Abstract
Lasers have gained rather broad application in the field of medicine, as well as in dentistry. Different wave lengths are appropriate for a variety of uses, such as hemostasis, antimicrobial effect, tissue section and excision. Low level laser therapy (LLLT) is particularly interesting, since it stands out for its biostimulatory effect on tissues.
The aim of the present review is the presentation of the broad spectrum of use of LLLT in the oral cavity, of the current literature on the matter and the dynamic lasers exhibit affecting different cell populations.
Without it having been completely clear, there is a mechanism that leads to analgesia after LLLT application. In the field of dentistry, LLLT is extremely useful for complementary use on the non-surgical treatment of periodontitis and peri-implant diseases, with very promising results. Experimentally, it seems to have a tremendously positive effect on the proliferation and differentiation of various cellular lines.
That event has a double benefit, both on the acceleration of wound healing and on the faster and better osseointegration of titanium implants. Although the use of lasers has yet to be broadly accepted for all potential applications, there is great field for further research that will lead to more reliable and long-term results. The great heterogeneity of settings, which are applied, implies the need for more studies, so that specific protocols can finally be announced. However, lasers certainly help with patient relief, which is after all a dentist’s main target. Undoubtedly, their correct use depends on the clinician’s deep theoretical knowledge and clinical skill, so that unfortunate events are avoided.
Keywords
Low Level Laser Therapy, LLLT, Human gingival fibroblasts, Soft laser therapy, Photobiomodulation, Wound healing, Inflammation
Introduction
Undoubtedly, the evolution of medicine is closely related to the field of basic sciences. Almost 70 years since the revolutionary work of Townes et al., at Columbia University, where forced emission of radiation was firstly achieved. When it comes to lasers used in the dental field, however, two general categories of treatment are available. First, there is High reactive-level Laser Treatment or HLLT which can be used in the treatment of periodontal diseases, for tissue ablation or tissue incision and excision. For example, Nd: YAG radiation is absorbed by haemoglobin, which makes it extremely useful when tissue ablation is necessary.
Lately, however, Low Level Laser Therapy or LLLT seems to be taking over the field. Many studies refer to it as ‘Photobiomodulation’, a term that comprises the promotion of cellular proliferation, collagen synthesis and analgesic action through various photoelectrical, photochemical and photophysical phenomena [1]. The biostimulatory nature of lasers had first been described by Gamaleya, back in 1977 [2]. In such therapies, the power applied reaches up to 500 mW or 0.5 W, while the energy density is usually between 2-4 J/cm2 [3]. Moreover, it is associated with small wavelengths, since the optimal results are achieved within the spectrum of red and near infrared radiation (540-1064 nm).
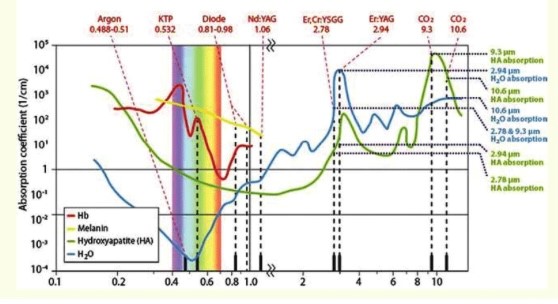
Figure 1: Absorption spectrum of laser wavelengths by various tissues.
According to the Arndt-Schultz law, ‘weak stimuli excite physiological activity, moderate stimuli favor it, and strong stimuli arrest it’. This means that ‘all desirable biological responses arise within a therapeutic window’ [4]. Lower energy will not lead to that result, whereas higher energies will have inhibitory action. This statement contains the logic behind LLLT, whose applications in the oral cavity will be discussed in this narrative review.
Mechanism of Action
Human eukaryotic cells contain some thousands of mitochondria, where the production of Adenosine triphosphate (ATP) takes place and are therefore, the main energy depository of the cell. The final step of the respiratory chain in mitochondria, which is Complex IV, transfers electrons from cytochrome c to heme a, to the a3-CuB centre and finally, to the molecular oxygen [5]. Under ischemic or stress conditions, nitrogen oxide (NO) is composed in the mitochondria, which antagonizes oxygen to the binding on cytochrome c oxidase. This chromophore molecule can absorb red and near infrared radiation, mainly due to the iron and copper ions that are parts of its subunits. Therefore, in a hypoxic cell, Low Level Laser Therapy causes the release of NO from cytochrome c oxidase, which leads to the re-binding of oxygen and ATP production, with the subsequent decrease of oxidative stress. Karu has also proposed ‘Retrograde Mitochondrial Signalling’, that starts with the absorption of a photon by Cox chromophore. Consequently, there is an increased ATP synthesis and alterations in the concentrations of ROS, NO and calcium. Moreover, modifications of the mitochondria structure, such as ATP synthesis and cAMP levels influence the nucleus [6].
An alternative theory, as far as the mechanism of action is concerned, regards the activation of photosensitive channels of ions, which allows the entrance of calcium in the cell [7]. Channelrhodopsins (ChRs) are the most researched of the kind, which can be found in several kinds of flora, such as the algae [8,9]. This family consists of seven transmembranedomain proteins, that upon activation with LLLT, open and induce membrane depolarization. Moreover, Transient Receptor Potential (TRP) channels are currently studied, which are calcium channels whose activity is mediated by phosphoinositides [10]. For example, Yang et al. have demonstrated that stimulation of mast cells with LLLT resulted in an increase of intracellular calcium ions and release of histamine. The role of those channels was shown, since upon use of TRPV4 inhibitor, the secretion of instamine was halted [ 11]. TRPV1 channels are also affected by photobiomodulation, which blocks their activation by capsaicin, thus restricting the production of pain stimuli in neurons in vitro [12].
Clinical Implications – Treatment of Periodontal Diseases and Conditions
Periodontal diseases, a major cause of tooth loss in adulthood, are treated with a series of therapeutic interventions that target bacteria, the main one being root scaling and planning. This process is highly intervening and causes trauma to the tissues, while their healing depends on various molecular and cellular responses.
As far as inflammation in general is concerned, Albertini et al. report its reduction after treatment with low-level laser irradiation [13]. Moreover, in vivo studies in rats have revealed a dose-dependent decrease in Tumor Necrosis Factor-a (TNF-a) levels with LLLT in acute inflammation [14]. Specifically, animals formerly injected with LPS, received four doses of 650nm-laser irradiation, with a total energy density of 5.2 J/cm2. LLLT seemed to reduce total TNF-a concentrations in a statistically significant manner. Bacteria, of course, are also cells and, based on this logic, Petrovic et al. searched the effect of LLLT on Agregibacter actinomycetecomitans, as well as Tannerella forsythia, Porphyromonas gingivalis and Treponema denticola. The latter three were characterized by Socransky et al., in 1998, as the most pathogenic bacteria in the oral cavity, mainly implicated in cases of severe periodontitis [15]. Petrovic used LLLT as an adjunctive to scaling and root planning, using 980 nm-laser irradiation on 30 patients, at 0.2 W power and 6 J/ cm 2 energy density. All the bacteria were significantly reduced in numbers (p<0.01) for the group that received LLLT [16]. This finding is particularly important as the target of periodontal therapy is not only the arithmetic decrease of bacteria, but also a qualitative switch of biofilms towards the domination of more symbiotic species.
The classical approach to periodontal therapy is scaling and root planning per quadrant and a subsequent four- to six-week healing period which may be followed by surgical intervention if required for pocket elimination. The adjunct role of LLLT is studied as far as its effect on the improvement of clinical signs of inflammation and pocket depth reduction is concerned, during the non-surgical phase of therapy. Quadri et al. have examined the results of laser irradiation, subjunctively after scaling and root planning. They used a diode laser, at 635 nm wavelength at 10 mW power for 90 s and at 820 nm wavelength at 70 mW for 25 s. Six consecutive sessions were performed, with a week’s time gap between them. All clinical parameters were significantly improved in the sites that received laser treatment. The authors have, therefore, concluded that adjunctive LLLT may indeed reduce inflammation of the periodontal tissues [17].
In a different study design, a comparison between a He-Ne laser at 632.8 nm wavelength and 3 mW power and a diode laser at 650 nm wavelength and 3 mW power was performed [18]. Apart from the wavelength, the rest of the parameters were common for both groups. There was a significantly greater reduction of the gingival index score and pocket depth where He-Ne laser was used. Therefore, wavelength certainly affects the outcome of the treatment, which is why each laser device must be used cautiously and with substantial knowledge of physics. Yang et al. have found many beneficial aspects to LLLT combined with non-surgical periodontal therapy, particularly in cases of smokers. The explanation for this lies on the positive effect of LLLT in microcirculation, collagen synthesis and cytokine secretion regulation [19].
However, the results of Riberio et al. are in absolute contrast to the above [20]. They used a diode laser of 780 nm wavelength at 70 mW power, for 20 seconds per site for analgesia and a 630 nm laser at 35 mW power for 10 seconds for healing. Patients were irradiated again after 24 and 48 hours. They did not reach similar conclusions since they found no clinical benefit for shallow to moderate pocket depths. Moreover, Calderin et al. have reported that LLLT in a single or more consecutive doses does not bring any significant reduction of clinical parameters [21].
The position of the American Academy of Periodontology is that there is still inadequacy of data to support the effectiveness of laser applications in non-surgical treatment of periodontal diseases (American Academy of Periodontology, Board of Trustees 2011).
Clinical Implications – Necrotizing Ulcerative Diseases of the Periodontium
Necrotizing diseases of the periodontium constitute a particularly unpleasant condition for the patient, since a common, basic symptom is acute pain. One case report is very engaging, since LLLT was performed, along with the rest therapeutic steps, in a patient with necrotizing ulcerative gingivitis. What was observed was the immediate pain relief, as well as a very uneventful healing process the next days. Despite there not being any reports on the clinical parameters, this study poses a great motive for clinicians to investigate the potential benefits of LLLT on such cases, where analgesia is key to the application of the rest therapeutic steps and to the quality of living of the patient [22].
However, in cases of common gingivitis, LLLT role has also been investigated. Igic et al. included 130 children in their study that revealed morphological alterations of epithelial cells, which had similar size of nucleus to that of healthy cells, after LLLT. A 635 nm-diode laser was used, with the parameters being 25 mW power and exposure time at 120 s, with a power density of 200 mW. Laser irradiation was performed for five consecutive days [23]. Gingivitis constitutes, however, a reversible disease and easily resolvable, within two weeks after professional prophylaxis and proper oral hygiene application, but this evidence is particularly useful for periodontitis cases as well, which is always the progression case of chronic gingivitis. Moreover, in cases of gingivitis where heavy symptoms are present, such as edema and spontaneous bleeding, the application of LLLT with the promotion of the healing process, could clinically facilitate the faster resolution of such signs, so that the patient can easily apply oral hygiene, which is the key component to therapy success.
Low-Level Therapy and Oral Cells - Osteoblasts
Implants constitute, nowadays, an integral part of everyday clinical practice in dentistry and patients’ requests regarding a faster rehabilitation are constantly growing. Therefore, research is targeted towards the achievement of a more rapid and effective osseointegration process.
The effect of low-level Nd:YAG laser irradiation on osteoblasts anchored on sandblasted and acid-etched (SLA) titanium surfaces appears to be very promising. In a recent study, the proliferation potential of osteoblasts was significantly increased after the irradiation, while a faster differentiation of cells on SLA surfaces has been noted 14 and 21 days afterwards. In addition to that, the expression of early markers, which were alkaline phosphatase (ALP) and Runx-2, was significantly higher within the first 7 days after irradiation. Lastly, an increase in osteocalcin levels was also observed, which is a non-collagen protein of the bone and the marker of the final differentiation of osteoblasts. Interestingly, increased proliferation was mostly present on Tissue Culture Plastic (TCPPolysterene) and smooth titanium surfaces, whereas when it came to SLA surfaces, increased differentiation was noted [24].
Khadra et al. report increased levels of calcium and phosphorus, as well as greater bone-to-implant contact, after LLLT irradiation with an 810 nm diode laser [25]. An interesting finding is that of Dortbudak et al., who have revealed a significantly greater viability of osteoblasts on the first stages of healing, after implant placement, using a 630 nm-diode laser. In another study by this group, osteoblasts derived from mesenchymal cells were irradiated in vitro on days 3, 5 and 7 of culture with a 690 nm-diode laser for 60s. Bone deposition was significantly more evident in lased cultures and, the authors concluded that LLLT could be proven to be very beneficial for the osseointegration of implants [26]. Bourouni et al. have also proceeded to irradiate co-cultures of immature osteoblastic cells (MG-63) and human gingival fibroblasts, with a single dose of 810 nm laser irradiation, with 15 J/cm2 energy density. Cell proliferation and viability were evaluated for the first three days after irradiation, as well as cell differentiation potential through Collagen Type 1a and ALP gene expression. The thought behind the co-culture was to create a model that was a simulation of flap elevation under clinical circumstances. Proliferation was significantly increased in both cell types, while Collagen 1a expression was also higher in the irradiated groups. However, ALP gene expression was not found to be statistically significant between the test and control groups [27]. Bone irradiation with a CO2 laser prior to the implant insertion promotes osseointegration and new bone formation [28]. Finally, a diode-laser driven increase of bone morphogenetic protein-2 (BMP-2) is associated with accelerated synthesis of bone and enhanced primary stability in mini-implants [29].
The research interest, however, is not only directed towards the osseointegration achievement, but also to very prevalent, late complications of implants, which are peri-implant diseases and most importantly, periimplantitis. In a systematic review of Albaker et al., the authors have found that the complementary use of low-level laser therapy on peri-implant mucositis treatment leads to further reduction of inflammation [30]. However, they have underlined the inadequacy of data to perform a meta-analysis, as well as the importance of the existence of confounding factors, such as smoking. Giannini et al. have also shown that low-level Nd: YAG laser irradiation has bactericidal effect in clinical conditions, without destroying the implant surface and osseointegration due to extreme increase of temperature [31].
Low-Level Therapy and Oral Cells – Epithelial Cells and Fibroblasts
Epithelial cells play an important role in tissue healing processes. It has been shown that after trauma, this cell group proliferates and migrates to that area, while concomitantly secreting growth factors that stimulate the underlying tissues. Low-level laser irradiation of such cells, in vitro, reveals a series of molecular events that are associated with inflammation control and healing acceleration. Near infrared radiation appears to have much greater penetration depth in the tissues, therefore stimulation of the substrate towards an inflammatory response. As far as infrared radiation is concerned, lower energy leads to greater proliferation and metabolic activity of cells.
Basso et al. have used a three-dimensional model for the culture of epithelial cells, an organotypic oral epithelium, as they call it. The reason behind this selection was the better morphological and phenotypic conditions it provides for cell culture, compared to monostromatic models. Moreover, it has been shown that tissue thickness may interfere with irradiation absorbance and reflection. The authors exposed human keratinocytes to780 nm-laser irradiation, in three doses, using 0.5, 1.5, 3, 5 and 7 J/cm2 energy doses. Exposure to 0.5 J/cm2 and 1.5 J/cm2 has resulted in greater stratification of cells, whereas in the group of 3 J/cm2, an epithelium of smaller thickness could be noted. Nevertheless, the greatest rate of proliferation was observed for the lowest energy density given., which was 0.5 J/cm2 [32].
Similarly, to keratinocytes, in vitro irradiation with infrared radiation, brings the optimal result as far as their proliferation, migration and viability are concerned [33]. A series of studies by the same group have also been performed to gingival fibroblasts cultures. Basso et al., have exposed fibroblasts to the same energy densities mentioned above and 40mW power density, for three consecutive doses, with an 780 nmdiode laser. Cell irradiation with 0.5 and 3 J/cm2 resulted in a significant increase in cell metabolism. Moreover, the number of viable cells for those two groups was significantly higher, meaning LLLT can promote biostimulation of fibroblasts. In another study, gingival fibroblasts were initially stimulated with several inflammatory cytokines (IL-1β, TNF-a, IL-6 and IL- 8) for 24 hours. Afterwards, all test groups received three doses of 780 nm-laser irradiation, with 25mW power density and 0.5 to 3 J/cm2 energy density. Cell migration, as an indicator of wound healing, was assessed, as well as cell proliferation, Collagen 1 and vascular endothelial growth factor (VEGF) synthesis. IL-6 and IL-8 resulted in a statistically significant alteration in cell migration and wound area, but overall, all cytokines decreased the migration potential of the cells. Moreover, these cytokines lead to reduced collagen and VEGF expression. LLLT with 0.5 J/cm2 energy density has significantly increased cell proliferation (1.2 times), as well as migration (0.3 times). LLLT also lead to greater gene expression of VEGF and EGF [34,35]. Martignago also has reported an increase in VEGF and Type I Collagen after exposure of mice fibroblasts to LLLT [36].
In a recent systematic review, Bakshi et al. have evaluated different diode lasers and the effect of irradiation on human gingival fibroblasts [37]. Overall, various wavelengths were used in the studies included and the authors have divided them in different categories, depending on the wavelength range. For all wavelengths used, which ranged between 600- 1000 nm, with the most common ones, among others, being 780 nm, 635 nm, 600 nm, 685 nm and 810 nm, for power between 10-30 mW, significant cell proliferation was observed in many studies. Power greater than 500 mW seems to reduce fibroblast population. Even though 940 nm irradiation did not influence cell growth, it resulted in higher expression levels of IGF, VEGF, TGF-β and Type I Collagen mRNA. The cells were irradiated with a single dose at a power of 300 mW for 20 s/ cm2. Moreover, in one study, included in the systematic review, where 685 nm laser irradiation was used, basic fibroblast growth factor (bFGF), except for the group where 660 nmlaser irradiation was used, insulin-like growth factor-1 (IGF- 1) and IGF-binding protein were significantly higher in the irradiated groups.
Interestingly, for the 810 nm irradiation, with power of 10 mW and exposure for 75, 150 and 300 s the stimulation of cell growth was more likely dose-dependent, since 48 and 72 hours after a single dose, the results were no longer statistically significant. However, Frozanfar et al. studied the effect of an 810 nm-diode laser irradiation on cell proliferation and Type-I Collagen gene expression of human gingival fibroblasts. The cells were irradiated for three consecutive days and the laser parameters selected were 50 mW power and 4 J/cm2 energy density. Despite there not being a significant difference in proliferation within the first 24 hours, the next two days revealed a significant increase in viable cells numbers. Overall, for the spectrum of 600-700 nm, 10-30 mW is the optimal power, while in the 700-800 nm spectrum, 25-50 mW power is preferred. Unfortunately, no other optimal parameters for each wavelength could be identified by the authors [38,39]. In a recently published study by Karoussis et al., gingival fibroblasts were stimulated with a single of 2, 4, 6 or 12 J/cm2. The effects of LLLT on EGF, TGF and VEGF were examined, and the investigators found that 12 J/cm2 is the optimal energy density, since it provoked a significantly higher expression of Collagen I, VEGF and EGF [40].
Other groups have associated cell response to LLLT with age. According to Pansani et al., who used 780 nm laser irradiation on young and elderly gingival fibroblasts, at 25 mW power, cell migration was greater in young fibroblasts. This was also observed when they stimulated such cells with EGF. However, only LLLT managed to promote this event on elderly fibroblasts. Interestingly, synthesis of VEGF was significantly reduced in the group of elderly cells after irradiation, but VEGF expression was much higher for both cells type after LLLT [41].
Choi et al. studied the implication of LLLT with inflammatory cytokines and the molecular pathways implicated. They found that 635 nm irradiation leads to the suppression of inflammatory cytokine expression on gingival fibroblasts. The expression of cyclooxygenase-2 (COX-2), PGE 2 production and phosphorylation of ERK, p38, JNK, which are integral parts of the MAPK pathway were evaluated. The authors have found that LLLT inhibited the gene expression of IL-6 and IL-8, whereas also decreased phosphorylation of p38 regardless of the presence of LPS. In the presence of LPS, LLLT also seemed to increase JNK phosphorylation. PGE 2 and COX-2 expressions were increased upon LPS stimulation but were significantly decreased after irradiation. This mediation of the pathway implicated in inflammatory cytokine expression is particularly important [42]. Lim et al. also report that 635 nm irradiation on immortalized hGFs lead to significant increase of COX-2 and PGE2 in previously LPS-treated cells [43].
Cardoso et al. have recently studied the effect of LLLT on cytokines IL-6 and IL-8 in a 3d culture model. They used 780 nm-laser irradiation at 0.025 W power, in cells that were formerly incubated with the cytokines. Photobiomodulation of the cells resulted in decreased synthesis of TNF-a and increased VEGF expression, which was reduced in the groups that received cytokines [44]. Kocherova et al. proceeded to compare two different wavelengths, 635 nm and 808 nm, as far as gingival fibroblast oxidative stress and inflammation markers were concerned. The parameters applied were 100 mW power and 4J/cm2 energy density. Irradiation with either wavelength led to the decrease of the genes related with apoptosis, which were p53, CASP9 and BAX. Furthermore, the authors report that the best results were retrieved after the third irradiation [45]. Pansani et al. cultured gingival fibroblasts on titanium and zirconia surfaces and stimulated them with E. coli derived LPS. They proceeded to irradiate the test groups with 780nm-laser irradiation at 0.5, 1.5 and 3 J/cm2. The two highest doses led to downregulation of IL-6 synthesis on both titanium and zirconia surfaces, while concomitantly reducing IL-8 synthesis on zirconia surfaces. For the highest energy density, in both groups, VEGF expression was also increased [46].
Apart from diode lasers, LLLT with Er:YAG laser also appears to increase the expression of galectin-7 protein in human gingival fibroblasts, which can lead to increased cell proliferation [47]. When it comes to Nd:YAG laser, Gkogkos et al. have investigated the effect of LLLT with this wavelength on fibroblasts, using energy densities of 2.6, 5.3, 7.9 and 15.8 J/cm2. They found that all doses led to higher cell proliferation, which was statistically significant in the group of the highest energy density. EGF was also increased in all groups, but its highest expression was at the lowest energy density [48]. Another study by the same group revealed the beneficial effect of Nd:YAG laser irradiation on human periodontal ligament cells. For the same energy densities, as in the previous study, cell proliferation was increased, but mainly for the groups that received 5.3 and 15.8 J/cm2 at 72 hours. The results regarding EGF, VEGF and bFGF expression remained unclear [49]. Finally, in another study, hFGs that were previously challenged with LPS, were irradiated either with 810 nm laser or Nd: YAG laser. LLLT with both wavelengths resulted in inhibition of expression increase of IL-6 and IL-8, within the first 48 hours [50]. These two wavelengths are very frequently used in clinical practice, with the 810 nm laser presenting the additional advantage of not being absorbed by water. Therefore, it does not cause excessive temperature increase and patient discomfort. Wada et al. also support that Nd:YAG laser irradiation at 0.5 W for 30 s, leads to the upregulation of CXCL8 and NFK-β1 and downregulation of STAT1 and NFK-βΙΑ [51]. On the contrary, according to the preliminary report of Sterczala et al., 635 nm at 64 J/cm2 and 405 nm at 25 J/cm2 laser irradiation presented the optimal results in cell metabolic activity, compared to 450 nm, 1064 nm and 980 nm or combinations [52].
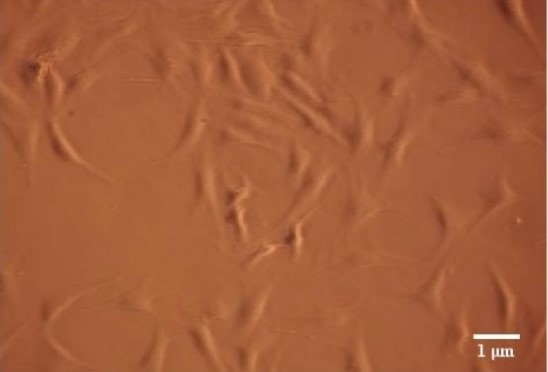
Figure 2: Microscopy photograph of human gingival fibroblasts 48 hours after treatment, that have not received LLLT and served as the control group. Courtesy of Gkogkos et al, 2015 [48].
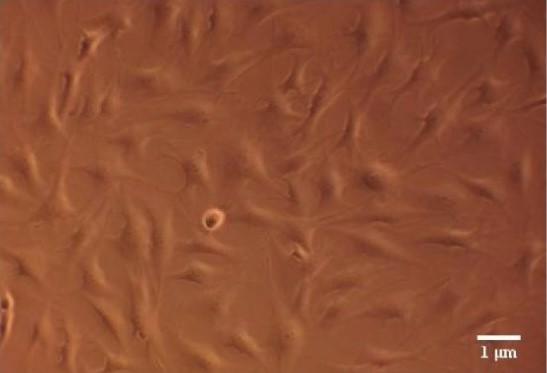
Figure 3: Microscopy photograph of human gingival fibroblasts 48 hours after low- level laser irradiation. Courtesy of Gkogkos et al, 2015 [48].
Conclusions
Growing in vitro and in vivo, as well as clinical evidence arise the past years regarding the benefits of Low-Level Laser Treatment in the oral cavity. It is vastly implicated in the treatment of periodontal and peri-implant diseases, playing a very important role on inflammation mediation, or used as an accelerator of the healing process or implant osseointegration. It is a great tool for the modern clinician, since several protocols based on its principles, lead to the reduction of treatment period, morbidity, since the need for surgery after periodontal therapy is lessened, as well as patient discomfort and pain during the therapeutic processes. Certainly, its clinical benefits and advantages to classical protocols need to be studied furtherly, with more clinical trials containing larger samples. Based on the in vitro results and since there are many available wavelengths to be selected and many laser parameters that have not yet been standardized due to the heterogeneity of the available studies, more targeted protocols need to be performed for solid results to be retrieved.
References
2. Gamaleya NF. Laser biomedical research in the USSR. InLaser applications in medicine and biology 1977 (pp. 1-173). Springer, Boston, MA.
3. Ioannis K Karoussis. Effect Of Nd: YAG Laser Irradiation In The Biological Behavior Of Osteoblasts Cultured On Titanium Surfaces. Research Thesis. Athens 2015. General Part.
4. Emrem Dogan G, Demir T, Orbak R. Effect of Low-Level Laser on Guided Tissue Regeneration Performed with Equine Bone and Membrane in The Treatment of Intrabony Defects: A Clinical Study. Photomedicine and Laser Surgery. 2014 Apr 1;32(4):226-31.
5. David L. Nelson, Michael M. Cox. Lehninger Basic Principles of Biochemistry. 4η Edition. 2011 BROKEN HILL PUBLISHERS.
6. Karu TI. Mitochondrial Signaling In Mammalian Cells Activated By Red And Near-IR Radiation. Photochemistry And Photobiology. 2008 Sep;84(5):1091-9.
7. Smith KC. The Photobiological Basis of Low-Level Laser Radiation Therapy. Laser Therapy. 1991;3(1):19-24.
8. De Freitas LF, Hamblin MR. Proposed Mechanisms of Photobiomodulation or Low-Level Light Therapy. IEEE Journal of Selected Topics in Quantum Electronics. 2016 Jun 9;22(3):348-64.
9. Lin JY. A User's Guide to Channelrhodopsin Variants: Features, Limitations and Future Developments. Experimental Physiology. 2011 Jan 1;96(1):19-25.
10. Rohacs T. Phosphoinositide Regulation of TRP Channels. Mammalian Transient Receptor Potential (TRP) Cation Channels. 2014:1143-76.
11. Yang WZ, Chen JY, Yu JT, Zhou LW. Effects Of Low Power Laser Irradiation on Intracellular Calcium and Histamine Release In RBL-2H3 Mast Cells. Photochemistry And Photobiology. 2007 Jul;83(4):979-84.
12. Gu Q, Wang L, Huang F, Schwarz W. Stimulation of TRPV1 By Green Laser Light. Evidence-Based Complementary and Alternative Medicine. 2012 Jan 1;2012.
13. Ryu JJ, Yoo S, Kim KY, Park JS, Bang S, Lee SH, et al. Laser Modulation of Heat and Capsaicin Receptor TRPV1 Leads to Thermal Antinociception. Journal Of Dental Research. 2010 Dec;89(12):1455-60.
14. Albertini R, Aimbire FS, Correa FI, Ribeiro W, Cogo JC, Antunes E, et al. Effects of Different Protocol Doses of Low Power Gallium–Aluminum–Arsenate (Ga–Al–As) Laser Radiation (650 Nm) On Carrageenan Induced Rat Paw Ooedema. Journal Of Photochemistry and Photobiology B: Biology. 2004 May 27;74(2- 3):101-7.
15. Aimbire F, Lopes-Martins RA, Castro-Faria-Neto HC, Albertini R, Chavantes MC, Pacheco MT, et al. Low-Level Laser Therapy Can Reduce Lipopolysaccharide-Induced Contractile Force Dysfunction And TNF-Α Levels in Rat Diaphragm Muscle. Lasers In Medical Science. 2006 Dec;21(4):238-44.
16. Socransky SS, Haffajee AD, Cugini MA, Smith CK, Kent Jr RL. Microbial Complexes In Subgingival Plaque. Journal Of Clinical Periodontology. 1998 Feb;25(2):134-44.
17. Petrovic MS, Kannosh IY, Milašin JM, Mihailovic DS, Obradovic RR, Bubanj SR, et al. Clinical, microbiological and cytomorphometric evaluation of low-level laser therapy as an adjunct to periodontal therapy in patients with chronic periodontitis. International Journal of Dental Hygiene. 2018 May;16(2):e120-7.
18. Qadri T, Miranda L, Tuner J, Gustafsson A. The short-term effects of low-level lasers as adjunct therapy in the treatment of periodontal inflammation. Journal of Clinical Periodontology. 2005 Jul;32(7):714-9.
19. Qadri T, Bohdanecka P, Tunér J, Miranda L, Altamash M, Gustafsson A. The importance of coherence length in laser phototherapy of gingival inflammation-a pilot study. Lasers in Medical Science. 2007 Nov;22(4):245-51.
20. Ren C, McGrath C, Jin L, Zhang C, Yang Y. The effectiveness of low-level laser therapy as an adjunct to non-surgical periodontal treatment: a meta-analysis. Journal of Periodontal Research. 2017 Feb;52(1):8-20.
21. Ribeiro IW, Sbrana MC, Esper LA, Almeida AL. Evaluation of the effect of the GaAlAs laser on subgingival scaling and root planing. Photomedicine and laser Surgery. 2008 Aug 1;26(4):387-91.
22. Calderín S, García-Núñez JA, Gómez C. Short-term clinical and osteoimmunological effects of scaling and root planing complemented by simple or repeated laser phototherapy in chronic periodontitis. Lasers in Medical Science. 2013 Jan;28(1):157-66.
23. Özberk SS, Gündouar H, senyurt SZ, Erciyas K. Adjunct Use of Low-Level Laser Therapy on the Treatment of Necrotizing Ulcerative Gingivitis: A. A case report. Journal of Lasers in Medical Sciences.2015 Sep;30(7):1855-66.
24. Igic M, Mihailovic D, Kesic L, Milasin J, Apostolovic M, Kostadinovic L, et al. Cytomorphometric and clinical investigation of the gingiva before and after low-level laser therapy of gingivitis in children. Lasers in Medical Science.2018 Winter;9(1):73-75.
25. Karoussis IK, Kyriakidou K, Psarros C, Lang NP, Vrotsos IA. Nd: YAG laser radiation (1.064 nm) accelerates differentiation of osteoblasts to osteocytes on smooth and rough titanium surfaces in vitro. Clinical Oral Implants Research. 2017 Jul;28(7):785-90.
26. Khadra M, Kasem N, Haanæs HR, Ellingsen JE, Lyngstadaas SP. Enhancement of bone formation in rat calvarial bone defects using low-level laser therapy. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology. 2004 Jun 1;97(6):693-700.
27. Dörtbudak O, Haas R, Mailath-Pokorny G. Effect of low-power laser irradiation on bony implant sites. Clinical oral Implants Research. 2002 Jun;13(3):288-92.
28. Bourouni I, Kyriakidou K, Fourmousis I, Vrotsos IA, Karoussis IK. Low Level Laser Therapy With an 810-nm Diode Laser Affects the Proliferation and Differentiation of Premature Osteoblasts and Human Gingival Fibroblasts In Vitro. Journal of Lasers in Medical Sciences. 2021;12:e33.
29. Naka T, Yokose S. Application of laser-induced bone therapy by carbon dioxide laser irradiation in implant therapy. International Journal of Dentistry. 2012 Jan 1;2012:409496.
30. Omasa S, Motoyoshi M, Arai Y, Ejima KI, Shimizu N. Low-level laser therapy enhances the stability of orthodontic mini-implants via bone formation related to BMP-2 expression in a rat model. Photomedicine and Laser Surgery. 2012 May 1;30(5):255-61.
31. Albaker AM, ArRejaie AS, Alrabiah M, Abduljabbar T. Effect of photodynamic and laser therapy in the treatment of peri-implant mucositis: A systematic review. Photodiagnosis and Photodynamic Therapy. 2018 Mar 1;21:147-52.
32. Giannini R, Vassalli M, Chellini F, Polidori L, Dei R, Giannelli M. Neodymium: yttrium aluminum garnet laser irradiation with low pulse energy: a potential tool for the treatment of peri-implant disease. Clinical oral Implants Research. 2006 Dec;17(6):638-43.
33. Basso FG, Pansani TN, Soares DG, Hebling J, de Souza Costa CA. LLLT effects on oral keratinocytes in an organotypic 3D model. Photochemistry and Photobiology. 2018 Jan;94(1):190-4.
34. Pansani TN, Basso FG, Turirioni AS, Kurachi C, Hebling J, de Souza Costa CA. Effects of low-level laser therapy on the proliferation and apoptosis of gingival fibroblasts treated with zoledronic acid. International journal of Oral and Maxillofacial Surgery. 2014 Aug 1;43(8):1030-4.
35. Basso FG, Soares DG, Pansani TN, Cardoso LM, Scheffel DL, de Souza Costa CA, et al. Proliferation, migration, and expression of oral-mucosal-healing-related genes by oral fibroblasts receiving low-level laser therapy after inflammatory cytokines challenge. Lasers in Surgery and Medicine. 2016 Dec;48(10):1006-14.
36. Basso FG, Soares DG, de Souza Costa CA, Hebling J. Low-level laser therapy in 3D cell culture model using gingival fibroblasts. Lasers in Medical Science. 2016 Jul;31(5):973-8.
37. Martignago CC, Oliveira RF, Pires-Oliveira DA, Oliveira PD, Pacheco Soares C, Monzani PS, et al. Effect of low-level laser therapy on the gene expression of collagen and vascular endothelial growth factor in a culture of fibroblast cells in mice. Lasers in Medical Science. 2015 Jan;30(1):203-8.
38. Bakshi PV, Setty SB, Kulkarni MR. Photobiomodulation of human gingival fibroblasts with diode laser-A systematic review. Journal of Indian Society of Periodontology. 2022 Jan 1;26(1):5-12.
39. Frozanfar A, Ramezani M, Rahpeyma A, Khajehahmadi S, Arbab HR. The effects of low level laser therapy on the expression of collagen type I gene and proliferation of human gingival fibroblasts (Hgf3-Pi 53): in vitro study. Iranian Journal of Basic Medical Sciences. 2013 Oct;16(10):1071-4.
40. Karoussis IK, Kyriakidou K, Psarros C, Afouxenides P, Vrotsos IA. Dosage Effects of an 810 nm Diode Laser on the Proliferation and Growth Factor Expression of Human Gingival Fibroblasts. Journal of Lasers in Medical Sciences. 2021;12:e25.
41. Pansani TN, Basso FG, Turrioni AP, Soares DG, Hebling J, de Souza Costa CA. Effects of low-level laser therapy and epidermal growth factor on the activities of gingival fibroblasts obtained from young or elderly individuals. Lasers in Medical Science. 2017 Jan;32(1):45-52.
42. Choi H, Lim W, Kim I, Kim J, Ko Y, Kwon H, et al. Inflammatory cytokines are suppressed by light-emitting diode irradiation of P. gingivalis LPS-treated human gingival fibroblasts. Lasers in Medical Science. 2012 Mar;27(2):459-67.
43. Lim W, Kim J, Kim S, Karna S, Won J, Jeon SM, et al. Modulation of Lipopolysaccharide-Induced NF-κB Signaling Pathway by 635 nm Irradiation via Heat Shock Protein 27 in Human Gingival Fibroblast Cells. Photochemistry and Photobiology. 2013 Jan;89(1):199-207.
44. Cardoso LM, Pansani TN, Hebling J, de Souza Costa CA, Basso FG. Photobiomodulation of inflammatory-cytokine-related effects in a 3-D culture model with gingival fibroblasts. Lasers in Medical Science. 2020 Jul;35(5):1205-12.
45. Kocherova I, Bryja A, Blochowiak K, Kaczmarek M, Stefanska K, Matys J, et al. Photobiomodulation with red and near-infrared light improves viability and modulates expression of mesenchymal and apoptotic-related markers in human gingival fibroblasts. Materials. 2021 Jan;14(12):3427.
46. Pansani TN, Basso FG, de Souza Costa CA. In vitro effects of photobiomodulation applied to gingival fibroblasts cultured on titanium and zirconia surfaces and exposed to LPS from Escherichia coli. Lasers in Medical Science. 2020 Dec;35(9):2031-8.
47. Ogita M, Tsuchida S, Aoki A, Satoh M, Kado S, Sawabe M, et al. Increased cell proliferation and differential protein expression induced by low-level Er: YAG laser irradiation in human gingival fibroblasts: proteomic analysis. Lasers in Medical Science. 2015 Sep;30(7):1855-66.
48. Gkogkos AS, Karoussis IK, Prevezanos ID, Marcopoulou KE, Kyriakidou K, Vrotsos IA. Effect of Nd: YAG low level laser therapy on human gingival fibroblasts. International Journal of Dentistry. 2015 Oct 4;2015:258941.
49. Prevezanos ID, Karoussis IK, Gkogkos AS, Marcopoulou KE, Kyriakidou K, Chernysheva A, et al. Effect of Nd: YAG Low Level Laser Therapy on human periodontal ligament cells: A preliminary in-vitro study. Biomedical Journal of Scientific & Technical Research. 2018;2(4):2797-802.
50. Papadelli A, Kyriakidou K, Kotsakis GA, Pepelassi E, Kallis A, Vrotsos IA, et al. Immunomodulatory effects of Nd: YAG (1064 nm) and diode laser (810 nm) wavelengths to LPS-challenged human gingival fibroblasts. Archives of Oral Biology. 2021 Feb 1;122:104982.
51. Wada Y, Suzuki A, Ishiguro H, Murakashi E, Numabe Y. Chronological Gene Expression of Human Gingival Fibroblasts with Low Reactive Level Laser (LLL) Irradiation. Journal of Clinical Medicine. 2021 Jan;10(9):1952.
52. Sterczala B, Grzech-Lesniak K, Michel O, Trzeciakowski W, Dominiak M, Jurczyszyn K. Assessment of human gingival fibroblast proliferation after laser stimulation in vitro using different laser types and wavelengths (1064, 980, 635, 450, and 405 nm)—preliminary report. Journal of Personalized Medicine. 2021 Feb;11(2):98.
