Abstract
The positive prognostic role of the immune environment in colorectal cancer is widely accepted. However, there are few data about the prognostic significance of interleukin-22 in human colorectal cancer which is still debated. In our study we could demonstrate for the first time a positive prognostic role of interleukin-22 in human colorectal cancer relying on its capacity to induce in tumor cells the production of chemokines recruiting into the tumor microenvironment neutrophils associated with a favorable clinical outcome.
Keywords
Human colorectal cancer, IL-22, Neutrophils, Tissue microarray, Prognosis, Chemokines
Commentary
Colorectal cancer (CRC) is the third most common cause of cancer related death worldwide [1]. Its outcome depends on different factors. On one hand there are cancer related features, including mutations, microsatellite status, and methylation alterations. In addition, the tumor microenvironment, which includes non-transformed stromal and tumor infiltrating cells, interacting with cancer cells, also significantly influences tumor biology and consequently patients’ survival [2]. Infiltration by cellular components of the adaptive immune system, and, in particular, by cytotoxic CD8+ and T-helper type 1 lymphocytes, has been shown to predict the survival of patients with CRC more efficiently than the tumor-nodemetastasis (TNM) staging [3].
In previous studies we could demonstrate that also cells of the innate immune system, such as neutrophilic granulocytes are associated with a favorable clinical outcome, possibly due to their co-stimulatory activity on CD8+ T cells [4]. However, the role of immune cells producing interleukin-22 (IL- 22) in CRC progression remains a matter of debate.
IL-22 can be produced by a variety of immune cells, including conventional T lymphocytes and innate lymphoid cells [5]. Instead, the IL-22 receptor, including IL-22Rα and IL-10Rβ chains, is uniquely expressed on non-hematopoietic cells, including keratinocytes and intestinal epithelial cells [6]. IL- 22 mediates pleiotropic functions. In different anatomical districts, such as skin and intestinal and bronchial epithelium, IL-22 synergizes with IL-17 and TNFα to promote host defense [7-9] and innate immunity to bacterial infections. On the other hand, IL-22 induces epithelial cell proliferation and up-regulation of genes encoding pro-survival molecules [10-13] and may protect liver, intestine and lung from tissue destruction [11-16]. Interestingly, IL-22 also plays a role in the maintenance of host-microbiota symbiosis [17].
Studies published so far, mainly based on murine models, support a tumor promoting role of IL-22 in hepatocellular carcinoma [18,19] and liposarcoma [20]. IL-22 production has also been shown to promote CRC development [21], possibly by direct effects on stem cells [22] or by enhancing cancer cell proliferation [23,24]. Most recently, however, IL-22 has been shown to play a key role in the control of genotoxic damage induced by carcinogens in colon epithelial stem cells, thereby limiting mutagenesis and cancer outgrowth [25]. Thus, in the intestine IL-22 might function as a double-edged sword, as on the one hand it mediates protective functions, through induction of epithelial regeneration and production of antimicrobial peptides, and on the other, it supports tumorigenesis, by increasing proliferation and apoptotic resistance of neoplastic epithelial cells [6,24]. Notably, most studies are based on mouse models of colitis-associated CRC, whereas in humans the majority of CRC develop in the absence of inflammatory conditions. On the other hand, there is paucity of data based on human studies. A specific polymorphism in IL-22 gene is associated with increased risk of developing CRC [26]. Furthermore, IL-22 synergizes with IFN-γ to induce iNOS production in human colon carcinoma cell lines with consequent production of pro-carcinogenic nitric oxygen species [27]. However, the overall impact of IL-22 on clinical outcome of CRC in humans has not been investigated so far.
We have evaluated the prognostic significance of IL-22 in human primary CRC, using two independent tissue microarrays (TMA), cumulatively including 514 samples. We observed that infiltration by IL-22 immune cells is significantly associated with an increased 5-year survival rate, independently of known prognostic factors including age, sex, T stage, N stage, tumor grade, vascular invasion, tumor border configuration, and microsatellite stability.
IL-22-producing cells, were detectable within normal colonic tissues and CRC, although, in the latter case, at a significantly higher density. Ex vivo phenotypic analysis revealed that they mainly consist of conventional T-helper cells (Th22), with a large majority also expressing IFN-γ and IL-17.
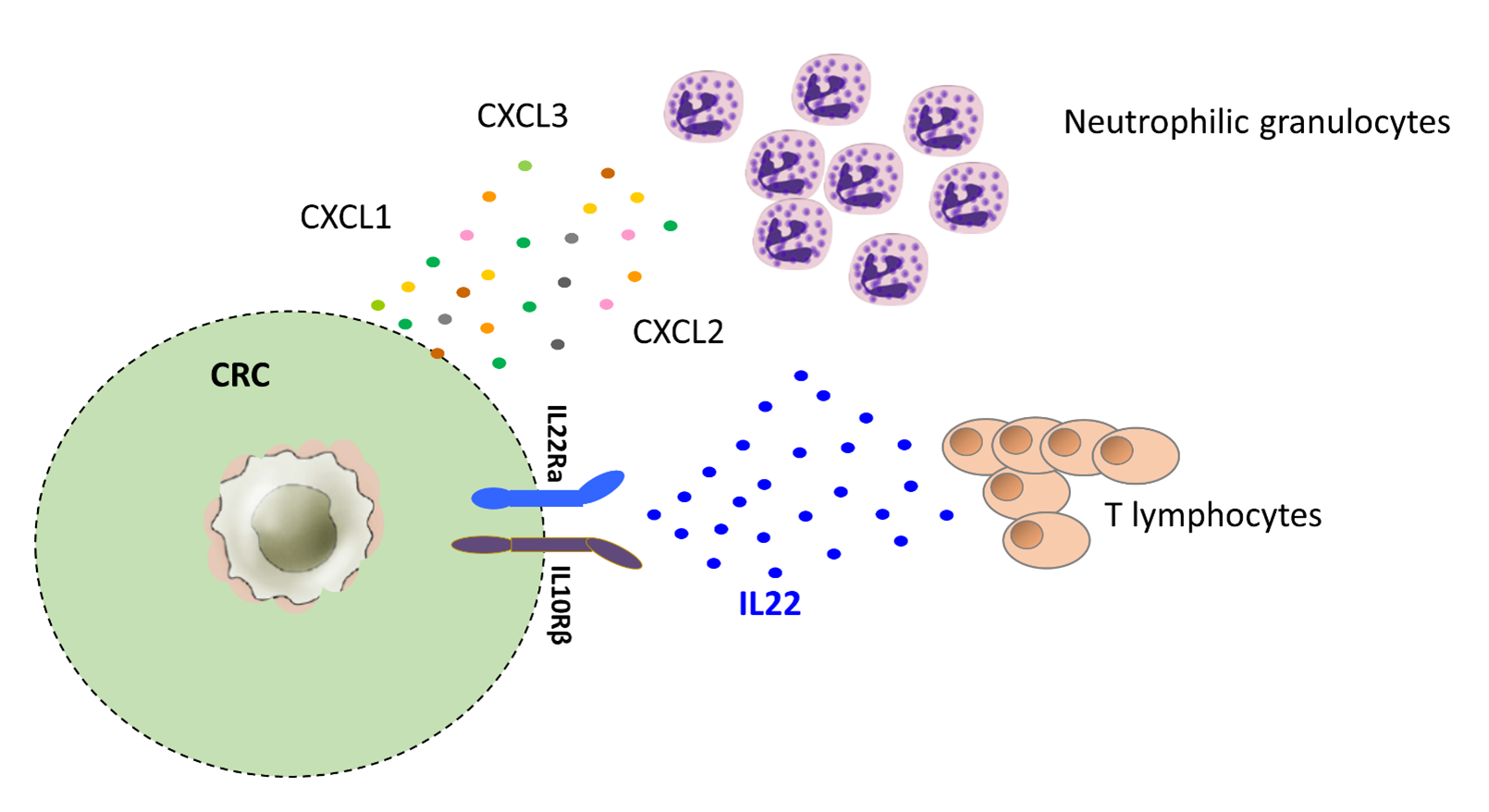
Interestingly, when we evaluated the direct effects of IL- 22 on CRC cells lines, we did not observe any significant effect on cell proliferation. In contrast, IL-22 treatment consistently increased in CRC cells the expression of CXCL1, CXCL2 and CXCL3 neutrophil recruiting chemokines at gene and protein level. Furthermore, culture supernatants from IL-22-exposed CRC cells enhanced neutrophil migration in vitro.
Consistent with the in vitro data, a significant correlation between expression of IL-22 and that of CXCL1, CXCL2 and CXCL3 genes was observed in 597 CRC specimens included in the TCGA database [28].
To the best of our knowledge, we demonstrated for the first time a positive prognostic impact of tumor infiltrating Th22 cells in human CRC. This beneficial effect relies on the capacity of Th22 cells to enhance tumor infiltration by neutrophilic granulocytes by favoring release of neutrophil recruiting chemokines from CRC cells (Figure 1).
Taken together our data delineate a complex crosstalk occurring between tumor cells, T lymphocytes and neutrophil granulocytes within the CRC microenvironment. Further studies are warranted to clarify the role of additional components of CRC microenvironment, including in particular gut microbiota, potentially contributing to Th22 recruitment [29] and activation, and to neutrophil functional modulation.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the Swiss National Science Foundation (grant# 133699), awarded to Dr. Giandomenica Iezzi.
References
2. Fridman WH, Zitvogel L, Sautès-Fridman C, Kroemer G. The immune contexture in cancer prognosis and treatment. Nature Reviews Clinical Oncology. 2017.
3. Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006 Sep 29;313(5795):1960-4.
4. Governa V, Trella E, Mele V, Tornillo L, Amicarella F, Cremonesi E, et al. The interplay between neutrophils and CD8+T cells improves survival in human colorectal cancer. Clinical Cancer Research. 2017 Jul 15;23(14):3847-3858.
5. Witte E, Witte K, Warszawska K, Sabat R, Wolk K. Interleukin-22: a cytokine produced by T, NK and NKT cell subsets, with importance in the innate immune defense and tissue protection. Cytokine & Growth Factor Reviews. 2010 Oct;21(5):365-79.
6. Sabat R, Ouyang W, Wolk K. Therapeutic opportunities of the IL-22-IL-22R1 system. Nature Reviews Drug Discovery. 2014 Jan;13(1):21-38.
7. Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi- Joannopoulos K, Collins M, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. The Journal of Experimental Medicine. 2006 Oct 2;203(20):2271-9.
8. Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nature Medicine. 2008 Mar;14(3):275-81.
9. Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, Pallotta S, et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. Journal of Clinical Investigation. 2009 Dec;119(12):3573- 85.
10. Sonnenberg GF, Nair MG, Kirn TJ, Zaph C, Fouser LA, Artis D. Pathological versus protective functions of IL-22 in airway inflammation are regulated by IL-17A. The Journal of Experimental Medicine. 2010 Jun 7;207(6):1293-305.
11. Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Karow M, Flavell RA. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity. 2007 Oct;27(4):647- 59.
12. Radaeva S, Sun R, Pan HN, Hong F, Gao B. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology. 2004 May;39(5):1332-42.
13. Sugimoto K, Ogawa A, Mizoguchi E, Shimomura Y, Andoh A, Bhan AK, et al. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. Journal of Clinical Investigation. 2008 Feb;118(2):534-44.
14. Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Stevens S, Flavell RA. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008 Dec 19;29(6):947-57.
15. Pickert G, Neufert C, Leppkes M, Zheng Y, Wittkopf N, Warntjen M, et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. The Journal of Experimental Medicine. 2009 Jul 6;206(7):1465-72.
16. Simonian PL, Wehrmann F, Roark CL, Born WK, O’Brien RL, Fontenot AP. gammadelta T cells protect against lung fibrosis via IL-22. The Journal of Experimental Medicine. 2010 Sep 27;207(10):2239-53.
17. Goto Y, Obata T, Kunisawa J, Sato S, Ivanov II, Lamichhane A, et al. Innate lymphoid cells regulate intestinal epithelial cell glycosylation. Science. 2014 Sep 12;345(6202):1254009.
18. Park O, Wang H, Weng H, Feigenbaum L, Li H, Yin S, et al. In vivo consequences of liver-specific interleukin-22 expression in mice: Implications for human liver disease progression. Hepatology. 2011 Jul;54(1):252-61.
19. Jiang R, Tan Z, Deng L, Chen Y, Xia Y, Gao Y, et al. Interleukin-22 promotes human hepatocellular carcinoma by activation of STAT3. Hepatology. 2011 Sep 2;54(3):900- 9.
20. Wang Z, Yang L, Jiang Y, Ling ZQ, Li Z, Cheng Y, et al. High fat diet induces formation of spontaneous liposarcoma in mouse adipose tissue with overexpression of interleukin 22. PLoSOne. 2011;6(8):e23737.
21. Huber S, Gagliani N, Zenewicz LA, Huber FJ, Bosurgi L, Hu B, et al. IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature. 2012 Nov 8;491(7423):259-63.
22. Kryczek I, Lin Y, Nagarsheth N, Peng D, Zhao L, Zhao E, et al. IL-22+CD4+ T cells promote colorectal cancer stemness via STAT3 transcription factor activation and induction of the methyltransferase DOT1L. Immunity. 2014 May 15;40(5):772-784.
23. Sun D, Lin Y, Hong J, Chen H, Nagarsheth N, Peng D, et al. Th22 cells control colon tumorigenesis through STAT3 and Polycomb Repression complex 2 signaling. Oncoimmunology. 2015 Sep 2;5(8):e1082704.
24. Kirchberger S, Royston DJ, Boulard O, Thornton E, Franchini F, Szabady RL, et al. Innate lymphoid cells sustain colon cancer through production of interleukin-22 in a mouse model. JExpMed. 2013 May 6;210(5):917-31.
25. Gronke K, Hernández PP, Zimmermann J, Klose CSN, Kofoed-Branzk M, Guendel F, et al. Interleukin-22 protects intestinal stem cells against genotoxic stress. Nature. 2019 Feb;566(7743):249-253.
26. Thompson CL, Plummer SJ, Tucker TC, Casey G, Li L. Interleukin-22 genetic polymorphisms and risk of colon cancer. Cancer Causes Control. 2010 Aug;21(8):1165-70.
27. Ziesche E, Bachmann M, Kleinert H, Pfeilschifter J, Muhl H. The interleukin-22/STAT3 pathway potentiates expression of inducible nitric-oxide synthase in human colon carcinoma cells. Journal of Biological Chemistry. 2007 Jun 1;282(22):16006-15.
28. Tosti N, Cremonesi E, Governa V, Basso C, Kancherla V, Coto-Llerena M, et al. Infiltration by IL22-Producing T Cells Promotes Neutrophil Recruitment and Predicts Favorable Clinical Outcome in Human Colorectal Cancer. Cancer Immunol Res. 2020;8(11).
29. Cremonesi E, Governa V, Garzon JFG, Mele V, Amicarella F, Muraro MG, et al. Gut microbiota modulate T cell trafficking into human colorectal cancer. Gut. 2018 Nov;67(11):1984-1994.