Abstract
Mitochondrial permeability transition pore (PTP) plays an important role in mitochondrial physiology and cell fate. Emerging studies highlight PTP forms from F-ATP synthase, but whether F-ATP synthase inhibitory factor 1 (IF1) regulates the activity of PTP is basically unknown. We have recently demonstrated that IF1 interacts with p53-CyPD complex and promotes opening of the PTP, and IF1 is necessary for the formation of p53-CyPD complex. IF1, a natural inhibitor of F-ATP synthase, acts as a main driver of metabolic switch to a Warburg phenotype. In this Commentary, we intend to discuss that the PTP may act as an alternative mechanism through which IF1 regulates metabolic reprogramming. The PTP participates in physiological Ca2+/ROS homeostasis and cell fate depending on the open state. The PTP-regulatory role of IF1 provides a clue that IF1 participates in metabolic plasticity probably involving modulation of PTP activity.
Keywords
Mitochondria, F-ATP synthase inhibitory factor 1, Permeability transition, Metabolic reprogramming, Cyclophilin D, ROS, p53, Transcription factors
F-ATP Synthase Inhibitory Factor 1 Regulates the Permeability Transition Pore
Mitochondrial permeability transition is a Ca2+-dependent increase of the inner membrane permeability mediated by the permeability transition pore (PTP) [1]. The molecular nature of PTP is a century mystery that remains contentious [2]. In the past decade, overwhelming evidences highlight that F-ATP synthase is a key component of PTP [3-14]. Cyclophilin D (CyPD) acts as the receptor for cyclosporin A (CsA), which is a well-known PTP inhibitor [15-19]. The binding of CyPD to PTP favors opening of the pore, and mitochondria devoid of CyPD or in presence of CsA become resistant to PTP inducers [19-21]. CyPD associates with F-ATP synthase through the lateral stalk of the complex including the oligomycin sensitivity conferral protein (OSCP) [4,22]. The most recent working model of PTP is that the conformational change upon Ca2+ binding to catalytic core is transmitted to subunit e and c-ring through subunits b and g via OSCP [1,7,13,14,23-27].
The p53 tumor suppressor, a well-known transcription factor induced by DNA damage and oxidative stress, is termed as the guardian of the genome [28]. p53 regulates a variety of key processes such as cell cycle arrest, DNA repair, apoptosis, senescence, and metabolism [29]. p53, a central stress sensor to multiple insults, is translocated to mitochondria in response to oxidative stress [30]. p53 protein can promote mitochondrial membrane permeabilization by direct activation of Bax and induce apoptosis [31,32]. Mitochondrial matrix p53 interacts with OSCP subunit and promotes the assembly of F-ATP synthase [33]. The PTP-regulatory activity of p53 in response to oxidative stress is CyPD dependent, and a robust p53-CypD complex formation triggers PTP opening during necrosis [30].
F-ATP synthase inhibitory factor 1 (IF1) is a regulatory subunit of F-ATP synthase, and the binding of IF1 to the enzyme depends on the matrix pH [34-36]. IF1 dimerizes at acidic pH where it is active and forms tetramer at alkaline pH where its inhibitory region is masked [37]. The active IF1 stabilizes the dimers of F-ATP synthase through F1-F1 bridging [38]. Overexpression of IF1 promotes the dimerization of F-ATP synthase and increases the density of mitochondrial cristae [39,40]. We have recently reported that IF1 regulates the PTP via interaction with the p53-CyPD complex [41]. Overexpression of IF1 activated caspase 3 and sensitized the pore to Ca2+, which was suppressed by CsA, while disruption of IF1 inhibited PTP opening and prevented cell death induced by oxidative stress [41]. The interaction of p53 with OSCP subunit via p53-CypD axis plays an important role in its tumor suppression activity, and p53-CypD complex is essential for opening of the PTP under oxidative stress [30,33]. The inducing effect of IF1 overexpression on PTP was abrogated by ablation of CyPD, and IF1 could interact with p53-CyPD complex, suggesting that IF1 facilitated PTP opening via p53-CyPD [41]. IF1 binding to p53-CyPD complex may cause a conformational change that transmitted to the inner membrane via OSCP subunit and eventually PTP formation [41]. However, the interaction between IF1 and p53-CyPD complex is direct or indirect and how they bind to each other await further studies.
F-ATP Synthase Inhibitory Factor 1 Regulates Metabolic Reprogramming
Mitochondria, known as “powerhouses of the cell”, generate ATP to drive energetically dynamical life processes. Mitochondrial dysfunction results in the decline of the mitochondrial membrane potential (Δψm), such as limitation of substrate or oxygen availability, impaired oxidative phosphorylation (OXPHOS), the activation of uncoupling proteins, or a leak of protons into the matrix through the PTP [42]. The underlying mechanism of Warburg effect is proposed to be impaired mitochondria, which forces metabolic reprogramming towards aerobic glycolysis [43]. Long-lasting openings of the PTP cause rupture of the outer membrane, mitochondrial depolarization, and loss of ATP production [44]. Depending on the open state, PTP is involved in metabolic plasticity, reprogramming and cell death [43].
The compromised mitochondrial function and the decline of Δψm lead to the reverse of F-ATP synthase, hydrolyzing ATP to pump protons out from the matrix [42]. In presence of proton motive force, the bound IF1 releases from F-ATP synthase and ATP synthesis recover, thus, IF1 plays a role in preventing futile ATP hydrolysis [45]. IF1 is upregulated in a variety of carcinomas and acts as a main driver of metabolic switch to a Warburg phenotype [46]. IF1 binding to p53-CyPD complex promotes opening of the PTP, and this finding may provide an alternative mechanism through which IF1 regulates metabolic reprogramming [41]. The relative IF1 expression level to F-ATP synthase varies between tissues and cell types, which may contribute to heterogeneous metabolic phenotypes of tumors [39,43]. Therefore, to elucidate the factors that dictate IF1 expression in different cell types or tissues is a critical issue. The expression of F-ATP synthase is regulated at both the transcriptional and post-transcriptional levels [47-49]. Our unpublished data suggested that the interaction of IF1 with transcription factors c-Myc and PGC1α might be involved in IF1-regulatory metabolic reprogramming. However, whether c-Myc and PGC1α could regulate IF1 expression awaits further investigation.
Role of the Permeability Transition Pore in Metabolic Reprogramming
Metabolic plasticity and reprogramming allow cancer cells to cope with different environments and treatments, increasing adaptability and developing chemoresistance [43]. ROS is critical to promote the tumor phenotype by regulation of oncogenic signaling and cellular metabolism, and metabolic deregulations lead to drug resistance [50]. Cytosolic Ca2+ activates several Ca2+-binding proteins that directly regulate many enzymes, transportome function, and gene expression [51]. Mitochondrial Ca2+ modulates mitochondrial energy machinery by activation of mitochondrial dehydrogenase enzymes and regulation of ETC function [51]. Ca2+ and ROS mutually influence each other, Ca2+ signaling is crucial for the generation of ROS while ROS regulate the activity of Ca2+ channels and transporters [52]. Transient opening of the PTP contributes to physiological Ca2+ and ROS homeostasis, indicating the role of PTP in regulation of metabolic reprogramming [43]. The discovery that the PTP forms from F-ATP synthase and the signaling pathways affecting its transition from an energy-conserving to an energy-dissipating device provide new therapeutic perspectives for carcinomas [53].
IF1-mediated inhibition of F-ATP synthase enhances the production of mitochondrial ROS [54]. IF1 is upregulated in some phenotypes of cancer resulting in an increase of ROS level, which activates pro-survival pathways and triggers proliferative response [40]. IF1 overexpression favors opening of the PTP involving in its interaction with p53-CyPD complex [41]. The production of ROS induced by IF1 overexpression further enhances the activity of PTP. In response to metabolic stress, p53 is activated to regulate metabolic pathways and to promote cell survival [55]. Mitochondrial ROS has been found to be an important component of the stress-induced activation of p53 [55]. After activation, p53 translocation to mitochondria may promote the flickering activity of PTP and contribute to metabolic reprogramming [41,43].
The peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α) synchronizes the mitochondrial and nuclear genomes and coordinates mitochondrial biogenesis [56,57]. Activation of PGC1α promotes OXPHOS in a transcription-dependent manner [58]. PGC1α plays a crucial role in regulating metabolic balance and chemoresistance, contributing to cancer progression [59]. The MYC/PGC1α balance acts as the main determinant for metabolic phenotype and plasticity in resistant cancer stem cells [60]. PGC1α can be translocated to mitochondria [61] and its binding to mitochondrial p53 regulates p53 transactivation of metabolic genes [62]. PGC-1α expression is induced by ROS, which in turn regulates mitochondrial biogenesis and activity [63]. PGC-1α is also co-induced with several key ROS-detoxifying enzymes under oxidative stress and acts as a broad and powerful regulator of ROS metabolism [64]. Whether PGC-1α regulates PTP activity and its role in metabolic reprogramming await further investigations.
Conclusions and Perspectives
We have recently reported that IF1 interacts with p53-CyPD complex and facilitates opening of the PTP, and IF1 is required for the formation of p53-CyPD complex. We propose that IF1 binding to p53-CyPD complex induces a conformational change transmitted to the inner membrane subunits via OSCP subunit and eventually PTP formation. As an intrinsic inhibitor of F-ATP synthase, IF1 has been well characterized to be a main driver of metabolic switch to a Warburg phenotype. The PTP-regulatory activity of IF1 provides a clue that IF1 may participate in maintenance of Ca2+/ROS homeostasis by regulating PTP opening, contributing to metabolic reprogramming (Figure 1). IF1-mediated inhibition of F-ATP synthase enhances the production of mitochondrial ROS, and ROS mediates the expressions of oncogenes and transcription factors like MYC/PGC1α mediating metabolic plasticity (Figure 1). The function of IF1 extends beyond that envisaged in literature, and we still have a great deal to learn about this fascinating little protein.
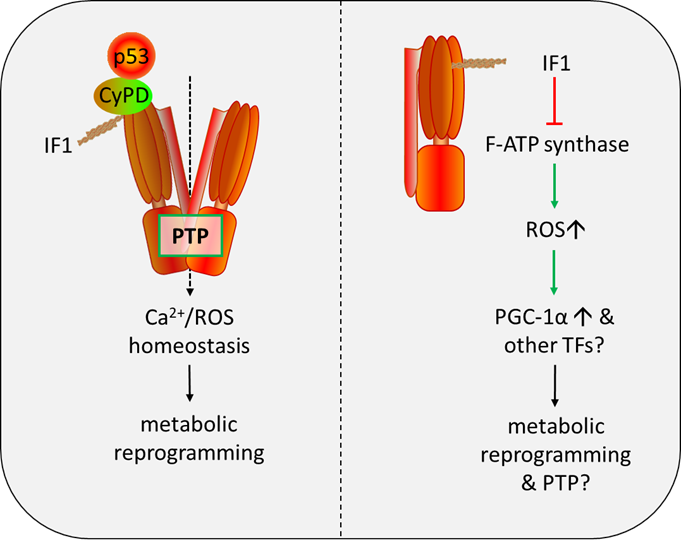
Figure 1. Role of IF1 in regulation of the PTP and metabolic reprogramming. IF1: F-ATP Synthase Inhibitory Factor 1; PTP: mitochondrial permeability transition pore; CyPD: Cyclophilin D; ROS: reactive oxygen species; TFs: transcription factors.
Funding
This work was sponsored by Shanghai Sailing Program (20YF1453200).
Conflicts of Interest
The author declares no conflict of interest.
References
2. Bernardi P, Carraro M, Lippe G. The mitochondrial permeability transition: Recent progress and open questions. The FEBS Journal. 2022 Nov;289(22):7051-74.
3. Alavian KN, Beutner G, Lazrove E, Sacchetti S, Park HA, Licznerski P, et al. An uncoupling channel within the c-subunit ring of the F1FO ATP synthase is the mitochondrial permeability transition pore. Proceedings of the National Academy of Sciences. 2014 Jul 22;111(29):10580-5.
4. Giorgio V, Von Stockum S, Antoniel M, Fabbro A, Fogolari F, Forte M, et al. Dimers of mitochondrial ATP synthase form the permeability transition pore. Proceedings of the National Academy of Sciences. 2013 Apr 9;110(15):5887-92.
5. Carrer A, Tommasin L, Šileikytė J, Ciscato F, Filadi R, Urbani A, et al. Defining the molecular mechanisms of the mitochondrial permeability transition through genetic manipulation of F-ATP synthase. Nature Communications. 2021 Aug 10;12(1):4835.
6. Galber C, Minervini G, Cannino G, Boldrin F, Petronilli V, Tosatto S, et al. The f subunit of human ATP synthase is essential for normal mitochondrial morphology and permeability transition. Cell Reports. 2021 May 11;35(6):109111.
7. Urbani A, Giorgio V, Carrer A, Franchin C, Arrigoni G, Jiko C, et al. Purified F-ATP synthase forms a Ca2+-dependent high-conductance channel matching the mitochondrial permeability transition pore. Nature Communications. 2019 Sep 25;10(1):4341.
8. Bonora M, Morganti C, Morciano G, Pedriali G, Lebiedzinska‐Arciszewska M, Aquila G, et al. Mitochondrial permeability transition involves dissociation of F1 FO ATP synthase dimers and C‐ring conformation. EMBO Reports. 2017 Jul;18(7):1077-89.
9. Carraro M, Giorgio V, Šileikytė J, Sartori G, Forte M, Lippe G, et al. Channel formation by yeast F-ATP synthase and the role of dimerization in the mitochondrial permeability transition*♦. Journal of Biological Chemistry. 2014 Jun 6;289(23):15980-5.
10. Carraro M, Jones K, Sartori G, Schiavone M, Antonucci S, Kucharczyk R, et al. The unique cysteine of F-ATP synthase OSCP subunit participates in modulation of the permeability transition pore. Cell Reports. 2020 Sep 1;32(9):108095.
11. Giorgio V, Burchell V, Schiavone M, Bassot C, Minervini G, Petronilli V, et al. Ca2+ binding to F‐ATP synthase β subunit triggers the mitochondrial permeability transition. EMBO Reports. 2017 Jul;18(7):1065-76.
12. Antoniel M, Jones K, Antonucci S, Spolaore B, Fogolari F, Petronilli V, et al. The unique histidine in OSCP subunit of F‐ATP synthase mediates inhibition of the permeability transition pore by acidic pH. EMBO Reports. 2018 Feb;19(2):257-68.
13. Guo L, Carraro M, Sartori G, Minervini G, Eriksson O, Petronilli V, et al. Arginine 107 of yeast ATP synthase subunit g mediates sensitivity of the mitochondrial permeability transition to phenylglyoxal. Journal of Biological Chemistry. 2018 Sep 1;293(38):14632-45.
14. Guo L, Carraro M, Carrer A, Minervini G, Urbani A, Masgras I, et al. Arg-8 of yeast subunit e contributes to the stability of F-ATP synthase dimers and to the generation of the full-conductance mitochondrial megachannel. Journal of Biological Chemistry. 2019 Jul 1;294(28):10987-97.
15. Fournier N, Ducet G, Crevat A. Action of cyclosporine on mitochondrial calcium fluxes. Journal of Bioenergetics and Biomembranes. 1987 Jun;19:297-303.
16. Crompton M, Ellinger H, Costi A. Inhibition by cyclosporin A of a Ca2+-dependent pore in heart mitochondria activated by inorganic phosphate and oxidative stress. Biochemical Journal. 1988 Oct 10;255(1):357-60.
17. Broekemeier KM, Dempsey ME, Pfeiffer DR. Cyclosporin A is a potent inhibitor of the inner membrane permeability transition in liver mitochondria. Journal of Biological Chemistry. 1989 May 15;264(14):7826-30.
18. Halestrap AP, Davidson AM. Inhibition of Ca2(+)-induced large-amplitude swelling of liver and heart mitochondria by cyclosporin is probably caused by the inhibitor binding to mitochondrial-matrix peptidyl-prolyl cis-trans isomerase and preventing it interacting with the adenine nucleotide translocase. The Biochemical Journal. 1990 May 15;268(1):153-60.
19. Basso E, Fante L, Fowlkes J, Petronilli V, Forte MA, Bernardi P. Properties of the permeability transition pore in mitochondria devoid of Cyclophilin D. Journal of Biological Chemistry. 2005 May 13;280(19):18558-61.
20. Connern CP, Halestrap AP. Chaotropic agents and increased matrix volume enhance binding of mitochondrial cyclophilin to the inner mitochondrial membrane and sensitize the mitochondrial permeability transition to [Ca2+]. Biochemistry. 1996 Jun 25;35(25):8172-80.
21. Nicolli A, Basso E, Petronilli V, Wenger RM, Bernardi P. Interactions of Cyclophilin with the Mitochondrial Inner Membrane and Regulation of the Permeability Transition Pore, a Cyclosporin A-sensitive Channel (∗). Journal of Biological Chemistry. 1996 Jan 26;271(4):2185-92.
22. Giorgio V, Bisetto E, Soriano ME, Dabbeni-Sala F, Basso E, Petronilli V, Forte MA, Bernardi P, Lippe G. Cyclophilin D modulates mitochondrial F0F1-ATP synthase by interacting with the lateral stalk of the complex. Journal of Biological Chemistry. 2009 Dec 4;284(49):33982-8.
23. Giorgio V, Burchell V, Schiavone M, Bassot C, Minervini G, Petronilli V, et al. Ca2+ binding to F‐ATP synthase β subunit triggers the mitochondrial permeability transition. EMBO Reports. 2017 Jul;18(7):1065-76.
24. Carraro M, Checchetto V, Sartori G, Kucharczyk R, di Rago JP, Minervini G, et al. High-conductance channel formation in yeast mitochondria is mediated by F-ATP synthase e and g subunits. Cellular Physiology and Biochemistry. 2018;50(5):1840-55.
25. Alavian KN, Beutner G, Lazrove E, Sacchetti S, Park HA, Licznerski P, et al. An uncoupling channel within the c-subunit ring of the F1FO ATP synthase is the mitochondrial permeability transition pore. Proceedings of the National Academy of Sciences. 2014 Jul 22;111(29):10580-5.
26. Pinke G, Zhou L, Sazanov LA. Cryo-EM structure of the entire mammalian F-type ATP synthase. Nature Structural & Molecular Biology. 2020 Nov;27(11):1077-85.
27. Gerle C. Mitochondrial F-ATP synthase as the permeability transition pore. Pharmacological Research. 2020 Oct 1;160:105081.
28. Lane DP. p53, guardian of the genome. Nature. 1992 Jul 2;358(6381):15-6.
29. Riley T, Sontag E, Chen P, Levine A. Transcriptional control of human p53-regulated genes. Nature reviews Molecular Cell Biology. 2008 May;9(5):402-12.
30. Vaseva AV, Marchenko ND, Ji K, Tsirka SE, Holzmann S, Moll UM. p53 opens the mitochondrial permeability transition pore to trigger necrosis. Cell. 2012 Jun 22;149(7):1536-48.
31. Chipuk JE, Maurer U, Green DR, Schuler M. Pharmacologic activation of p53 elicits Bax-dependent apoptosis in the absence of transcription. Cancer Cell. 2003 Nov 1;4(5):371-81.
32. Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, et al. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004 Feb 13;303(5660):1010-4.
33. Bergeaud M, Mathieu L, Guillaume A, Moll U, Mignotte B, Le Floch N, et al. Mitochondrial p53 mediates a transcription-independent regulation of cell respiration and interacts with the mitochondrial F₁F₀-ATP synthase. Cell Cycle. 2013 Sep 1;12(17):2781-93.
34. Pullman ME, Monroy GC. A naturally occurring inhibitor of mitochondrial adenosine triphosphatase. Journal of biological chemistry. 1963 Nov 1;238(11):3762-9.
35. Cabezon E, Butler PJ, Runswick MJ, Walker JE. Modulation of the oligomerization state of the bovine F1-ATPase inhibitor protein, IF1, by pH. Journal of Biological Chemistry. 2000 Aug 18;275(33):25460-4.
36. Walker JE. The regulation of catalysis in ATP synthase. Current Opinion in Structural Biology. 1994 Jan 1;4(6):912-8.
37. Cabezón E, Arechaga I, Jonathan P, Butler G, Walker JE. Dimerization of bovine F1-ATPase by binding the inhibitor protein, IF1. Journal of Biological Chemistry. 2000 Sep 15;275(37):28353-5.
38. García JJ, Morales-Ríos E, Cortés-Hernández P, Rodríguez-Zavala JS. The inhibitor protein (IF1) promotes dimerization of the mitochondrial F1F0-ATP synthase. Biochemistry. 2006 Oct 24;45(42):12695-703.
39. Campanella M, Casswell E, Chong S, Farah Z, Wieckowski MR, Abramov AY, et al. Regulation of mitochondrial structure and function by the F1Fo-ATPase inhibitor protein, IF1. Cell Metabolism. 2008 Jul 2;8(1):13-25.
40. Formentini L, Sánchez-Aragó M, Sánchez-Cenizo L, Cuezva JM. The mitochondrial ATPase inhibitory factor 1 triggers a ROS-mediated retrograde prosurvival and proliferative response. Molecular Cell. 2012 Mar 30;45(6):731-42.
41. Guo L. Mitochondrial ATP synthase inhibitory factor 1 interacts with the p53–cyclophilin D complex and promotes opening of the permeability transition pore. Journal of Biological Chemistry. 2022 May 1;298(5):101858.
42. Campanella M, Parker N, Tan CH, Hall AM, Duchen MR. IF1: setting the pace of the F1Fo-ATP synthase. Trends in Biochemical Sciences. 2009 Jul 1;34(7):343-50.
43. Guo L. Mitochondria and the permeability transition pore in cancer metabolic reprogramming. Biochemical Pharmacology. 2021 Jun 1;188:114537.
44. Criddle DN. Reactive oxygen species, Ca2+ stores and acute pancreatitis; a step closer to therapy?. Cell Calcium. 2016 Sep 1;60(3):180-9.
45. Walker JE. The ATP synthase: the understood, the uncertain and the unknown. Biochemical Society Transactions. 2013 Feb 1;41(1):1-6.
46. Sánchez-Cenizo L, Formentini L, Aldea M, Ortega AD, García-Huerta P, Sánchez-Aragó M, et al. Up-regulation of the ATPase inhibitory factor 1 (IF1) of the mitochondrial H+-ATP synthase in human tumors mediates the metabolic shift of cancer cells to a Warburg phenotype. Journal of Biological Chemistry. 2010 Aug 13;285(33):25308-13.
47. Scarpulla RC. Nuclear control of respiratory chain expression by nuclear respiratory factors and PGC‐1‐related coactivator. Annals of the New York Academy of Sciences. 2008 Dec;1147(1):321-34.
48. Scarpulla RC, Vega RB, Kelly DP. Transcriptional integration of mitochondrial biogenesis. Trends in Endocrinology & Metabolism. 2012 Sep 1;23(9):459-66.
49. Willers IM, Cuezva JM. Post-transcriptional regulation of the mitochondrial H+-ATP synthase: a key regulator of the metabolic phenotype in cancer. Biochimica et Biophysica Acta (BBA)-Bioenergetics. 2011 Jun 1;1807(6):543-51.
50. Bhardwaj V, He J. Reactive oxygen species, metabolic plasticity, and drug resistance in cancer. International Journal of Molecular Sciences. 2020 May 12;21(10):3412.
51. Dejos C, Gkika D, Cantelmo AR. The two-way relationship between calcium and metabolism in cancer. Frontiers in Cell and Developmental Biology. 2020 Nov 13;8:573747.
52. Görlach A, Bertram K, Hudecova S, Krizanova O. Calcium and ROS: A mutual interplay. Redox Biology. 2015 Dec 1;6:260-71.
53. Bernardi P, Rasola A, Forte M, Lippe G. The mitochondrial permeability transition pore: channel formation by F-ATP synthase, integration in signal transduction, and role in pathophysiology. Physiological Reviews. 2015 Oct;95(4):1111-55.
54. Esparza-Moltó PB, Cuezva JM. The role of mitochondrial H+-ATP synthase in cancer. Frontiers in Oncology. 2018 Mar 7;8:53.
55. Kh V, Ryan KM. p53 and metabolism. Nature Reviews Cancer. 2009;9(10):691-700.
56. O'Brien KM. Mitochondrial biogenesis in cold-bodied fishes. Journal of Experimental Biology. 2011 Jan 15;214(2):275-85.
57. Ryan MT, Hoogenraad NJ. Mitochondrial-nuclear communications. Annual Review of Biochemistry. 2007 Jul 7;76:701-22.
58. Puigserver P, Spiegelman BM. Peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α): transcriptional coactivator and metabolic regulator. Endocrine Reviews. 2003 Feb 1;24(1):78-90.
59. Tan Z, Luo X, Xiao L, Tang M, Bode AM, Dong Z, Cao Y. The Role of PGC1α in Cancer Metabolism and its Therapeutic ImplicationsPGC1α in Cancer Metabolism and Its Therapeutic Implications. Molecular Cancer Therapeutics. 2016 May 1;15(5):774-82.
60. Sancho P, Burgos-Ramos E, Tavera A, Kheir TB, Jagust P, Schoenhals M, et al. MYC/PGC-1α balance determines the metabolic phenotype and plasticity of pancreatic cancer stem cells. Cell Metabolism. 2015 Oct 6;22(4):590-605.
61. Aquilano K, Vigilanza P, Baldelli S, Pagliei B, Rotilio G, Ciriolo MR. Peroxisome proliferator-activated receptor γ co-activator 1α (PGC-1α) and sirtuin 1 (SIRT1) reside in mitochondria: possible direct function in mitochondrial biogenesis. Journal of Biological Chemistry. 2010 Jul 9;285(28):21590-9.
62. Sen N, Satija YK, Das S. PGC-1α, a key modulator of p53, promotes cell survival upon metabolic stress. Molecular Cell. 2011 Nov 18;44(4):621-34.
63. Kim B, Jung JW, Jung J, Han Y, Suh DH, Kim HS, et al. PGC1α induced by reactive oxygen species contributes to chemoresistance of ovarian cancer cells. Oncotarget. 2017 Sep 9;8(36):60299-311.
64. St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jäger S, et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006 Oct 20;127(2):397-408.
