Abstract
Aim: This study evaluates the sociodemographic, clinical, and anthropometric data of infants diagnosed with Food Protein-Induced Allergic Proctocolitis (FPIAP), categorized as exclusively breastfeeding or formula feeding at the onset of symptoms. This division allows us to compare the effects of different feeding on the development of FPIAP.
Methods: This retrospective, observational, single-center study included infants ≤36 months diagnosed with FPIAP. Exclusion criteria were chronic morbidities to ensure a focused study population. Data was collected using the Food Allergy Standard Protocol, meticulously coded, and stored in a secure database.
Results: Two hundred one were identified with FPIAP, breastfeeding (25%), and cow's milk formula (75%). The age at the onset of symptoms, at the first visit, and the diagnosis delay were lower for breastfeeding. for the first symptom, there was a higher proportion of breastfeeding in <180 days. Conversely, there was a higher proportion of cow's milk formula for children over 180 days. For Age at first visit, there was a higher proportion of breastfeeding in those <180 days and a higher proportion of cow's milk formula in those >180 days. Blood-streaked stools were present in 72% (breastfeeding) and 81% (cow's milk formula).
Conclusions: The findings of this study have implications for clinical practice. FPIAP, with its monotonous clinical presentation and lack of laboratory validity for diagnosis, should be considered in the differential diagnosis of hematochezia. The similarity of FPIAP presentation to studies in industrialized/Westernized countries is notable. However, the lower prevalence of FPIAP with breastfeeding suggests the need for a prospective study analyzing perinatal and environmental variables, which could provide valuable insights for future research.
Keywords
Food allergy, Proctocolitis, Exclusively breastfeeding, Formula feeding, Infant, Children, Hematochezia, Blood stools
Introduction
Food allergy (FA) is an adverse health response that occurs when exposed to specific food proteins [1–3]. Among the FA-specific subtypes, the recognition of gastrointestinal non-immunoglobulin e-mediated food allergies (Non-IgE-GIFAs) in infants is on the rise. These are classified into three different clinical entities: food protein-induced enterocolitis syndrome (FPIES), a severe gastrointestinal disorder; food protein-induced enteropathy (FPE), a condition that affects the small intestine; and food protein-induced allergic proctocolitis (FPIAP), an inflammation of the rectum and colon [4–6]. FPIAP is a primary concern in the pediatric gastroenterology clinic. Typically, it begins within the first weeks of life, with over 75% of cases diagnosed within 2.5 months [7–10]. It presents with blood-streaked and mucus stools in an otherwise healthy infant, often recognized as the earliest manifestation of FA in children.
Indeed, FA has undergone a significant shift over the last two decades, with a noticeable increase in the number of children diagnosed, the frequency of medical visits, and healthcare costs. This shift is concerning, mainly because FA disproportionately affects children in industrialized countries. According to the Euro-Prevall data, non-IgE-mediated FA has a prevalence of 1% in children. Furthermore, the rising allergy rates are beginning to strain healthcare services in developing countries as they adopt a more 'Westernized' lifestyle [11,12].
The NIAID-sponsored panel's first recommendation for managing Non-IgE-GIFAs involves the mother's diet, removing the offending protein or changing from breastmilk to the hypoallergenic formula [9,13,14]. This dietary modification aims to reduce the infant's exposure to the allergenic protein, thereby alleviating the symptoms. However, this can result in maternal dietary restrictions or expensive hypoallergenic infant formulas.
Therefore, clinical food allergy management is not just about recognizing allergic symptoms, but also about prompt treatment. It is crucial in the case of FPIAP, where early intervention can significantly improve outcomes. This approach is paramount in non-industrialized countries, where Breastfeeding predominates and early manifestation occurs, necessitating the modification of the mother's diet. Accordingly, the primary aim of this study is to provide a comprehensive understanding of the sociodemographic, clinical, and anthropometric data of infants diagnosed with FPIAP. A key aspect of our study design is the division of infants into two subgroups: Those who were exclusively breastfed and those who were formula-fed at the onset of symptoms. This division allows us to compare different feeding methods on the development and severity of FPIAP, a key area of interest for pediatric gastroenterologists and healthcare professionals.
Methods
Study design, setting, and selection of participants
The study was a retrospective, observational, single-center study that included consecutive cases of infants/pre-schoolers referred from the Brazilian Public Health System (SUS) for initial evaluation of FA, attended at a Pediatric Gastroenterology Outpatient Clinic between January 2012 and December 2021. The available infrastructure includes an outpatient clinic with weekly practice, care standardization, and regular clinical follow-up. Inclusion criteria: aged ≤36 months with a diagnosis of FA. Exclusion criteria include chronic co-morbidities, such as genetic, neurological, cardiological, endocrinological, and nephrological diseases, as well as undefined diagnoses of FA. Clinical Research Ethics Committees (protocol CAAE: 73269323.8.0000.5411) approved the study.
Data collection
Data were collected, coded in a pre-designed Food Allergy Standard Protocol, and stored in a database of Excel spreadsheets (Microsoft, Redmond, Washington). Specifically, the authors extracted data stored by children diagnosed with FPIAP for the current study. Parents/caregivers answered all the questions during the first clinic visit. The initial questionnaire takes information about the sociodemographics (age, sex, position of the child in the family; parents' age and education; caregiver's marital status, birth conditions, number of rooms, people and children in the household, and history of allergy in the family), clinical features, and laboratory variables (blood cell count, C-reactive protein (CRP), serum immunoglobulins, urinalysis, stool for ova & parasites). Additional tests were completed at the researcher's discretion. Feeding subgroups were: exclusive breastfeeding and cow's milk-based formula practices (only formula or mixed feeding) during the onset of symptoms. According to World Health Organization guidelines, experienced pediatric nurses obtained anthropometric data [15].
Diagnosis of FPIAP of Infants stored In an excel spreadsheet
Patients' final diagnoses of FPIAP are determined after four months of follow-up by two experienced pediatric gastroenterologists (MAC, NCM) based on a combination of the following conditions: 1) clinical features of a history of reproducible signs/symptoms on repeated exposure, 2) allergen elimination from infant's diet or mother's diet in breastfeeding with no symptoms elicited within 2–4 weeks, according to [3,16,17], 3) an open oral challenge (OFC) with direct observation of at least four hours, performed in the hospital under medical supervision, and after the elimination was performed at home [18], 4) negative serum food-specific IgE antibody levels for cow's milk, soy, egg, and other food antigens according to a specific indication of each case, 5) negative skin prick test using commercial food extract if a wheal diameter of 3 mm seen at 15 min in the presence of a 3 mm wheal to histamine, 6) laboratory data, and 7) Exclusion of possible causes of rectal bleeding, such as anal fissure, necrotizing enterocolitis, intussusception, infectious colitis (parasitic, bacterial, or viral), Meckel's diverticulum, coagulopathy, necrotizing enterocolitis, and very early onset inflammatory bowel disease. Colonoscopy and biopsy are generally not performed [19].
Statistical analysis
The study's findings were rigorously analyzed using statistical methods. Sociodemographic, clinical, and laboratory data were analyzed with GraphPad Prism version 8.4.0 for Windows (GraphPad Software, San Diego, California, USA, www.graphpad.com). Normality data was assessed using the Kolmogorov-Smirnov test to determine the differences between parametric and non-parametric tests. Categorical variables were expressed as numbers and percentages (%) and examined with Fisher's exact test. Continuous variables were expressed as median (25th-75th percentile), and the Mann-Whitney test made comparisons between groups. All tests were two-sided, and p<0.05 was statistically significant.
Results
The Excel spreadsheet database was meticulously filled with 629 children who had confirmed diagnoses of FA during the evaluated period. In this groundbreaking study, based on the combination criteria described in Methods, 201 (32%) were identified with the diagnosis of FPIAP, and data were extracted for analysis. Of this group, 50 patients were breastfeeding, and 151 used cow's milk-based formula. All data analyzed were from the first visit, providing an understanding of the initial stages of FPIAP. The comprehensive understanding of the disease's early progression enlightens us about the potential factors influencing its onset and development.
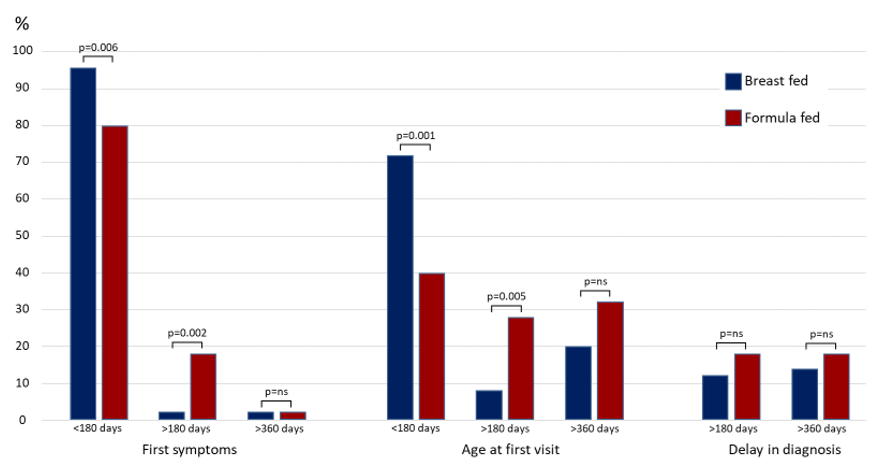
Table 1 displays the baseline characteristics of feeding children with FPIAP. More children were fed cow's milk-based formula (75%). When comparing the two types of feeding, the age at the onset of symptoms (p=0.002), age at the first visit (p=0.0008), and the diagnosis delay (p=0.02) were lower for breastfeeding. In addition, Figure 1 evaluates three distinct children age groups: younger than 180 days (≤180d), older than 180 days (>180d), and older than 360 days (>360d). For the age at the onset of symptoms, there was a higher proportion of breastfeeding in children ≤180d (p=0.006), and a higher proportion of formula feeding in >180d (p=0.02). Similarly, for age at the first visit, the proportion was higher in children ≤ 180 days on breastfeeding (p=0.001), and higher for formula feeding in > 180 days (p=0.005). There is no difference in the diagnosis delay for breastfeeding and formula feeding.
|
|
Breastfeeding (n=50) |
Formula (n=151) |
p< |
|
|
Median (IQR) |
||
|
Number of children, no. (% of total) |
50 (25) |
151 (75) |
|
|
Sex: Female, no. (%) |
19 (38) |
60 (40) |
ns* |
|
Onset age of Formula, days |
- |
60 (15-120) |
na |
|
Age at symptom onset, days |
45 (30-90) |
60 (30-150) |
0.002† |
|
Age at first visit, days |
120 (60-236) |
218 (120-390) |
0.0008† |
|
Diagnosis delay, days |
40 (15-210) |
90 (30-270) |
0.02† |
|
Delivery: cesarean section, no. (%) |
21 (42) |
68 (45) |
ns* |
|
First child, no. (%) |
20 (40) |
55 (36) |
ns* |
|
Number of siblings |
2 (1-2) |
2 (1-2.5) |
ns* |
|
Age of mothers, years |
26 (22-31) |
26 (21-30) |
ns* |
|
Age of fathers, years |
33 (26-36) |
29 (23-33) |
0.02† |
|
Number of people at home |
4 (4-5) |
5 (4-6) |
ns* |
|
Number of children at home |
2 (1-3) |
2 (1-3) |
ns* |
|
Reported family history of allergy |
|
||
|
Gastrointestinal, no. (%) |
02 (6.5) |
05 (05) |
ns* |
|
Respiratory, no. (%) |
09 (29) |
31 (31) |
ns* |
|
Dermatologic, no. (%) |
00 (00) |
03 (03) |
na |
|
*Fisher Exact Test, †Mann-Whitney U test, IQR: Interquartile Range; ns: not significant; na: not analysed |
|||
Figure 1. Comparisons of first symptoms, age at first visit, and delay in diagnosis between subgroups breastfeeding and formula, according three age groups.
Table 2 shows that the children presented with good health with no difference in weight, except for high length for formula feed at the first visit. Table 3 shows that blood-streaked stools were present in 72% of children under breastfeeding and 81% of children under formula, respectively. Loose stools/mild diarrhea were higher in breastfeeding (p<0.01). Eczema was slightly associated with FPIAP. Table 4 does not demonstrate statistically significant differences for all laboratory analyses between the feeding subgroups, providing reassurance about the robustness of our conclusions.
|
|
Breastfeeding (n=50) |
Formula (n=151) |
p< |
|
|
Median (IQR) |
||
|
Birth weight (grams) |
3050 (2770-3270) |
3218 (2870-3600) |
ns |
|
Birth weight, <2,500 grams no. (%) |
01 (02) |
03 (02) |
ns |
|
Birth Length (cm) |
48 (47-49) |
48.5 (47-50) |
ns |
|
Weight at first visit (grams) |
6170 (4650-8220) |
7240 (5345-9400) |
ns |
|
Length at first visit (cm) |
61 (56-67) |
67 (59-75) |
0.01† |
|
Good health during the first visit, no. (%) |
50 (100) |
151 (100) |
na |
|
†Mann-Whitney U test, IQR: Interquartile Range; ns: not significant; na: not analysed |
|||
|
|
Breastfeeding (n=50) |
Formula (n=151) |
|
|
no. (%) |
|
|
Blood-streaked stools |
36 (72) |
123 (81) |
|
Loose stools/mild diarrhea* |
10 (20) |
10 (6.6) |
|
Cry during defecation |
03 (06) |
09 (5.9) |
|
Hard stools |
01 (02) |
09 (06) |
|
Vomiting |
06 (12) |
17 (11.2) |
|
Poor weight gain |
01 (02) |
02 (1.3) |
|
Cry during feedings |
03 (06) |
07 (4.6) |
|
Food refusal |
00 |
04 (2.6) |
|
Rhinitis |
00 |
02 (1.3) |
|
Cough |
00 |
08 (5.2) |
|
Wheezing |
03 (06) |
05(3.3) |
|
Urticaria |
01 (02) |
06 (3.9) |
|
Morbilliform erythema |
00 |
02 (1.3) |
|
Eczema |
02 (04) |
11 (7.2) |
|
Recurrent infections |
00 |
03 (1.9) |
|
*p<0.01 using Fisher Exact Test. |
||
|
|
Breastfeeding (n=50) |
Formula (n=151) |
|
Median (IQR) |
||
|
Hemoglobin level (g/dL)* |
11.3 (10.3–12) |
11.3 (10.3–12) |
|
Hemoglobin level (g/dL≤10.9), no. (%)* |
07 (14) |
21 (14) |
|
Hemoglobin level (g/dL≤9.9), no. (%)* |
05 (10) |
21 (14) |
|
MCV (Mean Corpuscular Volume), fL* |
81 (73–85) |
78 (74–86) |
|
RDW (Red Cell Distribution Width, % )* |
14 (12–15) |
14 (12–15) |
|
Leukocytes, no./mm3 |
9900 (7,800–12,200) |
9400 (7700–11,900) |
|
Eosinophils, no./mm3 |
263 (162–547) |
280 (137–460) |
|
Eosinophils (>500/mm3), (%) |
24.3 |
17.6 |
|
Severe Eosinophilia (>1000/mm3 ), (%) |
2.7 |
4.4 |
|
Total Immunoglobulin E, IU/L |
7.7 (4.3–69.2) |
25 (25–43) |
|
Total Immunoglobulin A, IU/L |
29 (24–49) |
13.6 (4.3–39) |
|
There are no statistically significant results for all analysis using Fisher Exact Test, and Mann-Whitney U test. * evaluated according "WHO, UNICEF, UNU. Iron deficiency anemia: assessment, prevention and control, a guide for programme managers. Geneva, World Health Organization, 2001". Available at https://www.who.int/publications/m/item/iron-children-6to23--archived-iron-deficiency-anaemia-assessment-prevention-and-control |
||
Discussion
Unlike most publications that focus on clinical manifestations of FPIAP in industrialized countries, our study takes a unique approach. We evaluated FPIAP in a diverse environment, specifically comparing breastfeeding and cow's milk-based formula feeding in children at the onset of symptoms. Our retrospective analysis of 629 children diagnosed with FA identified 201 children (32%) with FPIAP, 50 patients (25%) who were breastfeeding, and 151 children (75%) who were on cow's milk-based formula. The early onset age of formula (median=60 days) could elucidate the lower prevalence of FPIAP in breastfeeding infants. In an extensive study conducted in an industrialized country, FPIAP was diagnosed in 17% (153 of 903) of cases [9], which contrasts with our findings. The main results were: for both age at the onset of symptoms and age at first visit, there was a higher proportion of breastfeeding in those ≤180 days and a higher proportion of cow's milk-based formula in those >180 days. The diagnosis delay was lower for breastfeeding. No difference was found for first symptoms after 360 days. These results are comparable with the classic publication that defined ages between 2 and 8 weeks in breastfed infants.
The diagnostic workup in a child with FA is comprehensive. It includes a detailed clinical history focused on food intake, symptom-inducing factors, the time gap from food ingestion to symptom onset, reproducibility, an apparent response to an elimination diet, and the exclusion of other medical causes. Although hundreds of foods can provoke a reaction, a few foods are specifically responsible for FA in FPIAP children: cow's milk, hen's egg, and soy. Several procedures could be adopted in children with gastrointestinal symptoms possibly related to FPIAP, including invasive (endoscopy with histologic evaluation) and non-invasive diagnostic tools.
In the current study, the diagnosis of FPIAP was defined when these criteria were satisfied.
- Small and bright red blood-streaked stools (breasfeeding =72%, and formula =81%). In developed countries, blood-streaked stools are common in exclusively breastfed infants with favorable weight gain [20]. Observe a fundamental aspect of average weight at birth and first visit, and a small number of birth weights, <2,500 grams, in both subgroups of feeding. These findings reinforce the similarity of the characteristics of children with FPIAP compared to studies in industrialized countries [21]. FPIAP was first defined in 1982 by Lake et al. in six breastfed infants [22] and usually starts during the first months of life in healthy infants. Nevertheless, they have visible streaks of blood or blood mixed with mucus in the stools [7]. Most infants are breastfed and become sensitized due to maternally ingested proteins-commonly cow's milk protein-excreted in breast milk. Previous publications demonstrated that symptoms appeared during breastfeeding in 53%-60% of the patients. Conversely, in the present study, only 25% of the infants started the symptoms during breastfeeding.
- Loose stools/mild diarrhea with mucus in a relatively healthy neonate or infant (100% with good health during the first visit). Some infants have increased bowel movements simulating diarrhea, which was present in a higher proportion in breastfed Infants than in those fed cow's milk-based formula (p<0.01). Therefore, these findings are comparable to those of developed countries [9].
- The present study evaluates any conditions that may cause blood in the stools. Infectious diarrhea, anal fissure, coagulation defects, vitamin K deficiency, intussusception, necrotizing enterocolitis, pseudomembranous colitis, inflammatory bowel diseases, failure to gain weight, weight loss, Hirschsprung disease, and other surgical abdominal conditions have been systematically investigated and ruled out [19,23]. Rectal bleeding is not a rare problem in healthy infants, and FPIAP is one of the most frequent causes of rectal bleeding and is broadly regarded as the most common type of colitis in infancy. The estimated prevalence ranges from 18% to 64% of infants with rectal bleeding [24–28]. Within the patient population referred to our unit for persistent or recurrent rectal bleeding, FPIAP represented 201/629 (32%)infants with confirmed diagnoses during the period evaluated. Cow's milk and soy are the most common triggers through breast milk or infant formula.
The disappearance of rectal blood after changing diet and the recurrence of rectal bleeding after the administration of offending food are crucial criteria. Indeed, the elimination diet is the cornerstone of treating FPIAP. Gross rectal bleeding should resolve within 72–96 hours. The oral food challenge (OFC) is crucial in diagnosing FPIAP. However, considering that it is a non-IgE-mediated form, the observation time for this form is longer than that of the IgE-mediated forms. In breastfeeding mothers, cow's milk, eggs, and soybeans were removed from the maternal diet. Once rectal bleeding disappeared, allergens were reintroduced sequentially to the diet. In formula-feeding infants, an amino acid-based formula must be offered.
The laboratory investigation resulted in a small contribution to the diagnosis of FPIAP. Haemoglobin levels <10.9 g/dL are present in 14% of infants, whether breastfeeding or on cow's milk-based formula. Peripheral eosinophilia (>500/mm3) was present in 24.3% of breastfeeding and 17.6% of cow's milk-based formula. No infants have thrombocytosis, which may be an additional feature of this immuno-inflammatory condition [29]. All patients had ESR and/or CRP and immunoglobulins, which gave entirely expected results. The biochemical parameters were all within the normal range. A few stool cultures for enteric pathogens and stool examination for ova and parasites also tested negative in patients. Experience in performing the diagnosis demonstrated that standard or negative exams provide valuable information for this diagnosis [7,30,31]. The role of healthcare professionals in interpreting these results and making informed decisions is crucial in the diagnosis and management of FPIAP.
Prick tests and RAST were standard for common food proteins (cow's milk, soy, egg, and other proteins according to each patient's suspicion). The tests were negative. Some authors' allergy testing is usually negative and insufficient for investigating non-IgE-GI-FA [32–34]. This study did not conduct atopy patch tests, which can explore cell-mediated reactions.
The operationalization of these steps and the monotony of the FPIAP presentation throughout the study period allowed for accurate diagnosis. Thus, by standardizing the search for the differential diagnosis, we will probably avoid overdiagnosis and unnecessary elimination diets [35]. The current study defined a correct diagnosis of FPIAP according to the European Academy of Allergy and Clinical Immunology food allergy [8,36–38] and the Guidelines for the Diagnosis and Management of Food Allergy [13]. These guidelines recommend using clinical history, prick tests, specific serum IgE testing, elimination diets, resolution of symptoms when the causative food is eliminated, and recurrence of symptoms for diagnosis. Remarkably, FPIAP mainly presented as an isolated disorder. In Table 3, vomiting, poor weight gain, food refusal, and respiratory and dermatological symptoms are of low prevalence.
There are limitations to this study. First, the single-center recruitment limits generalizability. Second, the infants were not evaluated with a Patch test. Thirdly, OFC was executed through the maternal rather than the infant diet. Strengths of this study are: First, the diagnosis of FPIAP was made according to international guidelines, which may lead to a significant reduction in overdiagnosis. Secondly, the patient's follow-up allowed a safe diagnosis. Thirdly, the study in a developing country allows a comparison with classical publications on FPIAP.
Conclusion
In conclusion, during the ten-year evaluation period, one-third of the children were identified with FPIAP, a benign disease with a clinical presentation that is often monotonous at the first visit. Most breastfeeding children had the onset of symptoms and the first visit before six months of life. The current guidelines allow an adequate and safe approach to the diagnosis. There is no evidence that a laboratory evaluation shows clinical validity for diagnosing FPIAP. FPIAP should always be considered in the differential diagnosis of hematochezia, particularly in exclusively breastfed infants. The current study established that the clinical presentation of FPIAP is very similar to data published in industrialized/Westernized countries. Compared to the literature, the lower prevalence of FPIAP with breastfeeding warrants a prospective study that analyzes a larger number of perinatal and environmental variables.
Author Contributions
Study design (NCM, MAC), Acquisition of data (JTD, GNH, CDFJ), Analysis and interpretation of data (NCM, JTD, TKW, GNH, MAC), Drafting of the manuscript (NCM, MAC, TKW), Critical revision of the manuscript (TKW).
Declarations
Conflicts of interest
The authors declare no conflicts of interest.
Funding
This research had no financial support.
Ethics approval
The Medical Institutional Review Board approved the study (Protocol CAAE: 81999317.7.0000.5411).
References
2. Valenta R, Hochwallner H, Linhart B, Pahr S. Food allergies: the basics. Gastroenterology. 2015 May 1;148(6):1120–31.
3. Munblit D, Perkin MR, Palmer DJ, Allen KJ, Boyle RJ. Assessment of evidence about common infant symptoms and cow’s milk allergy. JAMA Pediatrics. 2020 Jun 1;174(6):599–608.
4. Sicherer SH, Sampson HA. Food allergy. Journal of Allergy and Clinical Immunology. 2010 Feb 1;125(2):S116–25.
5. Mansoor DK, Sharma HP. Clinical presentations of food allergy. Pediatr Clin North Am. 2011;58(2):315–26.
6. Leonard SA. Non-IgE-mediated adverse food reactions. Curr Allergy Asthma Rep 2017; 17(12):84.
7. Nowak-Węgrzyn A. Food protein-induced enterocolitis syndrome and allergic Proctocolitis. Allergy Asthma Proc 2015;36(3):172–84.
8. Mennini M, Fiocchi AG, Cafarotti A, Montesano M, Mauro A, Villa MP, et al. Food protein-induced allergic proctocolitis in infants: Literature review and proposal of a management protocol. World Allergy Organization Journal. 2020 Oct 1;13(10):100471.
9. Martin VM, Virkud YV, Seay H, Hickey A, Ndahayo R, Rosow R, et al. Prospective assessment of pediatrician-diagnosed food protein–induced allergic proctocolitis by gross or occult blood. The Journal of Allergy and Clinical Immunology: In Practice. 2020 May 1;8(5):1692–9.
10. Martin VM, Virkud YV, Dahan E, Seay HL, Itzkovits D, Vlamakis H, et al. Longitudinal disease-associated gut microbiome differences in infants with food protein-induced allergic proctocolitis. Microbiome. 2022 Sep 23;10(1):154.
11. Gonçalves LC, Guimarães TC, Silva RM, Cheik MF, de Ramos Nápolis AC, Barbosa E Silva G, Segundo GR. Prevalence of food allergy in infants and pre-schoolers in Brazil. Allergol Immunopathol (Madr). 2016 Nov-Dec;44(6):497-503.
12. Chen J, Liao Y, Zhang HZ, Zhao H, Li HQ. Prevalence of food allergy in children under 2 years of age in three cities in China. Zhonghua er ke za zhi= Chinese Journal of Pediatrics. 2012 Jan 1;50(1):5–9.
13. Boyce JA, Assa'ad A, Burks A, Jones SM, Sampson HA, Wood RA, et al. Guidelines for the diagnosis and management of food allergy in the United States: Summary of the NIAID-sponsored expert panel report. Journal of Allergy and Clinical Immunology. 2010;126(6):1105–18.
14. Czerwionka-Szaflarska M, Łoś-Rycharska E, Gawryjołek J. Allergic enteritis in children. Gastroenterology Review/Przegląd Gastroenterologiczny. 2017 Jan 1;12(1):1–5.
15. World Health Organization. Expert Committee on Physical status: the use and interpretation of anthropometry (1993: Geneva, Switzerland) & World Health Organization (1995). Physical status: the use and interpretation of anthropometry, report of a WHO expert committee. World Health Organization Technical Report Series 854 (1995): 1-452. Available at: https://apps.who.int/iris/handle/10665/37003.
16. Fiocchi A, Schünemann HJ, Brozek J, Restani P, Beyer K, Troncone R, et al. Diagnosis and rationale for action against cow's milk allergy (DRACMA): a summary report. Journal of Allergy and Clinical Immunology. 2010 Dec 1;126(6):1119–28.e12.
17. Fiocchi A, Bognanni A, Brożek J, Ebisawa M, Schünemann H, Ansotegui IJ, et al. World allergy organization (WAO) diagnosis and rationale for action against cow's milk allergy (DRACMA) guidelines update–I–plan and definitions. World Allergy Organization Journal. 2022 Jan 1;15(1):100609.
18. Connors L, O’keefe A, Rosenfield L, Kim H. Non-IgE-mediated food hypersensitivity. Allergy, Asthma & Clinical Immunology. 2018 Sep 12;14(Suppl 2):56.
19. Fox VL. Gastrointestinal bleeding in infancy and childhood. Gastroenterology Clinics of North America. 2000 Mar 1;29(1):37–66.
20. Boné J, Claver A, Guallar I, Plaza AM. Allergic proctocolitis, food-induced enterocolitis: immune mechanisms, diagnosis and treatment. Allergologia et Immunopathologia. 2009 Feb 1;37(1):36–42.
21. Lozinsky AC, Morais MB. Eosinophilic colitis in infants. Jornal de Pediatria. 2014;90(01):16–21.
22. Lake AM, Whitington PF, Hamilton SR. Dietary protein-induced colitis in breast-fed infants. The Journal of Pediatrics. 1982 Dec 1;101(6):906–10.
23. Eigenmann PA. Mechanisms of food allergy. Pediatric Allergy and Immunology. 2009 Feb;20(1):5–11.
24. Xanthakos SA, Schwimmer JB, Melin‐Aldana H, Rothenberg ME, Witte DP, Cohen MB. Prevalence and outcome of allergic colitis in healthy infants with rectal bleeding: a prospective cohort study. Journal of Pediatric Gastroenterology and Nutrition. 2005 Jul;41(1):16–22.
25. Arvola T, Ruuska T, Keränen J, Hyöty H, Salminen S, Isolauri E. Rectal bleeding in infancy: clinical, allergological, and microbiological examination. Pediatrics. 2006 Apr 1;117(4):e760–8.
26. Elizur A, Cohen M, Goldberg MR, Rajuan N, Cohen A, Leshno M, et al. Cow's milk associated rectal bleeding: a population based prospective study. Pediatric Allergy and Immunology. 2012 Dec;23(8):765–9.
27. Caubet JC, Szajewska H, Shamir R, Nowak‐Węgrzyn A. Non‐IgE‐mediated gastrointestinal food allergies in children. Pediatric Allergy and Immunology. 2017 Feb;28(1):6–17.
28. Dehghani SM, Shahramian I, Ataollahi M, Bazi A, Seirfar N, Delaramnasab M, et al. A survey on rectal bleeding in children, a report from Iran. Turkish Journal of Medical Sciences. 2018;48(2):412–8.
29. Dame C, Sutor AH. Primary and secondary thrombocytosis in childhood. British Journal of Haematology. 2005 Apr;129(2):165–77.
30. Hideaki M, Ichiro N, Akio M, Kenji M. Gastrointestinal Food Allergy. Allergology International. 2013;62(3):297–307.
31. Meyer R, Fleming C, Dominguez-Ortega G, Lindley K, Michaelis L, Thapar N, et al. Manifestations of food protein induced gastrointestinal allergies presenting to a single tertiary paediatric gastroenterology unit. World Allergy Organization Journal. 2013 Dec;6(1):1–9.
32. Fenton M. Guidelines for the diagnosis and management of food allergy in the United States. Clinical and Translational Allergy. 2011 Dec 1;1(Suppl 1):S1–10.
33. Burks AW, Tang M, Sicherer S, Muraro A, Eigenmann PA, Ebisawa M, et al. ICON: food allergy. Journal of Allergy and Clinical Immunology. 2012 Apr 1;129(4):906–20.
34. Sampson HA, Aceves S, Bock SA, James J, Jones S, Lang D, et al. Food allergy: a practice parameter update—2014. Journal of Allergy and Clinical Immunology. 2014 Nov 1;134(5):1016–25.e43.
35. Hwang JB, Hong J. Food protein-induced proctocolitis: Is this allergic disorder a reality or a phantom in neonates? Korean Journal of Pediatrics. 2013 Dec 20;56(12):514.
36. Muraro A, Werfel T, Hoffmann‐Sommergruber K, Roberts G, Beyer K, Bindslev‐Jensen C, et al. EAACI food allergy and anaphylaxis guidelines: diagnosis and management of food allergy. Allergy. 2014 Aug;69(8):1008–25.
37. Meyer R, Chebar Lozinsky A, Fleischer DM, Vieira MC, Du Toit G, Vandenplas Y, et al. Diagnosis and management of Non‐IgE gastrointestinal allergies in breastfed infants—An EAACI Position Paper. Allergy. 2020 Jan;75(1):14–32.
38. Abrams EM, Hildebrand KJ, Chan ES. Non-IgE-mediated food allergy: evaluation and management. Paediatr Child Health. 2021;26(3):173–81.