Abstract
Background: Emergency situations can be personal and medical but also external and humanitarian (armed conflicts). In each situation a preparedness plan consisting of a risk assessment and gap analysis, emergency preparedness protocol, response and recovery protocol are needed.
Objective: To discuss the question “how to provide a sufficient supply of safe, effective and quality blood and blood components in emergency situations?’”
Study design: Literature review.
Results: To adequately respond to emergency situations in medicine it is important to prevent multiple tissue and organ failure by maintaining perfusion of tissues and organs. This can be secured maintaining a low blood viscosity by infusing crystalloids or plasma replacement fluids to allow red cells to deliver oxygen. To respond adequately to emergency situations health care institutions and blood establishments need an emergency preparedness plan based on a risk assessment and gap analysis, emergency preparedness protocol and response and recovery protocol.
Conclusion: Emergency situations, medical or external, need a preparedness plan based on a risk assessment and gap analysis and an anticipation on adequate response and resilience.
Keywords
Emergency, Preparedness plan, Response, Perfusion, Organ failure
Introduction
An emergency situation is an unforeseen occurrence or combination of circumstances that is potentially dangerous or even life-threatening and calls for immediate action.
It could happen medically on an individual basis, but also as an external incident to a more general, societal, environmental, natural origin, or influenced by human beings or directly caused by humans and present as a life-threatening event. It may happen in any field of life.
In the immediate aftermath of the Second World War, the newly established UN adopted the Universal Declaration of Human Rights, which includes the right to health [1]. In 1948, the General Assembly of the UN proclaimed
-
this Universal Declaration of Human Rights as a common standard of achievement for all peoples and all nations, to the end that every individual and every organ of society, keeping this Declaration constantly in mind, shall strive by teaching and education to promote respect for these rights and freedoms and by progressive measures, national and international, to secure their universal and effective recognition and observance, both among the peoples of Member States themselves and among the peoples of territories under their jurisdiction.
Article 25 of the Declaration reads:
- Everyone has the right to a standard of living adequate for the health and well-being of himself and of his family, including food, clothing, housing and medical care and necessary social services, and the right to security in the event of unemployment, sickness, disability, widowhood, old age or other lack of livelihood in circumstances beyond his control.
- Motherhood and childhood are entitled to special care and assistance. All children, whether born in or out of wedlock, shall enjoy the same social protection.
Development programs
Over the decades that followed, societies changed, decolonization began, and public awareness of the principle human rights started to grow. Yet there is a major difference between the advanced and industrial countries, home to around 16% of the global population, and the developing countries, home to the majority (84%) of the global population [2]. The UN Development Program (UNDP) together with the World Bank has mapped the world according to various development indices and indicators illustrating the socioeconomic gaps and the huge diversity of states of development and its consequences [3,4]. With the aim of rectifying this situation, the UN launched a major human development program at the turn of the last century—the Millennium Development Goals 2000–2015 [5]. This program includes a strong element of education intended to bridge the existing knowledge and socioeconomic gaps.
Because of the progress made, this work was continued in 2016 with another 15-year program: the UN Sustainable Development Goals 2016–2030 (Figure 1) [6].

Figure 1. Sustainable Development Goals: Goal 3 (dark green, on the right) = Good Health and Wellbeing.
The 2030 Agenda for Sustainable Development was unanimously adopted by the UN’s Member States at the historic UN General Assembly Summit in September 2015 [7]. The 17 Sustainable Development Goals (SDGs) and their 169 targets are part of this agenda. The SDGs are a bold, universal agreement to end poverty in all its dimensions and craft an equal, just and secure world – for people, planet and prosperity. The SDGs were developed through an unprecedented global consultative process that brought national governments and millions of citizens from around the globe together to negotiate and adopt this ambitious agenda [7].
The third goal: Good Health and Well-being is intended to Ensure healthy lives and promote well-being for all at all ages. This includes the availability and accessibility of safe and efficacious blood and blood components as essential medicines (EMs), both the cellular components and the plasma derived medicinal products (PDMPs) [6].
SDG Target 3.8 reads – Achieve universal health coverage (UHC), including financial risk protection, access to quality essential health-care services and access to safe, effective, quality and affordable essential medicines and vaccines for all.
This makes clear the importance of a high-quality blood supply as well as a professional and balanced approach to the clinical transfusion of blood, blood components, and PDMPs.
Concern
At the 2010 World Health Assembly (WHA) concern was expressed through resolution WHA63.12 about the unequal levels of access globally to blood products, in particular PDMPs. Such inequality of access left patients suffering without treatment, and many of those with severe congenital and acquired disorders without adequate plasma-derivative treatments [8]. In this resolution, the World Health Assembly urged WHO Member States:
-
To take all the necessary steps to update their national regulations on donor assessment and deferral, the collection, testing, processing, storage, transportation and use of blood products, and operation of regulatory authorities to ensure that regulatory control in the area of quality and safety of blood products across the entire transfusion chain meets internationally recognized standards.
Requirements for implementing an effective national blood regulation are described in the WHO Assessment criteria for national blood regulatory systems, which was replaced in 2019 by the WHO Global Benchmarking Tool + Blood (GBT + blood) [9].
According to resolution WHA63.12, it was recognized that achieving self-sufficiency in the supply of safe and efficacious blood is an important national goal in preventing blood shortages and meeting the transfusion needs of the patient population. Blood and blood components [whole blood, red blood cells (RBCs), platelets and fresh plasma] were therefore added to the eighteenth edition of the core list of the WHO Model List of Essential Medicines in 2013 [10]. Self-sufficiency in this context means that the national needs of patients for safe and clinically effective blood and blood components, as assessed within the framework of the national health system, are met in a timely manner particularly in emergency situations. It also entails equitable access for patients to safe and efficient blood for transfusion. Self-sufficiency can be accomplished by promoting 100% voluntary non-remunerated regular blood donation (VNRD). Defining blood and blood components as EMs could also contribute to self-sufficiency by:
- Drawing attention to the role of national governments in providing the necessary organizational and infrastructural support required for assuring a safe and adequate blood supply in periods of peace and stability as well as in situations of disasters and (humanitarian) emergencies; and
- Encouraging countries to develop and ensure compliance with safety and current quality standards as well as good practices in product preparation (cGMP) for transfusion, irrespective of the situation and environmental conditions.
Effective blood legislation and regulation [WHO Global Benchmarking Tool + Blood (GBT+blood)] is crucial for the establishment of blood components as EMS [9]. However, in some countries blood and blood components do not meet the legal definition of medicines, and this could require taking a different approach to assure their quality, safety, and availability from the one used for conventional medicines. In 2014, the International Conference of Drug Regulatory Authorities (ICDRA) recommended that WHO should undertake a project aiming to provide guidance on the management of blood and blood components as EMs [11]. Blood and blood components are either manufactured by blood establishments and distributed to hospitals and other facilities or prepared by hospital blood banks for use in the treatment of various diseases [12,13]. In some cases, the latter is perceived as part of medical practice rather than as the preparation of a biological therapeutic product. There is therefore a concern that blood and blood components could be prepared in facilities (including hospitals) that are not subject to appropriate regulatory oversight and where awareness of product liability is lacking. Consequently, the regulatory system should apply to all facilities whether private or public and should be focused on protection of patient and donor rights (safety, care) and product liability (quality, manufacturing or processing).
In 2018, WHO published the first edition of a Model list of essential in vitro diagnostics, which includes the essential diagnostics (reagents and test kits) needed for the mandatory quality testing of blood for transfusion – immunohematology (blood grouping, alloantibody detection and compatibility testing) and for screening for transfusion-transmissible infectious agents (TTIAs) (viral, bacterial, parasitic and others) [13].
Ethics
Ethical questions related to health, health care including supportive blood transfusion, and public health range from moral issues around reproduction, national obligations in the provision of quality health care services, and appropriate measures to diseases and mitigate disaster and emergency disparities.
Scholars and health care professionals have debated ethical questions related to health and health care since the earliest days of medicine, starting with the Hippocratic Oath. Recent formal efforts to articulate international standards of ethics applicable to health and health care can be traced to the Nuremberg military trials of 1945–1946, in the period immediately following the Second World War [14]. The principles that emerged from those trials, known as the Nuremberg Code, are applicable to many types of health-related situations involving human beings, including clinical trials. The growing breadth and complexity of contemporary health challenges has raised a range of difficult questions. Such questions cannot always be adequately addressed by relying exclusively on existing policies, guidelines or codes of conduct. There are ethical debates over access to new and expensive pharmaceuticals, specialized blood products, procedures and medical technologies in situations where infrastructure has been disrupted or are not yet developed [15]. These, together with increasing awareness of the gross health disparities that continue to exist both within and between countries of different states of development, have called attention to the need for an ethics of health policy and practice. The 1948 Universal Human Rights Declaration serves as the gold standard against which to measure ethical conduct and behavior, whatever the conditions and circumstances [4]. However, despite these principles, inequality persists among and between populations as illustrated by the 2018 UNDP’s Human Development Programme Statistical Update on Indices and Indicators, and the current politics that affect the entire globe [16].
Epidemics and pandemics, natural and human-made disasters, and emergencies raise many ethical issues for the people involved, who include responders, public health and health care professionals and other workers, and internal and external policy-makers. There are three main issues that need to be continuously addressed:
- The obligation to uphold principles and values embodied in international and national ethics guidelines, as well as human rights instruments. Economic and other sanctions and restrictions on essential supplies, for example, food, medication and medical consumables, cause a serious ethical conflict.
- Reluctance to ground the need for ethical oversight in the classical distinction between health practice and applied health research, recognizing that such a distinction easily becomes blurred during emergencies, especially when these are protracted and humanitarian.
- Adaptations of ethical principles, oversight and processes, focusing on the deliberations on the gravity of the situation and the urgency of the need.
During disastrous events and emergencies, one of the main objectives of medical and public health personnel is to minimize mortality and morbidity through maintaining current professional standards and adequate and justified prioritization. Because of constraints on time and resources, however, the ability to do so is limited, and a way must be found to choose who should be given what treatment. Effective planning and management of resources and personnel will significantly influence the duration and severity of the situation but also raises important ethical questions about setting priorities and allocating resources fairly. The process of triage involves prioritizing the use of scarce medical resources when these are insufficient to provide immediate treatment or diagnostic interventions for everyone. Effective triage should help determine who to treat first, and with what kind of treatment. In other words, triage involves assessing the nature and severity and emergency of an individual’s illness or injury to determine their health status and whether they can be saved. The information is also used to decide on the priority and type of care and blood product to give. In terms of blood procurement (collection, processing, and testing), decisions will have to be made on what the acceptable minimum is to preserve fundamental safety and efficacy of the supply. Triage can also be based on nonmedical factors, such as socioeconomic status, economic blockades, economic wars, and social utility, in deciding the priority to be given to whom and for what. Beyond decisions about care, triage can also involve decisions about priority for transport and facilities in which individuals will receive further care. Triage is used not only where the resources and/or medical personnel are insufficient to provide the necessary immediate care to everyone at the same time, but also, for example, in accident and emergency departments [17].
Rationing is a second means of effective resource management, which involves delaying or withholding immediate treatment for individuals for economic or scarcity reasons. Rationing, like triage, is not only used during emergencies. For instance, most health care systems ration the number of hip replacements performed each year, because there are not enough resources to treat everyone [18].
Ethical principles can be categorized as either substantive or procedural [19]. Substantive ethical principles include explanations of why a particular policy or course of action is ethically justified. Procedural ethical principles outline the way in which decisions or actions should be taken if they are to be considered ethically justified. There are ten substantive and five procedural ethical principles to be distinguished:
Substantive ethical values
Individual liberty: Restrictions to individual liberty should be proportional, necessary, relevant, be the least restrictive possible and be applied equitably.
Protection of the public from harm: Decision-makers should weigh the imperative for compliance, provide reasons for public health measures to encourage compliance and establish mechanisms to review decisions.
Proportionality: Restrictions to individual liberty and measures taken to protect the public from harm should not exceed those necessary to address the actual level of risk to or critical needs of the community.
Privacy: Individuals have a right to privacy in health care. In an emergency, it may be necessary to override this right to protect the public from serious harm.
Duty to provide care: Health care providers will have to weigh the demands of their professional roles against other competing obligations to their own health and to their families and friends. Moreover, health care workers will face significant challenges in resource allocation, scope of practice, professional liability and workplace conditions.
Reciprocity: Measures to protect the public goods are likely to impose a disproportionate burden on health care workers, patients and their families. This is particularly the case in a situation of massive displacement, evacuation or deportation of people which may cause an increase in demand.
Equity: During a pandemic or an emergency, difficult decisions will need to be made about which health services to maintain and which to defer. Depending on the severity of the event, this could curtail not only elective surgeries but could also limit the provision of emergency or other essential services.
Trust: Decision-makers will be confronted with the challenge of maintaining stakeholder trust while simultaneously implementing various control measures during an evolving emergency. Trust is enhanced by maintaining such process values transparently in a situation of protracted humanitarian emergency.
Solidarity: As the world learned during the severe acute respiratory syndrome (SARS) and COVID-19 outbreak, a pandemic outbreak of influenza or monkey pocks will require a new vision of solidarity among nations. A pandemic can challenge conventional ideas of national sovereignty, security or territoriality. It also requires solidarity within and among health care institutions. It calls for collaborative approaches that set aside traditional values of self-interest or territoriality among health care professionals, services or institutions like blood establishments and hospitals.
Stewardship: Inherent in stewardship are the notions of trust, ethical behavior and good decision-making, politically as well as professionally. This implies that decisions regarding resources are intended to achieve the best patient health and public health outcomes in the unique circumstances of, for example, a pandemic or an armed or economic conflict, violent or political aggression.
Procedural values
Reasonable: Decisions should be based on reasons (i.e., evidence, principles, and values) and made by people who are credible and accountable (competent leadership).
Open and transparent: The basis on which decisions are made should be publicly accessible.
Inclusive: Stakeholders should have opportunities to engage in the decision-making process.
Responsive: There should be mechanisms to address disputes and complaints as important elements of sustained quality management.
Accountable: Defense of actions and inactions should be grounded in the fundamental ethical values discussed.
Challenges like prioritizing, economic blockades, armed conflicts and wars, disrupted or inadequate infrastructure, displacement of people, aggressive migrant policies and unpredictability of disasters, and armed conflicts will have a major impact on the human response and on maintaining professional standards and ethics in practice. Regulatory flexibility in setting a balance and drawing a line of unacceptability together with resilience and inventiveness will provide solutions to cope with such devastating situations and continue the provision of basic care in accordance with the UN Declaration of Universal Human Rights [1].
Demand Planning
Routine demand planning considers the seasonal collection and distribution of blood based on local health care requirements and organization. Emergency planning builds on this essential background data. Guidelines are available for appropriate routine use [20]; however, special provision may need to be made for both emergency work and the transfusion-dependent communities during prolonged shortages. Various examples are referred to throughout this document. However, the following list is a useful prompt when planning for demand.
- Surgical. Limited access to safe blood can be a critical barrier to providing some elements of surgical care in resource-limited settings globally. The Lancet Commission on Global Surgery calls for global access to a safe and affordable blood supply by 2030 through a minimum collection of 15 units per 1,000 people/year. Low- and middle-income countries (LMICs) collect a range of 3.9–11.7 units per 1,000 people/year [2,21].
- Trauma. The foremost reason for death among under 40-year-olds is traumatic injury, which causes an estimated 5 million deaths per year globally. Of these deaths, an estimated 10–20% are preventable. Uncontrolled hemorrhage within 6 hours of injury is one of the prime emergency causes of avoidable death and has led many trauma specialists to seek ways to reduce early mortality due to severe injuries [22].
- Hemoglobinopathies and inherited coagulopathies. Hemoglobinopathies and inherited coagulopathies, such as the hemophilia A and B and von Willebrand disease, may be uncommon but people with severe disease are heavily dependent on transfusion (blood and PDMPs) support. These patients might suffer disproportionately during blood shortages [23].
- Hemato-oncology/cancer. Transfusion medicine plays a vital role in the supportive care of patients receiving therapy for hematological disorders or cancer, and hemopoietic stem cell transplants [24]. Mitigation strategies may be required, including choice and timing of treatment.
- Obstetric hemorrhage (OH). OH, which mostly occurs postpartum, is a leading cause of maternal mortality worldwide and accounts for one third of maternal deaths in Africa [21,25]. Multiple factors contribute to effective management, including timely access to emergency interventions, access to blood, availability of well-trained health care staff, financial and infrastructural provisions [26]. All of these may be further compromised during emergencies.
- Pediatric anemia. Severe anemia resulting in significant morbidity and mortality is common in children in sub-Saharan Africa, South-East Asia, and the Middle East and blood transfusion is a life-saving intervention. Blood shortages are common in LMICs and delays in delivery contribute significantly to in-hospital mortality of children with severe anemia, especially when associated with malaria [27] and helminthic infections.
- Anemia due to malnutrition. Globally, anemia is a leading cause of morbidity and mortality among women and children. In 2011, 43% of children under 5 years, 38% of pregnant women, and 29% of non-pregnant women between the ages of 15 and 49 years were living with anemia, chronic and untreated, which reduces physiological reserves.
- Access to and delivery of safe and sufficient supplies of effective blood and blood products, as well as PDMPs becomes more difficult in natural disasters and humanitarian emergencies.
WHO Efforts
Since 1975, a decade after the discovery of the Australia antigen by Blumberg present in Aboriginals, the causative agent of Hepatitis B and transmissible with blood and other body fluids, WHO has recognized being a surface antigen of a virus called Hepatitis B virus and named HBsAg. The World Health Assembly (WHA) adopted a resolution WHA28.72 ‘Utilization and Supply of Human Blood and Blood Products’ [28] urging the Member States to promote the development of national blood establishments based on voluntary and non-remunerated blood donation (VNRBD) and to enact effective legislation and regulation governing their operations. The resolution expressed serious concerns that commercial activities of private firms might interfere with the WHO efforts to establish safe and effective blood transfusion services based on non-remunerated voluntarism. The resolution also helped to recognize universal access to blood and blood components as one of the strategic areas of WHO and an integral part of WHO’s efforts towards achieving Universal Health Coverage (UHC) [29].
WHO has been and still is at the forefront of the movement to universal access to affordable, safe and quality-assured blood and blood products as mandated by successive WHA resolutions: WHA58.13 (Blood Safety: Proposal to Establish World Blood Donor Day); WHA63.12 (Availability, Safety and Quality of Blood Products) [30,31]. Consequently, blood and blood products were added to the WHO Model List of Essential Medicine [8,11]. In 2018 reagents and test kits focused on immunological testing and compatibility, and safety of donated blood were included in the first Model List of Essential Diagnostics [13].
Following the outbreak of the HIV/AIDS pandemic WHO created a Global Blood Safety Initiative and mapped the World for blood transfusion, started to supply governments with Aide Mémoires [32] and put together in 2000 a Quality Management Training (QMT) course that was implemented all over the World [33]. Distant Learning Material (DLM) was composed to boost basic transfusion medicine knowledge [34].
However, despite all these development focused initiatives did not result in a satisfactory and noticeable improvement as shown by the persisting challenges mentioned in the WHO Global Status Reports on Blood Safety and Availability published in 2016 and 2021 [35,36]. These have not changed:
- Deficiencies in national policy, governance and financing.
- Insufficient supply of safe, effective and quality-assured blood products for transfusion.
- Deficiencies in blood product safety, effectiveness and quality.
- Insufficient availability of PDMPs.
- Suboptimal clinical practices in transfusion of blood components.
- Insufficient access to blood during emergency situations.
That observation urged WHO to provide a practical ‘Action Framework to Advance Access to Safe, Effective and Quality-assured Blood Products 2020-2023’, consisting of six Strategic Objectives (SO) based on the six persisting major challenges and each ending with a number of High Level Outcomes as a measurable expectation [37,38]. Therefore, WHO drafted for the most relevant and important High Level Outcome of each SO a practical Guidance and added a special Guidance to Identify Barriers in Blood Services Using the Blood System Self-assessment (BSS) Tool [39]. With assistance of the Boston Consulting Group and financial aid from the then USAID the tool was developed as a Web access to the Guidance [40].
The six Strategic Objectives are:
- An appropriately structured, well coordinated and sustainably resourced national blood system.
- An appropriate national framework of regulatory controls, national standards and quality assessment programs.
- Functioning and efficiently managed blood services.
- Effective implementation of patient blood management to optimize clinical practice of transfusion.
- Effective surveillance, hemovigilance and pharmacovigilance, supported by comprehensive and accurate data collection systems.
- Partnerships, collaboration and information exchange to achieve key priorities and jointly address challenges and emerging threats at global, regional and national levels.
It has become clear that the daily bread-and-butter (donor issues, laboratory benchwork, and manufacturing of blood products) is just a part of Transfusion Medicine and will only sustain if implemented in a fertile environment and stimulating knowledge-based climate governed by the Ministry of Health and the underlying principles anchored in an adequate legal framework and regulatory system recognizing the two key legal principles–product liability and patient rights protection (Figure 2).
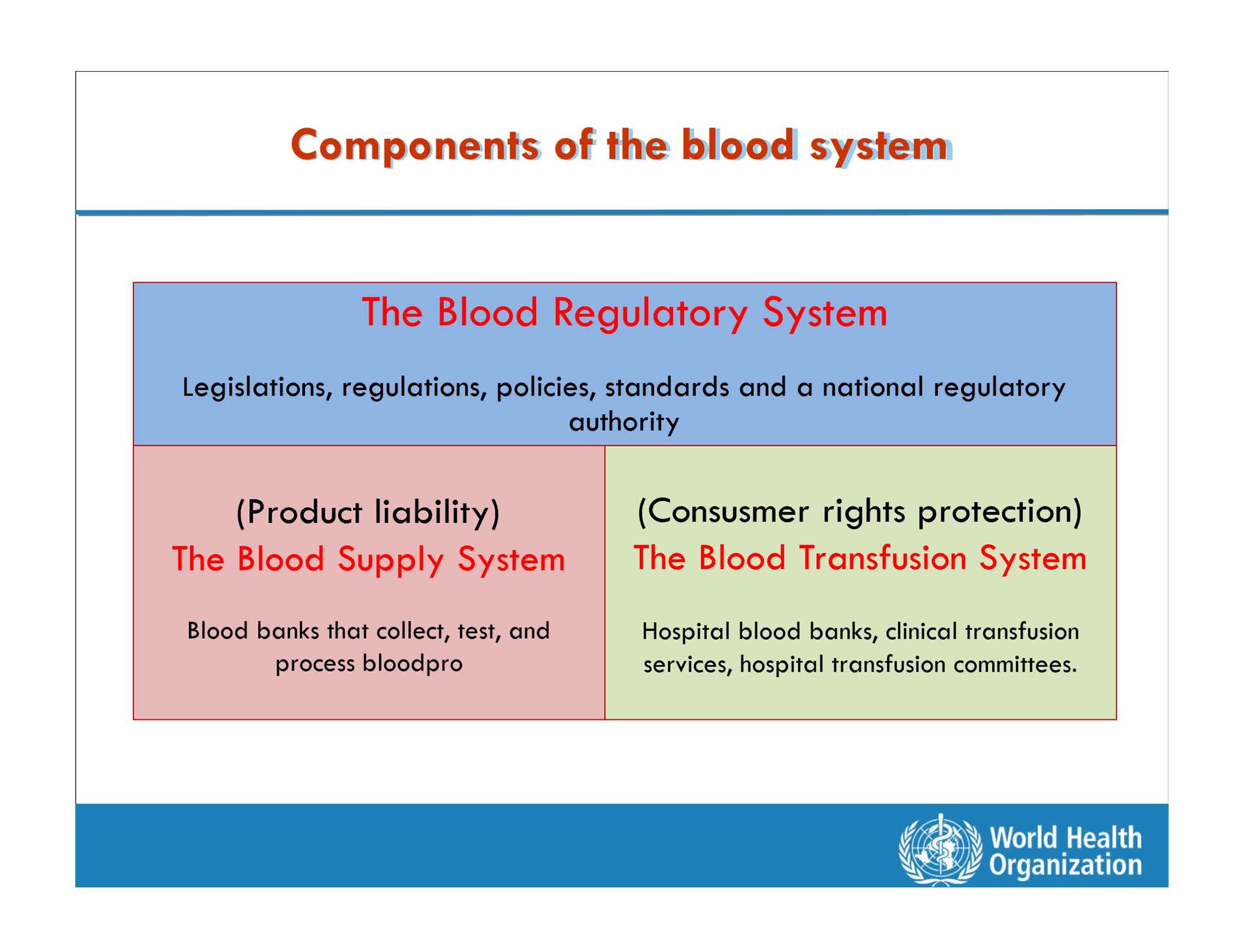
How to Ensure a Sufficient Supply of Safe, Quality-Assured Blood and Blood Components?
Fragmented blood systems, with blood services that are often operated by different players, pose a significant challenge in many countries. This situation often leads to problems with coordination and a lack of collaboration among different stakeholders. It may also lead to unnecessary competition for blood donors, which will not only increase the cost and effort needed to motivate and retain donors but may also have a negative effect on the principle of voluntary non-remunerated donation [41] and on the health of the blood donors. On the other hand, centralized operations during external emergencies, when communications and supply lines are disrupted, may not be optimal. Recent experience from conflicts in Ukraine and Bosnia suggest that decentralization allowed flexibility and the ability to operate autonomously, without reliance on supplies of blood or raw materials from other regions [42].
However, there are different types of emergency situations, medical (individual, pathophysiological) and external (humanitarian/armed conflict and industrial) and a mix of these two such as massive trauma due to e.g., chemical explosions and traffic clashes.
Whatever the type of emergency it is important to anticipate and plan in advance (contingency planning). That usually has several phases and aspects–risk assessment and gap analysis; emergency preparedness, and response with in case of external emergency the recovery [37,42].
Risk assessment and gap analysis
In some countries, blood services continue to operate under the responsibility of laboratory or pathology services, which are often not nationally coordinated.
Based on the 2015 data from the Global Database on Blood Safety (GDBS), the median number of annual donations processed per laboratory performing blood testing (immunohematology and microbiology) was 9,300, with an interquartile range (IQR) of 1,500–30,000 [34]. An analysis by WHO found major differences between the 6 regions, reflecting different organizational models and varying efficiencies and grades of effectiveness. An ineffective and inefficient blood supply system composed of many small-scale blood banks is a common barrier to implementation of quality screening using more sensitive assays for TTIAs and diseases. Data from the GDBS 2015 indicate that 25 out of 141 (18%) responding countries use rapid tests to screen all or part of their blood supply for infectious agents [36]. Although some of these rapid tests have a high sensitivity, they are handled manually, which leads to a greater potential for error both technical and administrative. Together with the widespread use of poor-quality and significantly less sensitive rapid tests in many LMICs, this contributes to an increased risk of transmission of bloodborne infections. Many countries lack a centralized reference laboratory system for evaluation and validation of test kits and reagents. There are often no minimum performance requirements to guide the selection and procurement of the test kits and reagents, and procurement decisions are too often based solely on price. Also, fragmentation in procurement or manufacturing of consumables often results in higher costs than can be obtained through centralized “bulk” purchasing and manufacturing.
Interruptions to the regular supply of test kits, reagents and consumables were reported by 21% of countries. These interruptions are usually due to insufficient budgetary allocation, economic restrictions or to ineffective and inefficient procurement systems and supply chain management. This may result in the procurement of poor-quality consumables tendered at the lowest price, and in the same test kits and reagents being procured for all laboratory services without any consideration of the specific needs of blood services (sensitivity for screening versus specificity for diagnostic purposes). Multiple supply contracts, for example for the same test kits, reagents and consumables, or contracts that are of insufficient duration, are also contributing factors. In addition, trade restrictions or blockades, customs and border clearance delays, and issues with transport and cold chain logistics, may limit the range of reagents and consumables available to the blood service under normal circumstances, let alone under disaster and humanitarian (external) emergency conditions.
Maternal and other health care policy interventions, concerning the national ambulance service, safe motherhood protocol, national health insurance scheme, medical supplies and health personnel, are often not well coordinated at the implementation level. These policies are often developed and rolled out without proper planning for the implementation phase. Damage to communication networks and road infrastructure during climate disasters and external emergencies makes it even more difficult to contact private transport operators, ambulance service providers, and the community health officers.
Medical emergencies are largely determined by the threat of multiple organ failure due to disrupted circulation, and tissue and organ perfusion.
Low viscosity of IV fluids allows a better perfusion and more effective oxygenation of less red cells, often enough to tip the balance back to normal [43].
Emergency preparedness
Following the risk assessment and gap analysis an emergency preparedness plan should be set up in a multidisciplinary way. The plan should be documented, validated, well communicated and regularly tested to ensure that it can address critical needs depending on the available resources. External and medical emergencies may affect and stress blood collection, screening, processing, storage, distribution, issuing and transfusion in different ways. There are four interwoven phases – mitigation, preparedness, response, and recovery.
Mitigation starts conducting a vulnerability or risk assessment to identify the critical points in the system that may be susceptible to risk [44,45]. Modelling and simulation methodology has been used by WHO [46] and many countries in deciding on the best strategies to mitigate the impact of emergencies, both the external and medical.
Preparedness covers actions that are taken prior to an emergency or disaster, including planning, training, and other educational activities to ensure mitigation or containment of incidents when they do occur.
Response has the purpose to:
- Enable continued safe supply of key products and services to meet routine demand as well as meeting the demands arising from the incident;
- Coordinate incident management to ensure the safety of individuals and to minimize adverse impacts on the products and services; and
- Minimize the impact of the incident on the organization and prepare for recovery.
An initial rapid assessment is needed to confirm the nature and impact of the emergency. The situation will evolve over time, especially if it is protracted, and the situation, and impact of the response will need regular reassessment. Some of the key initial questions and decisions related to blood transfusion include:
- What is the nature, severity and location of the emergency and how could it affect the blood supply including demand?
- What are the current local and regional levels of stocks, staff, and essential consumables?
- Can this external emergency be managed locally or provincially, or does it require a national emergency response with or without mutual support from other countries?
- Is the incident likely to be protracted? If so, its duration should be estimated.
- What needs to be done now and what can be done later?
Important is the grading of the severity in either situation. Grading triggers medical procedures and activities for the management of the response to the emergency. The grade assigned to an acute emergency for the transfusion community indicates the level of operational response required. The challenge is to deliver a timely response which is also proportionate and needs communication. An external emergency may be graded significant but only minor if the incident does not have an impact on blood supply or demand. In contrast, a business continuity incident affecting transfusion ICT, such as a cyber-attack, may be a major event due the potential impact on blood sufficiency and safety.
Mass Casualty Event (MCE) response
The causes of MCEs are varied and include transport incidents, infrastructure collapse or warlike activities. An MCE should trigger a multi-agency response. Many countries have formulated principles for joint working of emergency services (Joint Emergency Services Interoperability Programme) [47]. The same principles apply to working with other agencies including the armed forces.
It implies a surge in demand for blood and transfusion services following a traumatic event with many survivors. Transfusion services should be notified as early as possible but may have to wait for the details of casualty numbers and severity. Security and safety must come before health care, and emergency health care responders may initially be denied access to a scene until the dynamic risk assessment is complete. Therefore, it is essential that the public are familiar with first aid, especially early and effective hemorrhage control and tissue perfusion. Once prehospital staff have access to the site, casualties should be rapidly triaged to prioritize treatment and evacuation from the scene(s). Patients with minor injuries or unharmed rarely require blood. These patients are often managed outside the hospital system. Only patients admitted to hospital with severe bleeding and thread of organ failure would be expected to require blood. Demand planning for MCEs is best locally defined based on analysis of past events. Regular and systematic review of transfusion requirements during incidents informs the current and future response. Global reviews of available data suggest that the percentage of patients transfused following an incident varied from 7–67% (mean 25%) but only 5% (range 3–33%) received massive transfusion [48].
The amount of blood used per patient is often less than anticipated. MCE reviews in well-resourced environments recommend a two to four units of red cells for adult casualties admitted to hospital with bleeding. Children require proportionally less. However, the use of hemostatic components in trauma patients is increasing, including the use of whole blood [49], despite the fact that replacing what is lost is not necessary.
Blood allocation should consider patient distribution and the requirements of different hospitals. Most components are initially ordered as “universal” components and used within the first 6 hours. Blood group substitutions, such as group A plasma with low anti-B (typically <1:200), have been used successfully after local risk assessment to reduce the use of rare group AB [50].
Some trauma patients may have a continuing demand for blood over days and weeks, especially where repeat surgery is necessary [51]. In addition, the hospital demand for blood may increase once normal and catch-up surgical activity resumes. It is essential that the donor planning ensures a continuous supply of blood stock across all groups to support emergency work and “business as usual”, especially where incidents are protracted.
Conclusion
Emergency situations, medical and external, need a preparedness plan based on a risk assessment and gap analysis, and an anticipation on an adequate response and resilience to recover. In the personal medical emergency situation prevention of multiple organ failure through the maintenance of tissue and organ perfusion is most important. That can be achieved with crystalloids or other plasma replacement fluids and keeping the blood viscosity or hematocrit low allowing continuation of the circulation and oxygen delivery in the capillary beds (tissues and organs). Supply of blood products and selective PDMPs plays a vital role in the response and recovery phase. Blood establishments therefore need a sustainable structure, organization and resilience to be able to deal adequately with such situations without affecting the routine supply of safe, effective and quality blood products for the business-as-usual [36].
Conflict of Interest
The author has nothing to declare.
ORCID
Cees Th. Smit Sibinga – https://orcid.org/0000-0003-0156-5620.
References
2. Fact Sheet Blood safety and availability. Available at https://www.who.int/news-room/fact-sheets/detail/blood-safety-availability accessed 06 February 2025.
3. UNDP Human Development Index [online]. New York: United Nations. Development Programme. Available at: , accessed 06 April 2025.
4. World Bank Indicators [online]. Washington (DC): World Bank. Available at https://data.worldbank.org/indicator, accessed 06 April 2025.
5. Millennium Development Goals [online]. New York: United Nations. Available at: https://www.who.int/news-room/fact-sheets/detail/millennium-development-goals-(mdgs), accessed 06 April 2025.
6. Sustainable Development Goals [online]. New York: United Nations. Available at: https://www.sightsavers.org/policy-and-advocacy/global-goals/?gclid=EAIaIQobChMItsDbqqeJ9wIVyvDjBx2DxwlhEAAYAiAAEgKRh_D_BwE , accessed 06 April 2025.
7. UN Agenda 2030. Available at: www.un.org/sustainable developments/, accessed 06 April 2025.
8. World Health Organization. World health organization model list of essential medicines–23rd list. Geneva: World Health Organization; 2023 [Internet]. Available at: https://apps.who.int/iris/handle/10665/345533, accessed 06 April 2025.
9. WHO Global Benchmarking Tool + Blood [online]. Geneva: World Health Organization Available at: https://www.who.int/tools/global-benchmarking-tools, accessed 05 Aril 2025.
10. ICDRA – International Conference of Drug Regulatory Authorities. Available at: https://dcvmn.org/icdra-international-conference-of-drug-regulatory-authorities/, accessed 06 Aril 2025.
11. World Health Organization. WHO Expert Committee on Biological Standardization (sixty seventh report). Geneva: World Health Organization; 2017 Technical Report Series 1004 -Guidelines on Management of Blood and Blood Components as Essential Medicines.
12. World Health Organization. Guidance on centralization of blood donation testing and processing. Geneva: World Health Organization; 2021.
13. Second WHO Model List of Essential In Vitro Diagnostics. WHO/MVP/EMP; 2019.
14. The Nuremberg Trial and its Legacy, 2020. Available at: http://www.nationalww2museum.org/the-nuremberg-trial-and-its-legacy, Accessed 07 April, 2025.
15. Abdella Y, Hajjeh R, Sibinga CTS. Availability and safety of blood transfusion during humanitarian emergencies. East Mediterr Health J. 2018 Oct 10;24(8):778–88.
16. United Nations Development Programme Statistical update on human development indices and indicators. New York: United Nations; 2018.
17. Kajja I, Sibinga CTS. Seeking health care from a general hospital in Uganda following a fracture or a dislocation. Afr J Emerg Med. 2016 Dec;6(4):174-9.
18. Kajja I, Th Smit Sibinga C. Delayed elective surgery in a major teaching hospital in Uganda. International Journal of Clinical Transfusion Medicine. 2014 May 7;2:1-6.
19. World Health Organization. Ethics in epidemics, emergencies and disasters: research, surveillance and patient care: training manual. Geneva: World Health Organization; 2015. Available at: https://apps.who.int/iris/handle/10665/196326, accessed 07 April 2025.
20. World Health Organization. Educational modules on clinical use of blood. Geneva: World Health Organization; 2021 Available at: https://apps.who.int/iris/bitstream/handle/10665/350246/9789240033733-eng.pdf, accessed 4 September 2022.
21. Meara JG, Leather AJ, Hagander L, Alkire BC, Alonso N, Ameh EA, et al. Global Surgery 2030: evidence and solutions for achieving health, welfare, and economic development. Lancet. 2015 Aug 8;386(9993):569–624.
22. Chikwe J, Walther A, Jones P. Perioperative Medicine: Managing surgical patients with medical problems. Oxford: Oxford University Press; 2009.
23. Shah FT, Sayani F, Trompeter S, Drasar E, Piga A. Challenges of blood transfusions in β-thalassemia. Blood Rev. 2019 Sep;37:100588.
24. Elemary M, Seghatchian J, Stakiw J, Bosch M, Sabry W, Goubran H. Transfusion challenges in hematology oncology and hematopoietic stem cell transplant - Literature review and local experience. Transfus Apher Sci. 2017 Jun;56(3):317–21.
25. Elmardi KA, Adam I, Malik EM, Abdelrahim TA, Elhag MS, Ibrahim AA, et al. Prevalence and determinants of anaemia in women of reproductive age in Sudan: analysis of a cross-sectional household survey. BMC Public Health. 2020 Jul 17;20(1):1125.
26. Lohela TJ, Nesbitt RC, Pekkanen J, Gabrysch S. Comparing socioeconomic inequalities between early neonatal mortality and facility delivery: Cross-sectional data from 72 low- and middle-income countries. Sci Rep. 2019 Jul 5;9(1):9786.
27. Nabwera HM, Fegan G, Shavadia J, Denje D, Mandaliya K, Bates I, et al. Pediatric blood transfusion practices at a regional referral hospital in Kenya. Transfusion. 2016 Nov;56(11):2732–8.
28. World Health Organization. Twenty-eighth World Health Assembly, Geneva, 13-30 May 1975 WHA28. 72, Utilization and supply of human blood and blood products [Internet]. Geneva: WHO; 1975 [cited 2019 Jul 22] [Internet].
29. United Nations. Sustainable Development Goals. New York: United Nations; 2015. Available at https://sustainabledevelopment.un.org/?menu=1300.
30. Fifty-eighth World Health Assembly WHA58. 13. Blood safety: proposal to establish World Blood Donor Day. Fifty-eighth World Health Assembly, Geneva. 21 May 2010 A63/VR/8.
31. Sixty-third World Health Assembly WHA63. 12. Availability, safety and quality of blood products. Sixty-third World Health Assembly, Geneva. 21 May 2010 A63/VR/8.
32. World Health Organization. Aide Memoires for Blood Safety. World Health Organization 2002. WHO/BCT/02.03
33. World Health Organization. Quality management training for blood transfusion services: facilitator's toolkit [electronic resource].
34. World Health Organization. Safe blood and blood products. Geneva: World Health Organization; 2002.
35. World Health Organization. 2016 Global Status Report on Blood Safety and Availability. Geneva: World Health Organization; 2017.
36. World Health Organization. Global status report on blood safety and availability 2021. Geneva: World Health Organization; 2022 Jun 30.
37. World Health Organization. Action framework to advance universal access to safe, effective and quality-assured blood products 2020–2023. Geneva: World Health Organization; 2020. Available at https://apps/who/int/iris/handle/10665/331002, accessed 08 April 2025.
38. Abdella YE, Maryuningsih Soedarmo YS, Smit Sibinga CTh. Historical Background and Current Global Efforts. In: Eichbaum QG, Abdella YE, Amar S, Barnes LS, Bloch EM, Delaney M, et al. Eds Global Perspectives and Practices in Transfusion Medicine. Bethesda: AABB Press; 2023. P. 1-26.
39. World Health Organization. Guidance to identify barriers in blood services using the blood system self-assessment (BSS) tool. Geneva: World Health Organization; 2023.
40. Web annex Blood system self -assessment. In: Guidance to identify barriers in blood services using the blood system self-assessment (BSS) tool. Geneva: World Health Organization; 2023.
41. Flanagan P. Blood Donation–Incentives and Inducements: where to draw the line?. ISBT Science Series. 2020 Feb;15(1):19-22.
42. World Health Organization. Ukraine crisis strategic response plan for June–December 2022 (No. WHO/EURO: 2022-5778-45543-65230). Geneva: World Health Organization. Regional Office for Europe. Available at: https://apps.who.int/iris/handle/10665/358796.
43. Valeri CR, Crowley PJ. Indications for red blood cell transfusion. In: Smit Sibinga CTh, Das PC, Fratantoni JC, eds. Alternative Approaches to Human Blood Resources in Clinical Practice. Dordrecht/Boston/London: Kluwer Academic Publishers. 1997.
44. World Health Organization. Guidance on ensuring a sufficient supply of safe blood and blood components during emergencies. Geneva: World Health Organization; 2023 Apr 14.
45. Livestock in disasters. Unit 4. Disaster management in the United States. Hyattsville, MD: FEMA; 2013. Available at: https://training.fema.gov/emiweb/downloads/is111_unit%204.pdf, accessed 08 April 2025.
46. World Health Organization. The role of laboratories and blood banks in disaster situations. Geneva: World Health Organization; 2002. Available at: http://iris.paho.org/xmlui/handle/123456789/45853, accessed 08 April 2025.
47. Joint Doctrine: The Interoperability Framework. Welwyn Garden City: Joint Emergency Services Interoperability Programme (JESIP); 2016. Available at: http://www.jesip.org.uk/uploads/media/pdf/JESIP_Joint_Doctrine-The_Interoperability_Framework_[edition_2-July-2016].pdf, accessed 08 April 2025.
48. Ramsey G. Blood transfusions in mass casualty events: recent trends. Vox Sang. 2020 Jul;115(5):358–66.
49. Shinar E, Yahalom V, Silverman BG. Meeting blood requirements following terrorist attacks: the Israeli experience. Curr Opin Hematol. 2006 Nov;13(6):452–6.
50. Aylwin CJ, König TC, Brennan NW, Shirley PJ, Davies G, Walsh MS, et al. Reduction in critical mortality in urban mass casualty incidents: analysis of triage, surge, and resource use after the London bombings on July 7, 2005. Lancet. 2006 Dec 23;368(9554):2219–25.
51. World Health Organization. Protecting the blood supply during infectious disease outbreaks: Guidance for national blood services. Geneva: World Health Organization; 2019.
