Abstract
Our study aimed to examine the prevalence of atherosclerotic burden in a predominantly non-white cohort of patients from LA County who underwent coronary artery calcium scoring (CACS) and coronary CT angiogram (CCTA) for the evaluation of cardiomyopathy of ischemic origin. Ischemic cardiomyopathy, primarily caused by reduced blood supply to the heart, is the most common type of cardiomyopathy. CCTA is a well-established and non-invasive imaging test used to assess the extent of coronary artery disease (CAD) in individuals suspected of having it.
We analyzed data from 131 patients with cardiomyopathy who were referred to Harbor-UCLA Medical Center between August 2016 and September 2020. These patients had no chest pain, no q waves on electrocardiogram (ECG), and exhibited cardiomyopathy with reduced ejection fraction (EF) without wall motion abnormalities on transthoracic echocardiogram.
Among the 131 patients included in our study, the average age was 54.6 years, with 93 (71%) being male. The racial distribution was as follows: 71 (54%) Hispanic, 17 (13%) White, 24 (18%) African American, and 10 (8%) Asian. Various risk factors for coronary artery disease were present, including diabetes mellitus (30%), hypertension (75%), hyperlipidemia (45%), and smoking (46%).
We assessed CACS using the Agatston score, while atherosclerotic disease burden was evaluated using total plaque score (TPS), total segment stenosis score (TSSS), and segment involvement score (SIS). Of the patients, 55 (42%) had a CACS of zero, 28 (21%) had a CACS below 10, and the median (interquartile range) of TPS was 1 (1, 5). The median TSSS and SIS were both 1 (1, 5) and 0 (1, 4), respectively. A total of 23 patients (18%) exhibited 50% stenosis indicative of obstructive CAD, while 13 patients (10%) had even more severe stenosis of 75% or greater.
Our findings revealed that the majority of patients in our cohort had non-obstructive CAD, suggesting that non-ischemic factors were the primary cause of their cardiomyopathy. Additionally, our study demonstrated that CCTA is an effective non-invasive diagnostic tool for cardiomyopathy, allowing for the avoidance of more invasive procedures in a predominantly non-white population receiving care at a public hospital in LA County.
Keywords
Atherosclerosis, Cardiomyopathies, Cardiovascular imaging, Coronary artery disease, Coronary CTA, Ischemic evaluation
Abbreviations
Coronary CTA: Coronary CT Angiography; CAD: Coronary Artery Disease; HF: Heart Failure; CACS: Coronary Artery Calcium Score; TSSS: Total Segment Stenosis Score; TPS: Total Plaque Severity Score; SIS: Segment Involvement Score; SSS: Segment Stenosis Score; ICA: Invasive Coronary Angiography (ICA); MPI: Myocardial Perfusion Imaging
Introduction
Cardiomyopathies are a heterogeneous group of disorders characterized by myocardial structural or electrical alterations [1]. It remains a frequent encounter in inpatient and outpatient settings, with over 18000 instances where cardiomyopathy was reported as the principal diagnosis in inpatient hospitalizations, as reported by 2018 HCUP data [2].
Cardiomyopathies can manifest as either mechanical or electrical complications, and the mechanical complications are diastolic and systolic dysfunction, which manifests as the clinical syndrome of Heart Failure (HF) [1]. Annually, HF affects about 6 million Americans over the age of 20 and accounts for over 1.2 million hospitalizations [2]. The cost of care for HF in the US is projected to increase from $21 billion to $53 billion over the next 20 years [3].
Coronary artery disease (CAD) has succeeded hypertension and valvular heart disease as the leading cause of new-onset HF, accounting for more than two-thirds of the cases [3,6]. Establishing the etiology of HF has treatment implications [9], and patients with ischemic cardiomyopathy have poorer long-term outcomes [4]. In patients with heart failure with reduced ejection fraction secondary to CAD, percutaneous/surgical interventions, goal-directed medical therapy, and secondary prevention interventions have been shown to reduce mortality and morbidity in the long term [4,7,8]. There is a wide array of invasive and non-invasive modalities for ischemic evaluation. However, there remains a gross underutilization of these resources [10]. Between 2004-2020, only 34.8% of the patients with newly diagnosed HF underwent ischemic evaluation. Furthermore, older age, Black race, and female sex were associated with lower rates of CAD testing [11].
As there is a need for increased testing, it becomes essential to evaluate, utilize and validate non-invasive methods of assessing the extent of CAD in patients. This becomes even more significant in light of the existing racial and ethnic health disparities in the United States. Greater HF readmission rates and racial-ethnic disparities were seen in hospitals with predominantly black and Hispanic populations compared to hospitals with largely white populations [12,13].
Coronary CT angiography (Coronary CTA) is an excellent, validated, structural non-invasive test to assess the extent of coronary artery disease. Some of the coronary CTA images are represented in Figures 1-5. It has a greater than 90 to 95% sensitivity and specificity in patients with occlusive CAD, with a negative predictive value of 99% [14-16]. Although coronary artery calcium score (CACS) has excellent sensitivity, it has poor specificity as a standalone test for occlusive CAD [17]. Our study aimed to describe the prevalence of atherosclerotic burden in a largely non-white cohort of LA county patients who received coronary artery calcium (CAC) and CCTA for ischemic evaluation of cardiomyopathy.
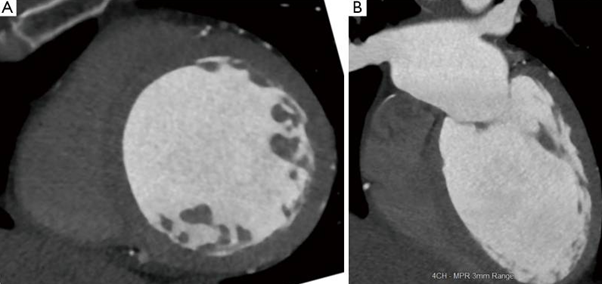
Figure 1: “Dilated cardiomyopathy. (A) Short-axis CT image shows severe dilation of the left ventricle with end-diastolic diameter of 7.1 cm; (B) 4-chamber CT image also shows severe dilation of the left ventricle. The ejection fraction in this patient was 15% and the constellation of findings is consistent with dilated cardiomyopathy. CT: Computed Tomography” [25].
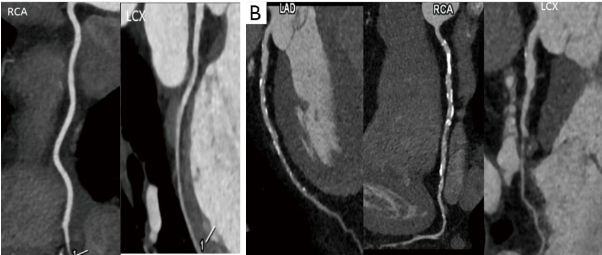
Figure 2. “Coronary artery evaluation. (A) Coronary CT angiography performed in a patient with new onset heart failure shows normal coronary arteries without any atherosclerotic plaque or luminal stenosis; (B) coronary CTA in another patient with heart failure shows multiple calcific and non-calcific atherosclerotic plaques in all the coronary arteries. CT: Computed Tomography; CTA: CT Angiography; LAD: Left Anterior Descending; RCA: Right Coronary Artery; LCx: Left Circumflex Coronary Artery” [25].
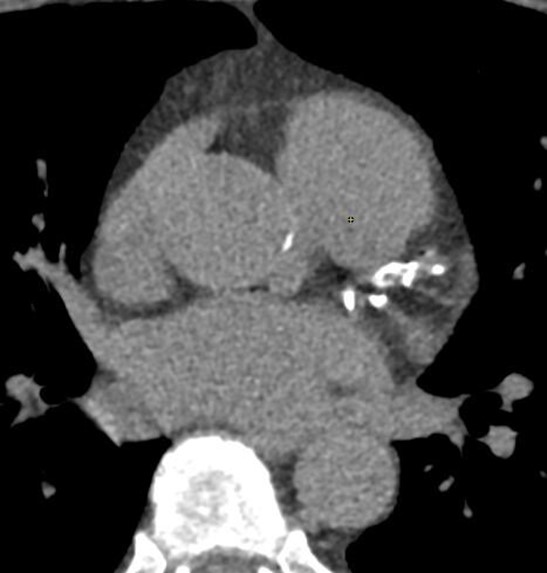
Figure 3. Axial image showing significant calcifications in the left main, proximal left anterior descending (LAD), and proximal Left Circumflex arteries (LCx).
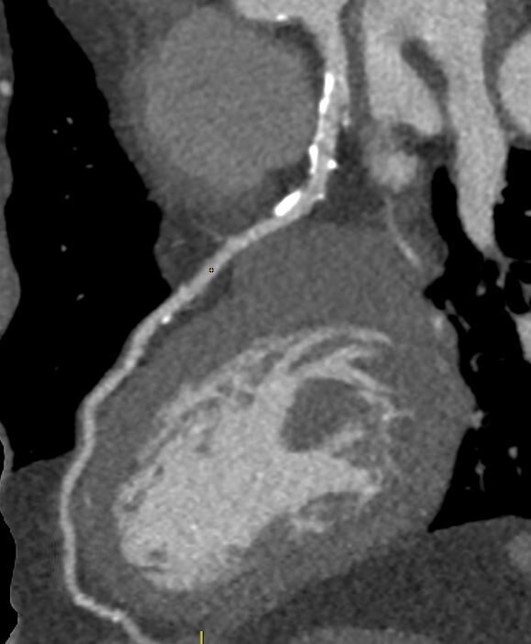
Figure 4. Sagittal image of the LAD showing significant non-obstructive calcifications.
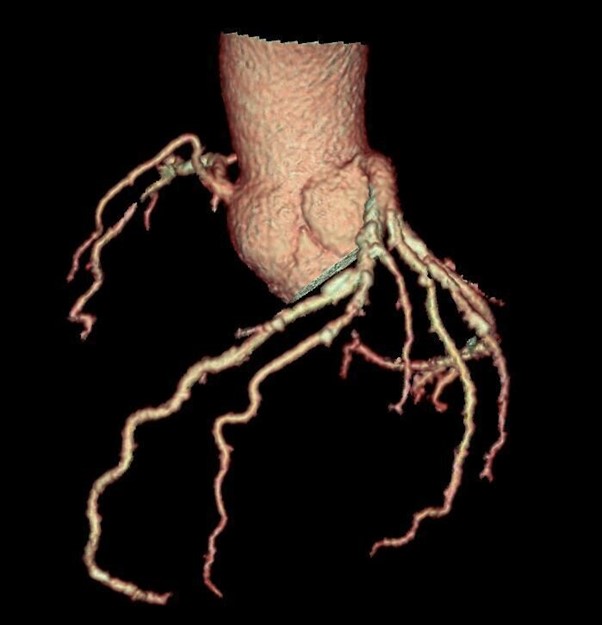
Figure 5. Tree VR view showing atherosclerosis by non-obstructive coronary artery disease.
Methods
Study population
This study is a retrospective observational review of patients referred to Harbor-UCLA, a public hospital serving South Los Angeles County, for ischemic evaluation from both outpatient and inpatient sources between January 2016 and September 2020. The patient population of Harbor-UCLA is largely non-white.
Patients referred had no chest pain, no q-waves on ECG, and had cardiomyopathy with depressed ejection fraction (EF) without regional wall motion abnormalities on transthoracic echocardiogram.
Informed consent was obtained from all participating patients. IRB approval was obtained prior to the commencement of data collection.
Study procedures
Coronary CT angiograms: Cardiac CT scans were interpreted with the use of a GE Advanced Workstation
(GE Healthcare, Milwaukee, WI). All images were used to evaluate each segment based on plaque type, amount of plaque, and severity of stenosis. The plaque was evaluated from axial source images and multiplanar reformat images of the long axis at each segment of the coronary arteries. The coronary plaque was measured on axial cross-sectional images. A total plaque score was developed to semi-quantitate the plaque in each participant using the 15-segment AHA model of the coronary arteries. Each plaque was assigned a score of 1 when plaque volume was small, 2 for medium plaque volume, and 3 for large plaque volume. The total plaque severity score (TPS) per person was determined by summing the number of interpretable coronary segments (maximum of 15 segments) with individual plaque scores (range 1–3). Segments were graded as normal (no stenosis), stenosis 1%–29%, 30%–49%, 50%–69%, <70% by visual semi-quantification method, with the assignment of scores of 0, 1, 2,3, or 4, respectively. Any stenosis greater than 70% was considered to be obstructive. Stenosis was not measured when the vessel diameter was less than 2 mm. The total segment stenosis score (TSSS) per person was calculated by summing all the 15 individual SSSs with a possible score ranging from 0 to 60 [24]. Segment Involvement Score (SIS) was calculated as the total number of coronary artery segments with plaque (range 0–15) [18,22,23].
Coronary calcium score: Coronary artery calcium score (CACS) was assessed using the Agatston score. Agatston Score: A semi-automated tool to assess the extent of coronary artery calcification on an unenhanced low dose CT of the heart. The data is reported as Agatston's total calcium score. The extent of CAD is graded as normal or mildly, moderately or severely abnormal for 0 Agatston Score [AS], 1–99 AS, 100–400 AS, and >400 AS, respectively [17,24]. Although CACS calculated through Agatston is a good indicator of the atherosclerotic burden, it does not account for the non-calcified plaque, which plays an important factor in non-obstructive CAD [18].
Statistical analysis
Results are presented as counts and frequencies (percent) for discrete variables and mean and standard deviations or median for continuous variables as appropriate. All descriptive analyses were performed using SAS for Windows, version 9.4 (SAS Institute, Cary, North Carolina).
Results
Demographics
As seen in Table 1, from the 131 patients, the mean age of 54.6 ± 12.3 years, 93 (71%) Male, 71 (54%) Hispanic, 17 (13%) White, 24 (18%) African American, and 10 (8%) Asian. Several predisposing factors for coronary artery disease were present including diabetes mellitus 39 (30%), hypertension 98 (75%), hyperlipidemia 59 (45%), and smoking 60 (46%).
|
Characteristics |
Patients (N=131) |
|
Mean Age |
54.6 ± 12.3 |
|
Gender |
|
|
Male |
93 (71%) |
|
Female |
38 (29%) |
|
Race |
|
|
Hispanic |
71 (54%) |
|
White |
17 (13%) |
|
African American |
24 (18%) |
|
Asian |
10 (8%) |
|
Risk Factors |
|
|
Diabetes Mellitus |
39 (30%) |
|
Hypertension |
98 (75%) |
|
Hyperlipidemia |
59 (45%) |
|
Smoking |
60 (46%) |
Furthermore, the mean age was 54.6 ± 12.3, the lowest age was 19, and the highest was 86 years. 25th percentile for age was 45, and the 75th was 61 years. Age was very normally distributed at the mean; it was not skewed higher or lower.
Imaging assessments
As seen in Table 2, patients with CAC of zero were 55 (42%), CAC less than 10 were 28 (21%), while median (interquartile range) of TPS was 1 (1, 5). TSS median was also 1 (1, 5) and median SIS was 0 (1, 4). There were 23 (18%) of patients with 50% stenosis consistent with obstructive CAD, and 13 (10%) had a more serious stenosis of 75% or greater.
|
Scores |
Patients (N=131) |
|
Mean CACS (coronary artery calcium score) |
1 |
|
CAC of 0 |
55 (42%) |
|
CAC Less than 10 |
28 (21%) |
|
Total plaque severity score (TPS) |
1 (1, 5) [mean (interqquartile range)] |
|
Total segment stenosis score (TSSS) |
1 (1, 5) [mean (interqquartile range)] |
|
Segment involvement score (SIS) |
0 (1, 4) [mean (interqquartile range)] |
|
Patients with 50% or greater stenosis |
23 (18%) |
|
Patients with 75% or greater stenosis |
13 (10%) |
Those with zero CAC were 49.7 ± 12.0 years, those with CAC 1-100 were 56.2 ± 11.4 years, and those with CAC > 100 were 59.2 ± 10.5 years.
Discussion
This study aimed at assessing the atherosclerotic burden in patients with newly diagnosed cardiomyopathy with reduced EF in the largely non-white LA county population using coronary CTA.
In the retrospective study by Silva et al., 168 patients were followed in the heart failure clinic, and one-third of the patients without any prior cardiac events or angina had CAD as the etiology of their newly diagnosed HF, which was proven angiographically [19]. There is wide variation in the evaluation of patients that are appropriate for ICA (invasive coronary angiography), with rates of appropriateness as high as 55% [20]. There is a potential 63.4% cost saving in the evaluation for CAD when following an evaluation pathway that involves coronary calcium scoring followed by coronary CTA and invasive coronary angiography (ICA) if needed; instead of the conventional pathway of MPI (myocardial perfusion imaging) followed by ICA [25].
Our cohort had several risk factors like diabetes 23 (30%), hypertension 57 (75%), hyperlipidemia 34 (45%), and smoking 35 (46%) that are predictive for CAD. Although these factors, in combination with Q waves or left bundle branch block, could be as sensitive as 98% for angiographically significant CAD [21].
Despite having several risk factors for CAD, the median CAC score in our cohort was only 1; furthermore, only 10% of the patients had significant obstructive CAD. We avoided potential invasive testing in more than 90% of the cohort. Thus, by using the screening criteria for the absence of chest pain, absence of Q waves on ECG, and absence of regional wall motion abnormalities on transthoracic echocardiogram, we can reliably screen patients who can be tested non-invasively. This method of ischemic evaluation in patients with cardiomyopathy is more cost-effective and has fewer complications than invasive testing. This becomes particularly important in an under-resourced setting like a public hospital serving predominantly non-white patients.
A relatively small cohort was included in the study. Despite being a well-validated tool, coronary CTA continues to have its limitations when it involves patients with irregular heart rates, pregnant women, severe obesity, elevated creatinine, and iodinated contrast allergy. There is also concern about radiation exposure.
Conclusions
In our cohort, most patients were found to have non-obstructive CAD, and that nonischemic etiology was the predominant cause of cardiomyopathy. Coronary CTA is an effective non-invasive test for the ischemic evaluation of cardiomyopathy that is used to avoid more invasive testing in a non-white population at a public hospital.
Conflicts of Interest
No conflicts to be declared.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
2. Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, et al. Heart disease and stroke statistics—2022 update: a report from the American Heart Association. Circulation. 2022 Feb 22;145(8):e153-639.
3. Lloyd-Jones DM, Larson MG, Leip EP, Beiser A, D’agostino RB, Kannel WB, et al. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002 Dec 10;106(24):3068-72.
4. Gheorghiade M, Sopko G, De Luca L, Velazquez EJ, Parker JD, Binkley PF, et al. Navigating the crossroads of coronary artery disease and heart failure. Circulation. 2006 Sep 12;114(11):1202-13.
5. Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circulation: Heart Failure. 2013 May;6(3):606-19.
6. Ling LF, Marwick TH, Flores DR, Jaber WA, Brunken RC, Cerqueira MD, et al. Identification of therapeutic benefit from revascularization in patients with left ventricular systolic dysfunction: inducible ischemia versus hibernating myocardium. Circulation: Cardiovascular Imaging. 2013 May;6(3):363-72.
7. Wolff G, Dimitroulis D, Andreotti F, Kołodziejczak M, Jung C, Scicchitano P, et al. Survival benefits of invasive versus conservative strategies in heart failure in patients with reduced ejection fraction and coronary artery disease: a meta-analysis. Circulation: Heart Failure. 2017 Jan;10(1):e003255.
8. DeVore AD, Velazquez EJ. Rethinking revascularization in left ventricular systolic dysfunction. Circulation: Heart Failure. 2017 Jan;10(1):e003770.
9. Lee HY, Oh BH. Paradigm shifts of heart failure therapy: do we need another paradigm?. International Journal of Heart Failure. 2020 Jul;2(3):145-56.
10. Doshi D, Ben-Yehuda O, Bonafede M, Josephy N, Karmpaliotis D, Parikh MA,
11. Zheng J, Heidenreich PA, Kohsaka S, Fearon WF, Sandhu AT. Variability in coronary artery disease testing for patients with new-onset heart failure. Journal of the American College of Cardiology. 2022 Mar 8;79(9):849-60.
12. Joynt KE, Orav EJ, Jha AK. Thirty-day readmission rates for Medicare beneficiaries by race and site of care. Jama. 2011 Feb 16;305(7):675-81.
13. Rodriguez F, Joynt KE, López L, Saldaña F, Jha AK. Readmission rates for Hispanic Medicare beneficiaries with heart failure and acute myocardial infarction. American heart journal. 2011 Aug 1;162(2):254-61.
14. Mahler SA, Miller CD. Diagnostic imaging to exclude acute coronary syndrome. Current Emergency and Hospital Medicine Reports. 2013 Mar;1:37-42.
15. Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey Jr DE, et al. 2011 ACCF/AHA focused update incorporated into the ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011 May 10;123(18):e426-579.
16. Park MJ, Jung JI, Choi YS, Ann SH, Youn HJ, Jeon GN, wt al. Coronary CT angiography in patients with high calcium score: evaluation of plaque characteristics and diagnostic accuracy. The International Journal of Cardiovascular Imaging. 2011 Dec;27:43-51.
17. Knipe, H. (2019, November 13). Agatston score. Radiology Reference Article | Radiopaedia.Org. https://radiopaedia.org/articles/agatston-score?lang=us
18. Gaur S, Øvrehus KA, Dey D, Leipsic J, Bøtker HE, Jensen JM, et al. Coronary plaque quantification and fractional flow reserve by coronary computed tomography angiography identify ischaemia-causing lesions. European Heart Journal. 2016 Apr 14;37(15):1220-7.
19. Silva F, Borges T, Ribeiro A, Mesquita R, Laszczynska O, Magalhães D, et al. Heart failure with reduced ejection fraction: Should we submit patients without angina to coronary angiography?. International Journal of Cardiology. 2015 Jul 1;190:131-2.
20. Chan PS, Patel MR, Klein LW, Krone RJ, Dehmer GJ, Kennedy K, et al. Appropriateness of percutaneous coronary intervention. Jama. 2011 Jul 6;306(1):53-61.
21. Doukky R, Shih MJ, Rahaby M, Alyousef T, Abusin S, Ansari NH, et al. A simple validated clinical tool to predict the absence of coronary artery disease in patients with systolic heart failure of unclear etiology. The American Journal of Cardiology. 2013 Oct 15;112(8):1165-70.
22. Pagali SR, Madaj P, Gupta M, Nair S, Hamirani YS, Min JK, et al. Interobserver variations of plaque severity score and segment stenosis score in coronary arteries using 64 slice multidetector computed tomography: a substudy of the ACCURACY trial. Journal of Cardiovascular Computed Tomography. 2010 Sep 1;4(5):312-8.
23. Budoff MJ, Mayrhofer T, Ferencik M, Bittner D, Lee KL, Lu MT, et al. Prognostic value of coronary artery calcium in the PROMISE study (Prospective Multicenter Imaging Study for Evaluation of Chest Pain). Circulation. 2017 Nov 21;136(21):1993-2005.
24. Wallis DE, O’Connell JB, Henkin RE, Costanzo-Nordin MR, Scanlon PJ. Segmental wall motion abnormalities in dilated cardiomyopathy: a common finding and good prognostic sign. Journal of the American College of Cardiology. 1984 Oct 1;4(4):674-9.
25. Kalisz K, Rajiah P. Computed tomography of cardiomyopathies. Cardiovascular Diagnosis and Therapy. 2017 Oct;7(5):539-56.
