Abstract
Background: The balance in the upright position becomes considerably more complex due to different changes, such as the decrease in the size of the support base and changes in the center of pressure (COP). However, there are no studies that relate differences between sex and ischemic preconditioning protocol (IPC).
Objective: The purpose of this investigation was to compare differences in plantar pressure distribution and center of pressure between males and females practitioners of resistance exercise.
Methods: All participants (male, n=16; female, n=15) were practitioners of resistance exercise and underwent four tests in the baropodometry platform before and after IPC protocol in static and dynamic conditions. The IPC protocol consists of 4 × 5-min occlusion 220 mmHg/reperfusion 0 mmHg in thighs.
Results: The forefoot and hindfoot pressures showed significant difference (p<0.001) between before vs. after intervention in the right plantar surface area in males. On the other hand, hindfoot pressures were significantly higher that forefoot before vs. after intervention in the right plantar surface area in females (p<0.01). Student’s t-test showed that during dynamic conditions occurred decrease significant (p<0.02) before vs. after intervention in the left plantar surface area maximal pressure in males. In contrast, the right plantar surface area mean pressure showed significant difference (p<0.01) during static conditions in males. The COP showed decrease significant (p<0.03) after intervention only left side in males.
Conclusion: This study showed decrease of the plantar surface area pressure and COP in dynamic and static conditions in the males after IPC protocol.
Keywords
Ischemic preconditioning protocol, Baropometry platform, Sex
Introduction
Hyperemia is the transient increase in organ blood flow that occurs following a brief period of ischemia (e.g., blood flow restriction), thus, promote greater shear stress against blood vessel walls on restoration of blood flow that occurs with release of occlusive pressure that contribute with a vasodilatation by increase in blood flow to a tissue due to the presence of metabolites (adenosine, prostaglandins, and oxide nitric) and myogenic effects [1-3].
The ischemic preconditioning (IPC) protocol consists of alternating periods of blood flow occlusion/reperfusion of limbs through a pneumatic tourniquet applied around the upper thighs or arms regions. This idea was based on clinical experimental research, where the effects of IPC protocol were related to an intervention that also affects organs other than those where it is applied [1,2]. Since IPC had shown positive protection effects on myocardial cells for events of limited blood flow [3], this maneuver became a target for several sports scientists, assuming that the hyperemia upon reperfusion could improve muscle performance in some way. Thus, IPC was shown to improve maximal cycling performance [ 4], sprint performance [5], enhance performance in resistance exercise [6,7] and reduce marathon-induced inflammation [8]. However, so far, no study has investigated the influence of the IPC protocol on balance.
The balance is defined as the condition in which all the forces acting on the body are balanced in the center of the mass and controlled by the support base, in a static or dynamic condition [9,10]. These balance conditions are characterized, respectively, by the absence or presence of speed. However, the ability to maintain balance during voluntary activities and to react to external perturbations becomes considerably more complex due to different changes, such as the decrease in the size of the support base, changes in the center of pressure [COP], and postural changes [9-11]. Hence, a body presents its state of normal balance when the sum of all external forces and all external torques is equal to zero [10-12]. It is noteworthy that the foot helps support, move the body and to absorb ground reaction forces during locomotion, which are the foundations of the human balance and posture, i.e these functions depends largely on the plantar vault, formed by the longitudinal medial and transverse arches [11,12].
Both static and dynamic balance is maintained by the vestibular (labyrinth, cochlear nerves, nuclei, pathways, and interrelations in the central nervous system), proprioceptive (sensory receptors located in joints, muscles, and tendons), and visual systems [11,12]. When these three systems are is a perfect spatial orientation that triggers ocular (vestibule-ocular, optokinetic, cervical-ocular) and spinal (vestibulospinal, vestibular-colic, cervical-colic, cervicalspinal) reflexes appropriate to the automatic and unconscious maintenance of postural control in the environment [11,12]. On the other hand, changes in one or more of these systems can cause imbalance. But the scientific literature still does not directly relate the IPC protocol and balance. Consequently, the absence of data supports the need for additional studies in this area. Hence, the purpose of this study was to compare the plantar surface area, COP and stabilometry variables between males vs. females trained. We hypothesized that males trained would show a better of the balance after IPC protocol.
Methods
Study design
This is a randomized comparative study. The sample size was determined by including all participants that complied with the eligibility criteria. All participants (male and female) were practitioners of resistance exercise and underwent four tests in the baropodometry platform before and after ischemic preconditioning protocol (IPC) in static and dynamic conditions without footwear. All tests were performed in a single assessment session to assess plantar surface area, center of pressure (COP), anteroposterior oscillation, laterolateral oscillation, maximum peak pressure, and mean pressure. In dynamic conditions, we assessed support plantar surface areas of both feet and pressure exerted on the ground. All assessments were taken in a temperature-controlled environment (temperature 21º C, 65% relative humidity) by a Hygro-Thermometer with Humidity Alert (Extech Instruments, Massachusetts, EUA). All assessments occurred between 2:00 and 4:00 P.M.
Participants
This study included 31 healthy and separated into two groups: male (age: 25.1 ± 3.8 years; height: 180.2 ± 5.5 cm; body mass: 80.3 ± 5.8 kg; body fat: 14.2 ± 3.5%; n= 16) and female (age: 24.5 ± 6.1 years; height: 165.2 ± 7.4 cm; body mass: 67.4 ± 8.2 Kg; body fat: 18.5 ± 4.2%, n = 15). The participants’ training frequency was 4.1 ± 0.3 days/week with a mean duration for each session training of 60 min-1 using resistance training programs.
The participants were eligible if they had not been smokers for the previous 3 months or more; had no cardiovascular or metabolic diseases, systemic hypertension (140/90 mm Hg or use of antihypertensive medication), recent musculoskeletal injury and surgery (in the last 6 months), or pain in any region of the body; and had not used anabolic steroids, drugs or any medication with the potential to impact physical performance (self-reported). This study was approved by the Ethical Committee for Human Experiments of the Augusto Motta University Center, Rio de Janeiro, Brazil (CAAE: 39675820.0.0000.5235). The present study was conducted at the Rehabilitation Science Center, Augusto Motta University Center, Rio de Janeiro, Brazil.
Anthropometric measurements
Body composition was measured following an 8-h overnight fast by bioelectrical impedance analysis using a device with built-in hand and foot electrodes (BIO 720, Avanutri, Rio de Janeiro, Brasil). The participants wore their normal indoor clothing and were instructed to stand barefoot in an upright position with both feet on separate electrodes on the device’s surface and with their arms ab¬ducted and both hands gripping two separate electrodes on each handle of the device. All biometric measurements were carried out in an air-conditioned room (21°C). No clinical problems occurred during the study.
Ischemic preconditioning protocol
The IPC session consisted of 4 cycles of 5 minutes of occlusion at 220 mm Hg of pressure using an 85 x 10-cm pneumatic tourniquet applied around the subinguinal region of the upper thighs (Avanutri, Rio de Janeiro, Brazil) alternated with 5 minutes of reperfusion at 0 mm Hg resulting in a total intervention of 40 minutes. The pressure used and cuff width were in accordance with previous studies to certify that subjects had the blood flow occluded during the intervention [6]. The occlusion and reperfusion phases were conducted with subjects remaining supine. The effectiveness of occlusion in the IPC session was checked by auscultation of the arteries around the ankle during the phases when the cuff was inflated and was deflated and controlled during the occlusion maneuver [6]. Five minutes after the interventions (IPC), subjects performed tests in the baropodometry platform.
Baropodometry assessment
The baropodometry platform consisted of a support with a 655 mm long and 534 mm wide (BaroScan®, Londrina, Brazil). The board contained 4096 platinums electronic sensors covered by an alveolar rubber captor that gives pressure information from each foot through a USB cable to the computer for an appropriate software (BaroSys). The sampling rate was set at 100 Hz for static assessment and 200 Hz for dynamic assessment.
Before assessments, all individuals remained in a standing, bipedal position with the arms pending along the body over the platform with their eyes open mirrored to a fixed point on the wall of the examination room. During static conditions, the subjects stood on the platform in an orthostatic position for 5 s (Figure 1). In dynamic conditions, the subjects walked on the platform during data collection (Figure 1). The following parameters were considered in static condition: support surface areas of both feet; the percentage distribution of the load between hindfoot and forefoot; and the pressure exerted upon the medial and lateral portions of each foot. In dynamic conditions, we assessed support surface areas of both feet and pressure exerted on the ground. The forefoot was assumed as the foot part anterior to the gravity center and the hindfoot as the part posterior to the center of gravity registered on the device.
Figure 1: Example of a baropodometry assessment.
Statistical analysis
All data are presented as mean ± SD. Statistical analysis was initially performed using the Shapiro-Wilk normality test and the homocedasticity test (Bartlett criterion). To test the reproducibility between the tests, the intraclass correlation coefficient (ICC) was used. Two-way analysis of variance (ANOVA) was used to test for main and interaction effects of the group (males vs. females) and timing of measurement for each outcome variable independently (right vs. left) and the post hoc Bonferroni was used to possibility a statistically significant. Student’s t-test was used to assess differences between tests in the baropodometry platform (before vs. after ischemic preconditioning protocol). The effect size (ES) of the difference between DL and NDL was assessed using Cohen’s d. Values of d<0.1, from 0.1 to <0.20, from 0.20 to <0.50, from 0.50 to <0.80, and ≥ 0.80 were considered as trivial, small, moderate, large and very large, respectively. The significance level was set at 0.05 and the software used for statistics was GraphPad® (Prism 6.0, San Diego, CA, USA).
Results
The two-way ANOVA yielded main effects for group in static left foot (F1,54= 31.16, p<0.0001), static right foot (F1,54= 39.29, p<0.0001), dynamic left foot (F1,28= 17.96, p<0.0002) and dynamic right foot (F1,28= 18.51, p<0.0002), such that Bonferroni post- hoc showed significant differences in plantar surface area (cm2) between males vs. females group (Table 1). Table 2 demonstrated that both forefoot and hindfoot pressures showed significant difference (p<0.001) between before vs. after intervention in the right plantar surface area (%) in males. On the other hand, hindfoot pressures were significantly higher that forefoot before vs. after intervention in the right plantar surface area (%) in females (Table 2). However, males showed a significant difference between hindfoot vs. forefoot right only before intervention.
| MALE | FEMALE | 95% CI | p< | ||
|---|---|---|---|---|---|
| Static (PRE) | Right | 100.5 ± 17.3 | 72.1 ± 16.5 | -29.4 (-44.3 to -14.5) |
0.0001 |
| Left | 97.8 ± 17.6 | 74.1 ± 18.9 | -25.1 (-40.1 to -10.1) |
0.001 | |
| Static (POST) | Right | 100.1 ± 16.3 | 73.1 ± 18.6 | -27.8 (-42.6 to -12.9) |
0.0001 |
| Left | 98.8 ± 16.7 | 73.2 ± 16.7 | -26.3 (-41.3 to -11.3) |
0.001 | |
| Dynamic (PRE) | Right | 138.7 ± 16.9 | 111.1 ± 19.7 | -27.6 (-43.1 to -12.2) |
0.001 |
| Left | 137.8 ± 18.3 | 107.4 ± 21.3 | -30.4 (-46.8 to -14.1) |
0.001 | |
| Dynamic (POST) | Right | 138.5 ± 18.5 | 109.4 ± 17.8 | -29.1 (-44.5 to -13.6) |
0.001 |
| Left | 136.1 ± 18.4 | 107.1 ± 19.7 | -29.1 (-45.5 to -12.7) |
0.001 |
| PRE | POST | 95% CI | p< | ES | ||
|---|---|---|---|---|---|---|
| Forefoot Static (MALE) | Right | 43.5 ± 9.1 | 50.5 ± 9.6 | -6.9 (-10.9 to -3.1) |
0.001 | 0.75 (large) |
| Left | 45.8 ± 11.5 | 46.9 ± 12.9 | -1.9 (-8.2 to -4.3) |
0.52 | 0.10 (small) |
|
| Hindfoot Static (MALE) | Right | 56.4 ± 9.1* | 49.4 ± 9.6 | 1.1 (-3.9 to -6.2) |
0.001 | 0.75 (large) |
| Left | 54.1 ± 11.5 | 53.1 ± 12.9 | 1.1 (-3.9 to 6.2) |
0.63 | 0.10 (small) |
|
| Forefoot Static (FEMALE) | Right | 37.2± 13.9 | 41.7 ± 10.2 | 4.5 (-1.7 to -10.7) |
0.14 | 0.37 (moderate) |
| Left | 44.1 ± 10.3 | 46.1 ± 12.9 | 1.9 (-4.3 to 8.2) |
0.52 | 0.16 (small) |
|
| Hindfoot Static (FEMALE) | Right | 62.7 ± 13.9** | 58.2 ± 10.2** | -4.5 (-10.7 to 1.7) |
0.14 | 0.37 (moderate) |
| Left | 55.8 ± 10.3 | 53.9 ± 12.9 | -1.9 (-8.3 to 4.3) |
0.52 | 0.17 (small) |
|
| *p<0.02 = Hindfoot vs. Forefoot right male. **p<0.01 = Hindfoot vs. Forefoot right female. |
||||||
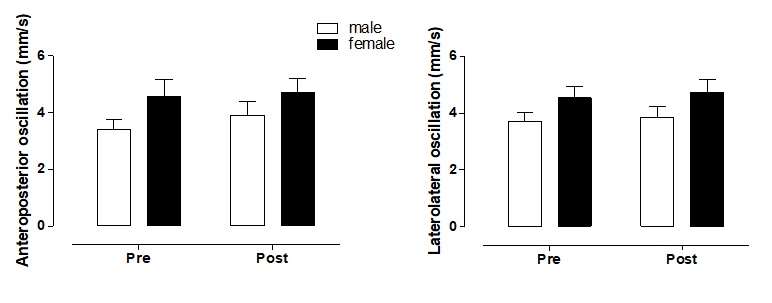
Table 3 compares the plantar surface area maximal pressure between males vs. females. Student’s t-test showed that during dynamic conditions occurred decrease significant (p<0.02) before vs. after intervention in the left plantar surface area maximal pressure in males (Table 3). In contrast, the right plantar surface area means pressure showed significant difference (p<0.01) during static conditions in males (Table 4). The center of pressure (COP) showed decrease significant ( p<0.03) after intervention only left side in males (Table 5). However, none of the stabilometry variables (anteroposterior and laterolateral oscillation) showed a significant difference between males vs. females (p>0.05) (Figure 2).
Figure 2 : Comparisons of stabilometry variable s between males vs. females trained.
| PRE | POST | 95% CI | p< | ES | ||
|---|---|---|---|---|---|---|
| Static position (MALE) | Right | 1.1 ± 0.3 | 1.1 ± 0.5 | -0.04 (-0.27 to 0.17) |
0.64 | 0.15 (small) |
| Left | 1.1 ± 0.2 | 1.2 ± 0.4 | 0.11 (-0.14 to 0.3) |
0.36 | 0.44 (moderate) |
|
| Dynamic position (MALE) | Right | 1.4 ± 0.4 | 1.4 ± 0.6 | -.003 (-0.30 to 0.29) |
0.97 | 0.01 (trivial) |
| Left | 1.8 ± 0.7 | 1.4 ± 0.6 | -0.30 (-0.56 to -0.04) |
0.02 | 0.45 (moderate) |
|
| Static position (FEMALE) | Right | 1.4 ± 0.4 | 1.3 ± 0.3 | -0.15 (-0.33 to 0.02) |
0.08 | 0.40 (moderate) |
| Left | 1.3 ± 0.3 | 1.4 ± 0.4 | 0.06 (-0.16 to 0.29) |
0.53 | 0.17 (small) |
|
| Dynamic position (FEMALE) | Right | 1.8 ± 0.4 | 1.9 ± 0.8 | 0.13 (-0.38 to 0.66) |
0.58 | 0.18 (small) |
| Left | 1.7 ± 0.4 | 1.8 ± 0.4 | 0.07 (-0.25 to 0.40) |
0.62 | 0.17 (small) |
| PRE | POST | 95% CI | p< | ES | ||
|---|---|---|---|---|---|---|
| Static position (MALE) | Right | 0.38 ± 0.07 | 0.36 ± 0.07 | -0.02 (-0.03 to -0.006) |
0.01 | 0.32 (moderate) |
| Left | 0.37 ± 0.05 | 0.37 ± 0.05 | -0.003 (-0.01 to 0.009) |
0.59 | 0.06 (trivial) |
|
| Dynamic position (MALE) | Right | 0.35 ± 0.06 | 0.35 ± 0.05 | -0.001 (-0.01 to 0.01) |
0.87 | 0.02 (trivial) |
| Left | 0.35 ± 0.05 | 0.35 ± 0.05 | 0.002 (-0.008 to 0.01) |
0.62 | 0.05 (trivial) |
|
| Static position (FEMALE) | Right | 0.40 ± 0.1 | 0.39 ± 0.09 | -0.003 (-0.03 to 0.03) |
0.82 | 0.04 (trivial) |
| Left | 0.38 ± 0.07 | 0.37 ± 0.07 | -0.004 (-0.02 to 0.01) |
0.67 | 0.06 (trivial) |
|
| Dynamic position (FEMALE) | Right | 0.33 ± 0.06 | 0.34 ± 0.05 | 0.001 (-0.01 to 0.01) |
0.86 | 0.03 (trivial) |
| Left | 0.35 ± 0.06 | 0.34 ± 0.05 | -0.003 (-0.02 to 0.01) |
0.72 | 0.06 (trivial) |
| PRE | POST | 95% CI | p< | ES | ||
|---|---|---|---|---|---|---|
| MALE | Right | 12.4 ± 1.5 | 12.6 ± 1.9 | 0.17 (-0.63 to 0.98) |
0.65 | 0.10 (small) |
| Left | 13.1 ± 1.8 | 12.2 ± 2.1 | -0.78 (-1.4 to -0.07) |
0.03 | 0.39 (moderate) |
|
| FEMALE | Right | 11.3 ± 1.3 | 11.4 ± 2.1 | 0.14 (-0.9 to 1.1) |
0.77 | 0.08 (trivial) |
| Left | 11.7 ± 2.4 | 12.1 ± 2.8 | 0.39 (-0.77 to 1.5) |
0.47 | 0.15 (small) |
|
| Abbreviations: 5-HT: Serotonin; CNTL: Control; DI: Distal Intestine; DIO: Diet-Induced Obesity; MI1: Mid Intestine part 1; MI2: Mid Intestine part 2; PI: Proximal Intestine . | ||||||
Discussion
This study aimed at investigating the contribution of the IPC protocol in the static and dynamic balance between males and females trained. The main results obtained with this study were that [a] males showed greater plantar surface area (cm2) when compared to females, [b] males showed similar right plantar surface area between forefoot and hindfoot after IPC protocol, [c] females revealed a significant increase of hindfoot in the right plantar surface area (%) before and after IPC protocol, [d] in dynamic conditions was observed decrease of the left plantar surface area maximal pressure in males after IPC protocol, [e] in static conditions were observed decrease of the right plantar surface area mean pressure in males after IPC protocol, and [f ] COP reduced significantly in the left side in males after IPC protocol.
Anatomical or biomechanical variations between males and females can directly intervene in balance and plantar pressure. Some studies reported differences in feet and gait-related anatomy and habits between males and females [13,14]. Other studies showed that males had a foot longer, higher plantar fascia and heel fat pad thickness compared with females [15,16]. In general, male and female feet are different to varying degrees with respect to arch lateral side of the foot, the first toe, heel-to-toe length, ball length, ball width, ball circumference, malleoli height, and arch dimensions [15,16]. These differences should be taken into account in relation greater plantar surface area in static and dynamic conditions in males when compared to females.
Differences were observed in the fore-/Hind-foot in the right foot load distribution parameters between males and females. Our results showed that independent of the IPC protocol was observed plantar load distribution in right hindfoot in the females. Our findings agree with the ideal load values reported in scientific literature, i.e suggested that 60% of the weight should rest on the hindfoot and 40% on the forefoot [17,18]. On the other hand, females showed asymmetric distribution of plantar load distribution during static condition. We may hypothesize that way of loading and setting of the foot is often the result of biomechanical variations from structural changes in the spine that can cause of asymmetry of foot loads, weakening of their muscle, ankle stabilization and gait asymmetry [19]. However, males showed similar right plantar surface area between the forefoot and hindfoot after IPC protocol. This result can be associated to biochemical messengers, enhanced of the motor-evoked potential and activation of the nerve pathways, or a combination of these, in response to IPC protocol [20,21]. In addition, we hypothesized that the decrease plantar surface area pressure (maximal and mean) and COP in males can be result from the reduced vertical component of ground reaction force after IPC protocol. However, we did not explore the mechanism and future studies comparing neurologic response, such as motor-evoked potential and activation of the nerve pathways, are warranted. In view of these concepts, the observed fading of the initial improvements in performance over time could be interpreted as some kind of habituation to the IPC interventions.
The limitations of the study include the absence of measures of physiological parameters, which would be interesting; this, yet, does not limit the answer to the study question. In addition, longitudinal studies are needed to define a causeand- effect relationship differences between sex.
Conclusion
This study showed decrease of the plantar surface area pressure and COP in dynamic and static conditions in the males after IPC protocol. These data contribute to the qualitative and quantitative understanding of the differences between sex in balance condition with using of the IPC protocol.
Conflict of Interest
The author states no conflict of interest.
Acknowledgments
The investigators would like to thank the 40 healthy male and female that participated in the study. The study was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil [CAPES], Finance Code 001.
Authors Contribution
Bruno Guimarães de Oliveira: a. Design and analysis and interpretation of data; b. Writing of the manuscript; c. Final approval of the version to be published. Alex Souto Maior: a. Design and analysis and interpretation of data; b. Writing of the manuscript; c. Final approval of the version to be published.
References
2. Xie Y, Jiang D, Xiao J, Fu C, Zhang Z, Ye Z, et al. Ischemic preconditioning attenuates ischemia/reperfusion-induced kidney injury by activating autophagy via the SGK1 signaling pathway. Cell Death Dis. 2018;9(3):338.
3. Turrell HE, Thaitirarot C, Crumbie H, Rodrigo G. Remote ischemic preconditioning of cardiomyocytes inhibits the mitochondrial permeability transition pore independently of reduced calcium-loading or sarcKATP channel activation. Physiol Rep. 2014;2(11):e12231.
4. Paradis-Deschênes P, Joanisse DR, Mauriège P, Billaut F. Ischemic Preconditioning Enhances Aerobic Adaptations to Sprint-Interval Training in Athletes Without Altering Systemic Hypoxic Signaling and Immune Function. Front Sports Act Living. 2020;2:41.
5. Lindsay A, Petersen C, Blackwell G, Ferguson H, Parker G, Steyn N, et al. The effect of 1 week of repeated ischaemic leg preconditioning on simulated Keirin cycling performance: a randomised trial. BMJ Open Sport Exerc Med. 2017;3(1):e000229.
6. Marocolo M, Willardson JM, Marocolo IC, da Mota GR, Simão R, Maior AS. Ischemic Preconditioning and Placebo Intervention Improves Resistance Exercise Performance. J Strength Cond Res. 2016;30(5):1462-9.
7. Marocolo M, Marocolo IC, da Mota GR, Simão R, Maior AS, Coriolano HJ. Beneficial Effects of Ischemic Preconditioning in Resistance Exercise Fade Over Time. Int J Sports Med. 2016;37(10):819-24.
8. Mieszkowski J, Stankiewicz B, Kochanowicz A, Niespodziński B, Borkowska A, Antosiewicz J. Effect of Ischemic Preconditioning on Marathon-Induced Changes in Serum Exerkine Levels and Inflammation. Front Physiol. 2020;11: 571220.
9. Lee CH, Sun TL. Evaluation of postural stability based on a force plate and inertial sensor during static balance measurements. J Physiol Anthropol. 2018;37(1):27.
10. Yiou E, Caderby T, Delafontaine A, Fourcade P, Honeine JL. Balance control during gait initiation: State-of-the-art and research perspectives. World J Orthop. 2017;8(11):815-28.
11. Riemann BL, Lephart SM. The sensorimotor system, part I: the physiologic basis of functional joint stability. J Athl Train. 2002;37(1):71-9.
12. Ivanenko Y, Gurfinkel VS. Human postural control. Frontiers in Neuroscience. 2018;12:171.
13. Putti AB, Arnold GP, Abboud RJ. Foot pressure differences in men and women. Foot and Ankle Surgery. 2010; 16: 21–4.
14. Cho SH, Park JM, Kwon OY. Sex differences in three dimensional gait analysis data from 98 healthy Korean adults. Clin Biomech [Bristol, Avon]. 2004;19(2):145-52.
15. Taş S. Effect of Sex on Mechanical Properties of the Plantar Fascia and Heel Fat Pad. Foot Ankle Spec. 2018;11(5):403-9.
16. Luo G, Houston VL, Mussman M, Garbarini M, Beattie AC, Thongpop C. Comparison of male and female foot shape. J Am Podiatr Med Assoc. 2009 S;99(5):383-90.
17. Sarpong NO, Swindell HW, Trupia EP, Vosseller JT. Metatarsal Fractures. Foot & Ankle Orthopaedics 2018 3(3):1-8.
18. Nyska M, McCabe C, Linge K, Klenerman L. Plantar foot pressures during treadmill walking with high-heel and low-heel shoes. Foot Ankle Int. 1996;17(11):662-6.
19. Buldt AK, Menz HB. Incorrectly fitted footwear, foot pain and foot disorders: a systematic search and narrative review of the literature. J Foot Ankle Res. 2018;11:43.
20. Herajärvi J, Anttila T, Sarja H, Mustonen C, Haapanen H, Mäkelä T, et al. Exploring Spinal Cord Protection by Remote Ischemic Preconditioning: An Experimental Study. Ann Thorac Surg. 2017;103(3):804-11.
21. Haapanen H, Herajärvi J, Arvola O, Anttila T, Starck T, Kallio M, et al. Remote ischemic preconditioning protects the spinal cord against ischemic insult: An experimental study in a porcine model. J Thorac Cardiovasc Surg. 2016;151(3):777-85.