resistome.
To consider the first statement, it is necessary to define what we mean by the term ‘resistance.’ It refers to a microbe’s ability to adapt to a drug so that it is no longer effective in its host or those acquiring the microbe or its progeny. Bacteria develop resistance to drugs over time by 1) modification of the drug target; 2) increased cell efflux of the drug; 3) reduced uptake of the drug; or 4) inactivation of the drug [5], with similar mechanisms described in parasites [6]. Fungi, however, have shown epigenetic changes after repeat exposures to the current antifungal agents [7]. Antiviral agents on the other hand, damage the DNA causing mutation of the nuclear enzyme thymidine kinase (TK) [8]. Future drug treatments may introduce additional mechanisms of resistance.
Liu et al. [9] have stated that bacteria cannot develop resistance to a photosensitiser used for PDT because the druglight interval is too short. These pharmacokinetic constraints are similar for bacteria, viruses, fungi, and parasites, reflected by similar incubation periods during clinical applications [10]. The incubation period can be minimised by using cationic photosensitisers which are taken up rapidly by microbial cells compared to anionic versions [11] especially in Gramnegative bacteria [12]. Other authors have found that low concentrations of a photosensitiser can cause incomplete damage to bacteria and the development of inheritable resistance [13].
Mechanisms of resistance to photosensitisers in bacteria are known to be similar to those observed to antibiotics. When considering the combination of drugs and light, it has been proposed that metabolic adaptations cannot happen because bacteria are unable to sense where the oxidative stress comes from [14]. Or ROS created during PDT target such a variety of bacterial cell structures and metabolic pathways that it will take longer for tolerance to develop at any one site [14]. Tolerance results in the need for longer light exposures during PDT to achieve the same degree of microbial killing given a photosensitiser dose [14]. Increasing the irradiance of the light source within safe limits [15-16] could increase fluence without extending the duration of exposure, and there is a need for experiments identifying which method minimises the development of further tolerance.
One mode of resistance during PDT occurs as a result of tissue hypoxia secondary to vascular damage and photochemical oxygen consumption [17]. It would be interesting to know how modes differ between microbial classes- it is postulated that fungi and viruses possess few routes to death and so could build up tolerance quite quickly. Type 1 and 2 reactions are simply the different ways in which oxygen, a physiological substrate, light and drugs interact to destroy the microbe. The yield of type I and type II reactions during PDT varies by substrate, photosensitiser and the local level of oxygen [18]. So, for an infection requiring repeat treatments, varying the photosensitiser and the available oxygen will ensure a variety of ROS are created and the full range of microbial targets are enabled and reduce the rate at which tolerance develops. Additionally, the use of salts maximises the effect of ROS on a given target [19], presumably minimising treatment duration and the likelihood of tolerance developing.
The second statement often made by those endorsing PDT is that its utility is unaffected by the drug resistance status of microbes [3]. However, microscopic lab observations of Deuteroporphyrin PDT of Staphylococcus Aureus report a larger light fluence and adjuvant Oxacillin during eradication of antibiotic resistant isolates of S. Aureus compared to susceptible ones [20]. It has also been found that while PDT of sufficient dose can inactivate both susceptible and resistant bacterial strains, the likelihood of selecting the latter is much lower [21].
The six ESKAPE pathogens were those most common in nosocomial infection up to 2019 [22] -‘ESKAPE’ being an acronym of their first letters (Table 1). A 33% change to this list has occurred since 2019 (Table 2) [1] and while the latter study only considered bacteria, it is assumed that the other microbial classes remain outside the top six. The deadliest pathogen in 2019, Gram-positive Enterococcus Faecium, no longer appears in the list, implying it has been managed successfully. Escherichia Coli replaced it suggesting it in 2021 may have been overlooked when treatments strategies focussed exclusively on the original top six. Independent of resistance status, Gram-negative bacteria are more difficult to kill than Gram-positive bacteria [11] and this status could add to the challenge of E. coli eradication.
| EnterococcusFaecium | Gram-positive |
| Staphylococcus Aureus | Gram-positive |
| KlebsiellaPneumoniae | Gram-negative |
| Actinebacter Baumanni | Gram-negative |
| Psuedomonas Aeruginosa | Gram-negative |
| Enterobacter Spp | Gram-negative |
| Escherichia Coli | Gram-negative |
| Staphylococcus Aureus | Gram-positive |
| KlebsiellaPneumoniae | Gram-negative |
| Streptococcus Peneumoniae | Gram-positive |
| Actinebacter Baumanni | Gram-negative |
| Pseudomonas Aeruginosa | Gram-negative |
While bacteria dominate rankings of lethal microbes, urinary tract infection (UTI) can be caused by Candida spp [23] and is a common cause of Sepsis [24]. Additionally, Twenty percent of the population are affected by mycosis, which is often chronic [25] and therefore prone to resistance to treatment. Mycosis also predisposes the sufferer to bacterial infection [25] making sustainable treatments for fungi vital.
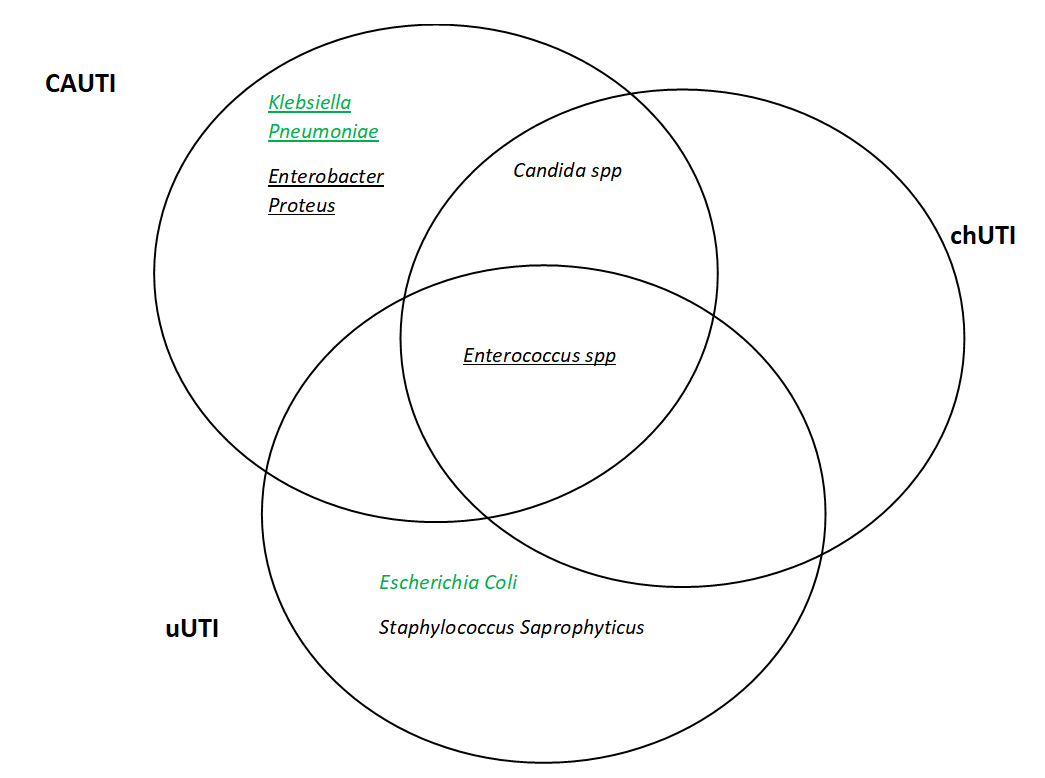
Focussing on the bladder, the Venn diagram in Figure 1 one categorises microbes by their presence in the three recognised categories of UTI and illustrates which appear in the lists of the six deadliest pathogens (Tables 1 and 2). The Enterococcus genre are common to all types of infection but have proven to be manageable since these data were presented, perhaps by virtue of appearing on the ESKAPE list as stated earlier. The Gram-negative Enterobacter proteus has also reduced since 2019, implying treatment methods might have improved too. However, Klebsiella Pneumoniae (also Gram-negative) endures as the third most deadly pathogen in keeping with the burden of respiratory infection [1]. The relative difficulty in accessing the lungs for treatment compared to the bladder may provide an explanation for its persistence.
Figure 1. Pathogens associated with the three types of urinary tract infection (UTI: uncomplicated UTI (uUTI); Catheter Associated UTI (CAUTI); Complicated Human UTI (chUTI). The underlined organisms are on the 2019 list of ESKAPE pathogens. The green text highlights those that appear in the Lancets 2022 list.
In summary, microbial resistance to PDT is complex and defined terminology and methodology are proposed before taking further steps [13]. Alternative methods are desirable to prevent an upsurge in resistant microbes if PDT were to be overused. Recent dermatological work has summarised alternatives [4] but for infections affecting less accessible organs like the lungs, neuroimmunological signalling interception has proven to be effective [26]. The ranking list of deadly pathogens provides one method of monitoring the progress of an array of treatments on the antibiotic resistome. A future list describing the PDT resistome may also have a place.
Acknowledgements
Thank you to my parents Alistair and Margaret Mackay for feeding me and keeping me company. Also to my flatmate Ewan Hopes for being an excellent sounding board.
Funding
None.
Conflicts of Interest
None.
References
2. Maisch T, Eichner A, Späth A, Gollmer A, König B, Regensburger J, et al. Fast and effective photodynamic inactivation of multiresistant bacteria by cationic riboflavin derivatives. PloS One. 2014 Dec 3;9(12):e111792.
3. Hamblin MR. Antimicrobial photodynamic inactivation: a bright new technique to kill resistant microbes. Current Opinion in Microbiology. 2016 Oct 1;33:67-73.
4. Mackay AM. The evolution of clinical guidelines for antimicrobial photodynamic therapy of skin. Photochemical & Photobiological Sciences. 2022 Feb 7:1-1.
5. Reygaert WC. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiology. 2018;4(3):482.
6. Ertabaklar H, Malatyali E, Ertug S. Drug resistance in parasitic diseases. European Journal of Therapeutics. 2020 Mar 1;26:1-5.
7. Torres-Garcia S, Yaseen I, Shukla M, Audergon PN, White SA, Pidoux AL, et al. Epigenetic gene silencing by heterochromatin primes fungal resistance. Nature. 2020 Sep;585(7825):453-8.
8. Strasfeld L, Chou S. Antiviral drug resistance: mechanisms and clinical implications. Infectious Disease Clinics. 2010 Sep 1;24(3):809-33.
9. Liu Y, Qin R, Zaat SA, Breukink E, Heger M. Antibacterial photodynamic therapy: overview of a promising approach to fight antibiotic-resistant bacterial infections. Journal of Clinical and Translational Research. 2015 Dec 30;1(3):140.
10. Mackay AM. Antimicrobial Photodynamic Therapy of Human Skin. Journal of Dermatology & Skin Science. 2022;4(2):20-23.
11. Sperandio F, Huang YY, R Hamblin M. Antimicrobial photodynamic therapy to kill Gram-negative bacteria. Recent Patents on Anti-Infective Drug Discovery. 2013 Aug 1;8(2):108-20.
12. Hamblin MR, Hasan T. Photodynamic therapy: a new antimicrobial approach to infectious disease?. Photochemical & Photobiological Sciences. 2004;3(5):436-50.
13. Rapacka-Zdonczyk A, Wozniak A, Nakonieczna J, Grinholc M. Development of antimicrobial phototreatment tolerance: Why the methodology matters. International Journal of Molecular Sciences. 2021 Feb 23;22(4):2224.
14. Rapacka-Zdonczyk A, Wozniak A, Pieranski M, Woziwodzka A, Bielawski KP, Grinholc M. Development of Staphylococcus aureus tolerance to antimicrobial photodynamic inactivation and antimicrobial blue light upon sub-lethal treatment. Scientific Reports. 2019 Jul 1;9(1):1-8.
15. International Commission on Non-Ionizing Radiation Protection. ICNIRP Guidelines on Limits of Exposure to Laser Radiation of Wavelengths between 180 nm and 1,000 μm. Health Physics. 2013 Sep 1;105(3):271-95.
16. International Commission on Non-Ionizing Radiation Protection. ICNIRP guidelines on limits of exposure to incoherent visible and infrared radiation. Health Physics. 2013 Jul 1;105(1):74-96.
17. Casas A, Di Venosa G, Hasan T, Batlle A. Mechanisms of resistance to photodynamic therapy. Current Medicinal Chemistry. 2011 Jun 1;18(16):2486-515.
18. Robertson CA, Evans DH, Abrahamse H. Photodynamic therapy (PDT): a short review on cellular mechanisms and cancer research applications for PDT. Journal of Photochemistry and Photobiology B: Biology. 2009 Jul 17;96(1):1-8.
19. Kasimova KR, Sadasivam M, Landi G, Sarna T, Hamblin MR. Potentiation of photoinactivation of Gram-positive and Gramnegative bacteria mediated by six phenothiazinium dyes by addition of azide ion. Photochemical & Photobiological Sciences. 2014 Nov;13(11):1541-8.
20. Iluz N, Maor Y, Keller N, Malik Z. The synergistic effect of PDT and oxacillin on clinical isolates of Staphylococcus aureus. Lasers in Surgery and Medicine. 2018 Jul;50(5):535-51.
21. Gilaberte Y, Rezusta A, Juarranz A, Hamblin MR. Antimicrobial Photodynamic Therapy: A New Paradigm in the Fight Against Infections. Frontiers in Medicine. 2021:2084.
22. Mulani MS, Kamble EE, Kumkar SN, Tawre MS, Pardesi KR. Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance: a review. Frontiers in Microbiology. 2019 Apr 1;10:539.
23. Mackay AM. Sustainable Treatment of Female UTI. International Continence Society 2022.
24. Sabih A, Leslie SW. Complicated urinary tract infections. In StatPearls. Treasure Island (FL): StatPearls Publishing; 2022.
25. Shen JJ, Jemec GB, Arendrup MC, Saunte DM. Photodynamic therapy treatment of superficial fungal infections: A systematic review. Photodiagnosis and Photodynamic Therapy. 2020 Sep 1;31:101774.
26. Baral P, Umans BD, Li L, Wallrapp A, Bist M, Kirschbaum T, et al. Nociceptor sensory neurons suppress neutrophil and γδ T cell responses in bacterial lung infections and lethal pneumonia. Nature medicine. 2018 Apr;24(4):417-26.