Abstract
Purpose: As long-acting reversible contraceptive choices are becoming more popular among young women, proper informed consent over potential complications should be addressed when such decisions are made. A local case series was analyzed to consider the risk of intrauterine device (IUD) fracture.
Materials and Methods: A retrospective review of provider experience with the intrauterine device over a seven-year period was undertaken, to specifically analyze the incidence of IUD embedment and fracture in the studied population. The type of IUD, length of time that it was in place, and the age and parity of the individual, were examined.
Results and Conclusion: The apparent incidence of IUD fracture was notable, with a suggestion of IUD type and individual parity as being correlated to fracture. A 1.25 % incidence rate of IUD fracture for the ParaGard was found, in contrast to what was seen for the Mirena IUD (0.03%), in our studied population.
Keywords
Copper IUD, LNG-IUS, IUD side effects, IUD perforation
Introduction
As gynecologic providers continue to provide intrauterine devices (IUDs) for long-acting reversable contraception (LARC) with insertion of those devices, the associated complication rate needs to be well-understood, so that patients can make properly informed shared healthcare decisions. There appears to be IUD embedment into the uterine wall that can occur over time, which can cause its fracture when retrieving it with grasp of its string. The authors previously published a case series [1] that described situations in which fractured IUDs were encountered after attempts at their removal occurred. Given that patients continue to be referred to the Director of Gynecologic Sonography at our institution for assistance in managing such complications when they are encountered, this case series was continued, and the authors believe it important to report these findings at this time.
Case Series
The cases of these IUD fractures are listed in Table 1, revealing some relevant patient and IUD characteristics. Since these findings are retrospective in nature, and not prospectively collected, the proportion of IUD placement that this complication represents can only be estimated. For the population of patients described in this case series, we were able to approximate the number of IUD insertions, by our provider group practicing at multiple sites. The IUD insertions which were performed over the 7-year duration of this case series (2013 – 2019) are listed in Table 2, for the different types of devices, for this provider group, consisting of 18 different attending physicians. While some of the IUD insertions were performed by resident physicians, only 2 of the cases listed in Table 1 had an IUD inserted by a resident. None of the patients presented with complaints of pain at the time when IUD fracture was identified. There were also no visual or histologic evidence of inflammation or infection in the cases for which hysteroscopy was performed.
| # | Insertion | Removal | Duration of Use | Parity Status | Type of IUD | Management |
| 1 | 2007 | 2013 | 6 years | Multiparous | ParaGard® | Spontaneous expulsion |
| 2 | 2007 | 2013 | 6.5 years | Multiparous | ParaGard® | Hysteroscopic removal of IUD arm |
| 3 | 2003 | 2013 | 10 years | Parous | ParaGard® | Hysteroscopic removal of IUD |
| 4 | 2006 | 2013 | 7 years | Multiparous | ParaGard® | Hysteroscopic removal of IUD arm |
| 5 | 2006 | 2013 | 7 years | Multiparous | ParaGard® | Incomplete hysteroscopic removal of IUD |
| 6 | 2006 | 2013 | 7 years | Parous | ParaGard® | Hysteroscopic removal of IUD arm |
| 7 | 2006 | 2014 | 8.5 years | Parous | ParaGard® | Hysteroscopic removal of IUD arm |
| 8 | 2006 | 2015 | 8.5 years | Multiparous | ParaGard® | Hysteroscopic removal of IUD arm |
| 9 | 2008 | 2018 | 10 years | Multiparous | ParaGard® | Hysteroscopy – IUD fragments not identified intraoperatively |
| 10 | 2009 | 2019 | 10 years | Parous | ParaGard® | Hysteroscopic removal of IUD arm and hook |
| 11 | 2015 | 2019 | 4 years | Multiparous | Mirena® | Hysteroscopy – unable to remove embedded IUD arm |
| 12 | 2008 | 2019 | 11 years | Nulliparous | ParaGard® | Hysteroscopic removal of retained IUD arm |
| 13 | 2015 | 2019 | 4 years | Nulliparous | ParaGard® | Hysteroscopic removal of retained IUD arm |
| 14 | 2015 | 2019 | 4 years | Nulliparous | ParaGard® | Hysteroscopic removal of retained IUD arm |
| IUD = Intrauterine device | ||||||
| IUD type | IUD insertions | IUD fractures | Incidence of IUD fractures per 100 insertions |
| ParaGard | 1,120 | 14 | 1.25 |
| Mirena | 3,780 | 1 | 0.03 |
| Skyla/Kyleena | 1,239 | 0 | 0 |
| All Types | 6,139 | 14 | 0.23 |
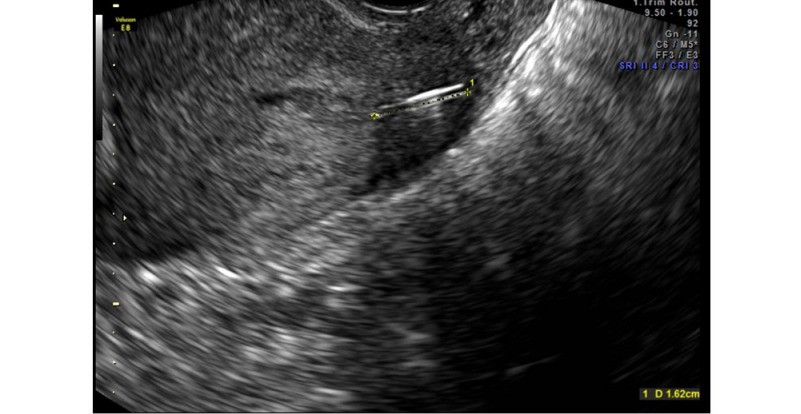
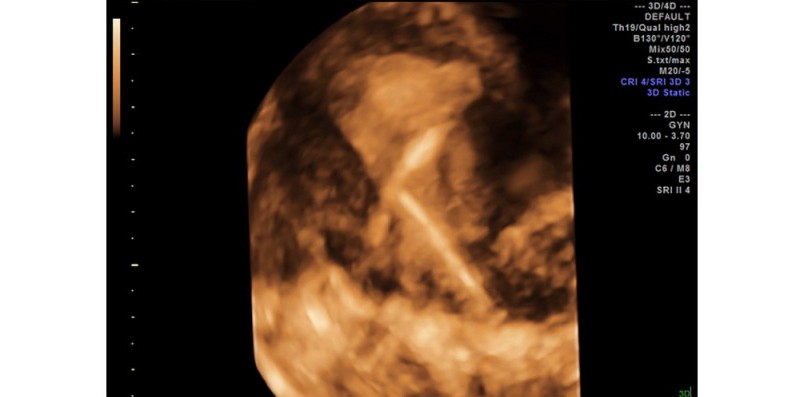
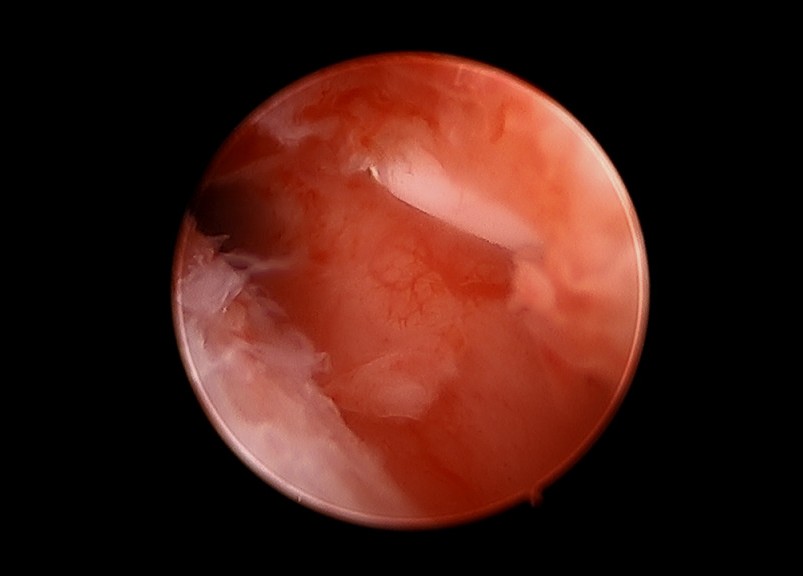
Whether an IUD is a non-hormone containing copper device (e.g., ParaGard®) or a levonorgestrel intrauterine system (LNG-IUS, e.g., Mirena®), the IUD term is applied. Each of these cases were identified as fractured IUDs, found at the time of removal from the uterus. The IUD fractures appear to have occurred after being embedded into the uterine wall, fracturing when the usual effort was exerted for the IUD removal (i.e., pulling on the string). It is presumed that embedment of the IUD into the uterine wall must have occurred, to result in the fractured IUD which was found. This phenomenon may be similar to the process of IUD perforation, alternatively referred to as “migration”,[2] in that it may occur over time, and is not necessarily temporally related to the IUD insertion. Figure 1 shows this with the use of 2D ultrasound, and Figure 2 illustrates this with the application of three-dimensional transvaginal sonography (3DTVS). Figure 3 shows a hysteroscopic view of an embedded IUD arm, at the time of its hysteroscopic removal.
Figure 1. The arm of an IUD is shown as embedded in the left cornual portion of the uterus.
Figure 2. 3D ultrasound showing a combination of sagittal and rendering coronal views of the cervix, demonstrating an arm of the IUD within the anterior lip of the cervix at the level of the external os.
Figure 3. Hysteroscopic image of the last case, illustrating the embedment of an arm of the IUD which was fractured at its removal attempt.
Comment
As more young nulliparous women are using this LARC method [3], the number of larger frame devices being used, along with the duration of their potential use (5 years vs. 10 years), may be a relevant factor leading to the complication described in this case series. The size difference between a ParaGard® or Mirena® (having a diameter of 32 mm) and a Skyla® or Kyleena® (smaller frame size, having a diameter of 28 mm) may be clinically important. A difference in the intrauterine interostial diameter also exists between a nulliparous and a multiparous woman [4], which may also be clinically relevant.
While embedment of an IUD into the uterine wall may not commonly occur, this case series describes its approximate incidence of occurrence. Though this embedment cannot be anticipated, there may be factors relevant to its occurrence [5]. The patient and IUD features which are listed in Table 1, may represent identifiable conditions worthy of note. The duration of IUD use, as well as the IUD type, appears to be especially pertinent. The counting of the IUD insertions among all of the physicians who would typically refer for imaging of such complications, as well as the patients who underwent hysteroscopy for resolving this complication, yielded approximately 1000 IUD insertions per year over the course of 7 years. Naturally, the number of IUD insertions by this provider group, may not exactly correlate with the entire number of IUDs which were in place during the course of this case series, with some resulting in this complication. Nonetheless, in spite of the temporal discordance, these numbers may represent a close approximation of the risk of this phenomenon. It should be additionally noted that there may be limited continuation rates of IUD users [6], making an estimate of the true prevalence of IUD fracture to have limited accuracy in this dataset. Though it may be more ideal to present the described phenomenon in a prospectively followed investigation, that is unlikely to ever be practically performed. Thus, this case series is presented, for its valuable clinical information, to inform the patient (and physician) population. The low IUD complication rate that has been reported [7] may not be consistent with the experience in this case series, as we found the prevalence of this phenomenon (i.e., embedment and IUD fracture) to be about 1 per 500 IUD insertions for any IUD type, and specifically, slightly more than 1 per 100 ParaGard IUD insertions. The calculated relative risks of this particular IUD complication in our experience are described in Table 2, and this phenomenon may occur more commonly than what has been previously reported [8]. This complication may also be replicated by others who have the capability of this type of surveillance with sonography in skilled hands. The information contained in this present case series should be considered when obtaining informed consent to a patient considering this LARC method.
Acknowledgement
Chris Kabir, MS – for assistance with the statistical analyses performed.
None of the authors have any financial conflicts of interest.
Author Contributions
Elliot M. Levine, MD: Conducted the extensive supporting literature review and data analysis.
Carlos M. Fernandez, MD: Performed the sonography for each described case in data set.
Marie Cabiya, MD: Instituted the original case report and identified the clinical problem described.
Imaan Ansari, MD: Resident physician who helped organize data in presented table.
Leah Delfinado, MD: Assistance in writing and editing manuscript.
References
2. Santos AP, Wetzel C, Siddiqui Z, Harper DS. Laparoscopic removal of migrated intrauterine device. Case Reports. 2017 Sep 27;2017.
3. Prescott GM, Matthews CM. Long-acting reversible contraception: a review in special populations. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy. 2014 Jan;34(1):46-59.
4. Benacerraf BR, Shipp TD, Lyons JG, Bromley B. Width of the normal uterine cavity in premenopausal women and effect of parity. Obstetrics & Gynecology. 2010 Aug 1;116(2):305-10.
5. Shipp TD, Bromley B, Benacerraf BR: The width of the uterine cavity is narrower in patients with an embedded IUD. The Journal of Ultrasound in Medicine 2010;12:1848.
6. Agostini A, Godard C, Laurendeau C, Benmahmoud Zoubir A, Lafuma A, et al. Two year continuation rates of contraceptive methods in France: a cohort study from the French national health insurance database. The European Journal of Contraception & Reproductive Health Care. 2018 Nov 2;23(6):421-6.
7. Jatlaoui TC, Riley HE, Curtis KM. The safety of intrauterine devices among young women: a systematic review. Contraception. 2017 Jan 1;95(1):17-39.
8. Korber PE, Goldstein BH. The Management of a patient with a fragmented intrauterine device embedded within the cervical canal. Contraception. 2019 Jan 1;99(1):67-9.