Abstract
Sepsis is highly prevalent, and is one of the main causes of mortality among hospitalized patients. Ethanol consumption in large quantities compromises the normal functioning of the body, leading to dysfunction of multiple different organ systems. The association between sepsis and ethanol is not fully understood, but it is well accepted that ethanol consumption plays a role in the development of sepsis. Both sepsis and ethanol cause inflammatory dysfunction and promote oxidative stress. Antioxidant agents may be highly relevant targets to abrogate the effects of sepsis in patients who also consume large amounts of ethanol. This review focuses on presenting the main mechanisms involved between sepsis and ethanol consumption, and to describe the main antioxidants that have been used as therapeutic agents.
Keywords
Ethanol consumption, Sepsis, Oxidative stress, Antioxidant defense, Inflammation, Antioxidant agents, Sepsis mortality
Introduction
Sepsis is considered one of the main causes of death in the world, and can be aggravated by acute or chronic exposure to ethanol [1], directly affecting vascular function [2]. According to the World Health Organization (WHO), 43% of the world population consumes ethanol [3]. Chronic ethanol consumption increases risk for the development of sepsis , sepsis mortality and costs of patient hospitalization [1,4].
Increased mortality in sepsis after ethanol consumption has also been observed in animal models of induced sepsis. Alterations in permeability in the hepatic, vascular, pulmonary, renal, and gastrointestinal systems are caused primarily by ethanol consumption [2,5,6]. The main pathogenic mechanisms implicated in both sepsis and ethanol consumption involve the activation of the immune system, releasing anti-inflammatory and pro-inflammatory mediators, as well as the activation of the antioxidant system [1,5,7,8], which has been demonstrated in both animal and human studies [2,5,9,10]. Experimental studies have suggested that antioxidant agents may be used to try to reverse these pathologic mechanisms [9-12].
The focus of this review is to address the main mechanisms between increased sepsis mortality associated with ethanol consumption, giving an overview of the inflammatory processes involved. In particular, we will focus on the oxidative processes that occur as well as the possible benefits of using antioxidant compounds to prevent organ dysfunction in the setting of sepsis and ethanol consumption.
We performed an active literature search using the terms "ethanol and sepsis", "ethanol, sepsis and pathologies", "ethanol and organ disorders", “sepsis and organ disorders”, “epidemiology of ethanol”, “sepsis and ethanol and antioxidants”, “sepsis and ethanol and oxidative stress” using Medline, Scopus, Web of Science, ScienceDirect, PLoS databases, between 2001 to 2021. Only articles published in English language were included. Analysis of the information started with the title, abstract and then the full report.
Sepsis and Ethanol Consumption Epidemiology
Alcohol use disorders are considered a serious public health problem [13,14]. According to the most recent data from the WHO, it is estimated that in the last year about 43% of the global population consumed alcohol, with an average of 6.4 liters of pure alcohol per capita. In Brazil, it is estimated that 40% of the population consumed alcohol in the last 12 months, with the estimated consumption per person in 2016 7.8 liters of pure alcohol, higher than the world average. In addition, Brazilian men have a higher prevalence in the consumption of alcohol when compared to women [3].
Excessive alcohol consumption may lead to chronic organ dysfunction, particularly in the gastrointestinal tract, liver and pancreas. It is a potent modulator of the immune system that can lead to chronic dysregulation [4]. Ethanol can modulate the antioxidant system, leading to increased production of reactive oxygen species (ROS) [2]. The extent of the effects caused by ethanol is influenced by factors such as consumption pattern, quantity ingested, gender and organ system affected [15].
Chronic consumption of ethanol is directly related to susceptibility to infections due to direct toxic effects on the immune system [4,16,17]. Bacterial infections are the main complication in patients with liver disease related to alcoholism in a hospital environment [18]. According to one study, between 20 to 40% of hospitalized patients have at least one condition associated with chronic ethanol consumption [19].
Studies have shown that rats that chronically consumed ethanol and later developed sepsis had a higher mortality rate compared to control animals. Furthermore, the mortality rate is directly related to the amount of alcohol consumed, with higher consumption associated with increased mortality [4,6,19]. If mice that were subjected to sepsis were given ethanol, their overall survival was decreased at 72 hours [20]. Mice that chronically consume alcohol have fewer infections during periods of abstinence, with fewer mice developing sepsis during abstinent periods [17].
Alcohol consumption is associated with impaired immune responses patients requiring intensive care unit (ICU) level of care, with increased ICU length of stay and a high risk of death [13,14]. In addition, alcoholic patients are more likely to develop sepsis [13,14], with higher hospital mortality and increased hospitalization days [4,21]. According to the latest data from the WHO, 12.9% of deaths from infectious diseases were attributed to the ethanol consumption [3].
Sepsis is considered an inflammatory response in response to infection that causes cell damage in many tissues. This condition is highly prevalent, especially in the population over 85 years old, in addition to being one of the main causes of morbidity and mortality in the intensive care unit worldwide [1,4,5,22]. This pathology can be divided into 4 categories: systemic inflammatory response syndrome, which does not yet verify an infectious process itself; sepsis, where the installation of the infectious process has already been confirmed [22]; severe sepsis, which is mainly associated with multiple organ failure [5,22]; and septic shock, characterized by low blood pressure and organ failure with a more imminent risk of death [22].
During sepsis, an increase in the levels of pro-inflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α) [4,15], and decrease in CD4+ and CD8+ lymphocytes [6] was observed. There is an induction of intestinal epithelial apoptosis, which favors an increase in the permeability of the intestine, thus causing an increase in the systemic inflammatory process, since the intestine is closely linked to the immune system [6].
The association of ethanol consumption with sepsis and the mechanisms involved between increased mortality seen in alcoholic patients to sepsis is still quite controversial. It seems to be associated with the attenuation of the innate and acquired immune response and, consequently, a decrease in the bactericidal activity of macrophages and neutrophils [1,4]. Furthermore, these patients may have chronic malnutrition, leading to [1,4] and marked apoptosis, particularly in intestinal cells [6].
Mechanisms Mediated by the Immune System in Ethanol Consumption and Sepsis
The human immune system has two mechanisms to protect itself, innate and adaptive immunity. Macrophages are the primary cell associated with innate immunity associated with damage caused by sepsis [7,23]. Macrophages ingest microbes as well as release pro-inflammatory cytokines, classically TNF-α and IL-6 [7,23,24]. Additionally, sepsis promotes a marked decrease in CD4+ and CD8+ lymphocytes, and ethanol consumption further reduces this process [6]. CD4+ lymphocytes are responsible for robust inflammation and activation of host defenses with production of TNF-α and interferon-gamma (IFN-γ) [25].
Sepsis may increase the levels of IL-6 and TNF-α [4,15], damaging different organs [5,26] culminating in septic shock [22]. Increased levels of blood urea nitrogen and creatinine may occur during sepsis, important markers of kidney damage [4]. Ethanol may also contribute to high creatinine and urea levels [15], leading to kidney damage and acute tubular necrosis [5]. Up to 50% of patients develop acute kidney injury in sepsis [1,5].
Sepsis among patients with alcohol abuse is also associated with worsening of intestinal cell apoptosis, increased gut permeability and reduced villous length, all of which are linked to increased mortality [27]. It is believed that there is greater translocation of bacteria and bacterial products through the intestinal lumen. In studies of animals subjected to sepsis by cecal ligation and puncture that maintain the intestinal barrier, there is better prognosis [6].
The ways in which ethanol can aggravate sepsis are not fully elucidated. Ethanol consumption directly affects immune function, leading to a decrease in the lymphocytic response, as well as alteration in cytokine production by lymphocytes and macrophages [15]. Other studies have demonstrated that the administration of ethanol suppresses the elimination of bacteria present in sepsis, with decreased pro-inflammatory chemokine levels (TNF-α and IL-6) [1,4,26]. The decrease in TNF-α itself may be responsible for the decreased recruitment of neutrophils, resulting in a less robust immune response [4].
The association of ethanol with sepsis increases the levels of TNF-α and IL-6 in plasma [2] and renal tissue [5]. Yoseph et al. observed that mice subjected to sepsis and exposed to ethanol for 12 weeks demonstrated an upregulation in the production of TNF-α and IFN-γ in CD4+ cells [6]. However, theoretical differences may be temporally associated with the sepsis, since the initial phase of sepsis is characterized by hyperinflammation and in the late phase, hypoinflammation is observed, characterized by a decrease in pro-inflammatory mediators. These divergences may also be related to the ethanol concentrations used or gender [8,15].
Mechanisms Mediated by Oxidative Stress in Ethanol Consumption and Sepsis
Oxidative stress is defined by the disequilibrium between oxidizing compounds and the antioxidant system, culminating in cellular and tissue oxidative damage. The main ROS important in sepsis and ethanol consumption are the superoxide anions (O2•-), the hydroxyl radical (OH•), and the hydrogen peroxide (H2O2). Furthermore, O2•- can react with NO (nitric oxide) to form peroxynitrite (ONOO-), an important marker of lipid peroxidation, detected through the presence of malonaldehyde [28,29].
Antioxidant enzymes are responsible for inhibiting or reducing the damage caused by oxidizing agents, particularly superoxide dismutase (SOD), Catalase and Glutathione Peroxidase (GPx). SOD converts O2•- into H2O2 and molecular oxygen. Catalase and GPx prevent the formation of H2O2 [28-30]. In addition, GPx is important for the balance of the glutathione redox cycle, controlling reduced and oxidized glutathione [28,29]. Therefore, changes in oxidized glutathione levels are an indicator of increased oxidative stress [31].
The increase in reactive oxygen species and lipoperoxidation leads to the activation of metalloproteinase (MMPs), especially MMP-2 and MMP-9, which are closely linked with the development of tissue pathology, as they promote the remodeling and degradation of the extracellular matrix [5,11]. MMP-2 is usually of mesenchymal origin, while MMP-9 is derived from macrophages during inflammatory processes [32].
Ethanol consumption promotes a modulation of the antioxidant system through the activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and increased expression of inducible nitric oxide synthase. This mechanism causes an increase in reactive oxygen species, with greater lipid lipoperoxidation as well as vascular dysfunction [2,5]. Studies have indicated an increase in malonaldehyde levels in different organs, confirming this pathway [12,33].
Ethanol consumption can modulate oxidizing enzymes in different ways, depending on the organ being studied. Some studies show a decrease in GSH levels in the vascular tissue of animals and an increase in catalase activity [2]. In the renal system, increases in MMPs, ROS and pro-inflammatory cytokines [5,34] suggest a poor prognosis in individuals with sepsis, since half of those affected by sepsis develop acute kidney injury [5]. Decreased catalase and SOD activity have been noted in the liver [33].
There is a strong interaction between inflammatory processes and the formation of reactive oxygen species, favoring redox imbalance in sepsis [11]. Animals submitted to sepsis showed increases in MMP-2 [11] and MMP-9 [32,35], with decreased SOD and catalase activity [30]. In addition, MMP-9 can be used as a marker of sepsis severity [32]. Furthermore, macrophages present in inflammation resulting from sepsis act by producing ROS (O2•-, OH•, H2O2) and NO during the phagocytosis process. NO, which is absent in resting macrophages, is activated by the presence of INF-γ, which is also an important proinflammatory mediator [7,30] and favors the production of inflammatory cytokines such as IL- 6 and TNF-α [23].
Chronic ethanol consumption increases the risk of acute lung injury by depleting glutathione. Resulting tissue alterations, such as decreased surfactant processing, decreased barrier integrity, and increased apoptosis are associated with an increase in the susceptibility to sepsis [36].
Oxidative stress induced by ethanol chronic consumption plays a role in several aspects of alcohol-related liver disease. Ethanol metabolism produces excessive acetaldehyde, reactive oxygen species and protein and DNA adducts. These alterations lead to the expression of pro-inflammatory mediators in the liver, structural and functional dysregulation of mitochondria, causing hepatocyte apoptosis and necrosis [37]. Sepsis increased lipidic peroxidation and oxidative stress in the liver of rats submitted to acute ethanol consumption. These early changes in oxidative stress caused in the liver by ethanol were associated with increased sepsis-related organ damage [38]. In addition, chronic and acute ethanol consumption are associated with enhanced cardiac oxidative and nitrative stress, damage in mitochondrial function, cardiomyocyte hypertrophy and cardiac steatosis [16,39].
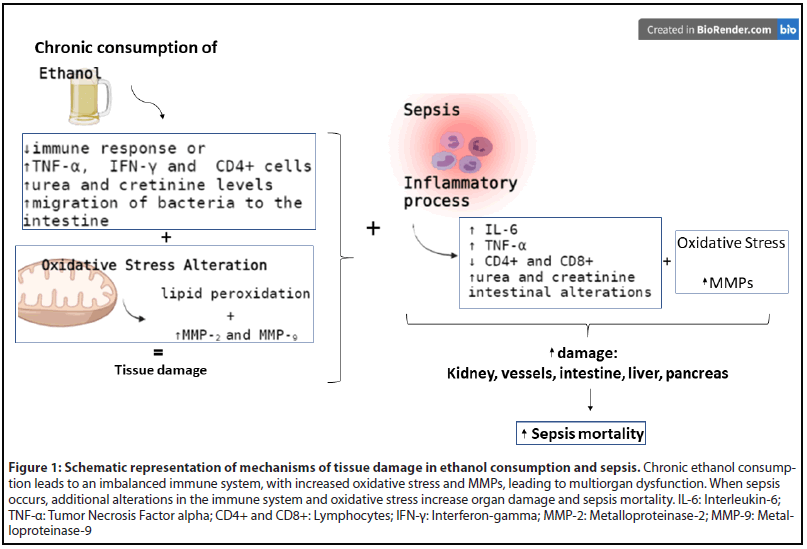
Figure 1 summarizes the of mechanisms of tissue damage in chronic ethanol consumption and sepsis, focused in the immune system alterations and oxidative stress.
Antioxidants as an Alternative Treatment in Ethanol Consumption and Sepsis
Antioxidant agents are defined as substances that, in the presence of an oxidizing compound, effectively delay or inhibit oxidation. Most of these compounds have both antioxidant and anti-inflammatory properties [28,29]. Currently, different studies aim to evaluate the use of antioxidant substances for the treatment of chronic consumption of ethanol and sepsis.
Ethanol consumption increased O2•- and increased IL-10 levels in the mesenteric arterial bed of chronic ethanol treated rats. Ethanol also increased plasma levels of TBARS, TNF-α, IL-6, IL-1β, and IL-10. Apocynin, an antioxidant inhibitor of the enzyme NADPH oxidase, prevented these alterations and reduced endothelial dysfunction induced by ethanol in resistance arteries [24]. In another experimental study, ethanol chronic consumption increased O2 generation and lipid peroxidation in the aorta of rats and apocynin prevented these responses [40].
The antioxidant ascorbic acid or vitamin C prevented endothelial dysfunction in the aorta of rats with acute ethanol intake. However, vitamin C was not able to revert the increased O2•- generation in the aorta of ethanol treated mice. However,an increase in SOD activity was observed with the use of the antioxidant [41].
Rats submitted to chronical ethanol consumption were treated with melatonin, a hormone with indirect antioxidant activities by increasing antioxidant enzymes activities, and vitamin C. Ethanol increased aortic and plasma lipidic peroxidation and treatment with melatonin and vitamin C reduced these alterations, but not the alterations in SOD and catalase activities caused by ethanol [42]. In another study, the antioxidant effect of melatonin and vitamin C was evaluated by renal injury induced by chronic alcohol consumption in rats. In this study, ethanol increased the formation of lipid peroxides, SOD and catalase activities in the kidney, while melatonin treatment reduced only the lipidic peroxidation in the kidneys [43].
Hepatic mitochondria are injured by both acute and chronic ethanol consumption. Manganese superoxide dismutase (MnSOD) overexpression in mice prevented liver mitochondrial DNA depletion, ROS formation, decreased mitochondrial glutathione and increased inducible nitric oxide synthase (NOS) expression induced by acute ethanol exposure. However, when mice were subjected to chronic ethanol consumption, the mitochondrial damage was worsened by the overexpression of this antioxidant enzyme [44].
Regarding sepsis, treatment with antioxidants seems to have interesting results. Mice subjected to cecal ligation and puncture (CLP) model of sepsis treated with the antioxidant curcumin presented less inflammatory damage in the lung and kidney. This response was associated with increased plasmatic IL-10 levels and inhibition of the secretion of plasma TNF-α and IL-6 septic mice after the treatment with the antioxidant [9]. Furthermore, the administration of blackberry extracts in a mouse model of sepsis induced by LPS decreased leukocytes in bronchoalveolar lavage, decreased TNF levels in the serum and decreased GPx and MMP-2 activity of septic animals. These results were associated to an improvement in the survival rate in sepsis [11].
Treatment of septic models with melatonin reduced the lipidic peroxidation in the liver, brain, lung and kidneys and attenuated pulmonary inflammation of LPS-induced and CLP-induced septic models. The attenuation of the cytokine response observed with the treatment improved the survival rate of septic models [45].
Vitamin C is emerging as a potential treatment to sepsis. The administration of high intravenous dose of vitamin C attenuated oxidative stress, inflammation, improved the synthesis of vasopressor and enhanced immune cell function in septic patients [46]. Several recent clinical trials intend to prove the benefits and the safety of vitamin C in the treatment of sepsis, including potential improvements in mortality rates [47-49].
Fewer studies investigate the role of antioxidants in the treatment of chronic ethanol consumption associated with increased sepsis susceptibility and mortality. Decreased melatonin levels in alcoholic individuals have been associated with increased intestinal permeability endotoxemia in patients with alcohol abuse [50]. Pretreatment of mice with melatonin stabilized erythrocyte membranes and decreased oxidative stress [51]. In order to further investigate the effect of antioxidants in ethanol consumption and sepsis, a clinical trial was recently registered to investigate the effect of Vitamin C Infusion for treatment in Sepsis and Alcoholic Hepatitis (CITRIS-AH) and started in 2019 which will be completed in 2022 (ClinicalTrials.gov Identifier: NCT03829683) [52]. The use of antioxidants as an alternative treatment in the increased susceptibility to sepsis induced by ethanol consumption remains a poorly explored theme in the literature and requires further research.
Conclusions
Sepsis is a public health problem that requires new, more effective therapies. Ethanol consumption increases sepsis mortality by mechanisms that have not been fully elucidated. The imbalance of the antioxidant system could be a potential target for the treatment of sepsis and ethanol consumption. However, studies demonstrating the effect of antioxidants on the association of ethanol with sepsis are scarce in the literature. Therefore, further studies, both experimental and clinical, to analyze the effect of antioxidants on organ damage and increased susceptibility to sepsis observed in ethanol consumption are needed.
Conflicts of Interest
There is no conflict of interest.
Acknowledgements
This study was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Capes), Conselho Regional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG). Figure was created with BioRender.com.
Author Contributions
Silva A.O., Conceptualization and Original draft preparation.
Prohaska C.C., Reviewing and editing.
Ceron C.S., Conceptualization, Original draft preparation, Reviewing and editing.
References
2. Ceron CS, do Vale GT, Simplicio JA, Ricci ST, De Martinis BS, de Freitas A, et al. Chronic ethanol consumption increases vascular oxidative stress and the mortality induced by sub-lethal sepsis: Potential role of iNOS. Eur J Pharmacol. 2018;825:39-47.
3. Hammer JH, Parent MC, Spiker DA. Mental Help Seeking Attitudes Scale (MHSAS): Development, reliability, validity, and comparison with the ATSPPH-SF and IASMHS-PO. Journal of Counseling Psychology. 2018;65(1):74-85.
4. Barros FR, Castro-Faria-Neto HC, Castro CL, Aguiar Nemer AS, Rocha EM, Silva Fonseca VA. Effects of chronic ethanol consumption in experimental sepsis. Alcohol Alcohol. 2012;47(6):677-82.
5. do Valle GT, Ricci ST, Silva AO, Tirapelli CR, Ceron CS. Ethanol consumption increases renal dysfunction and mortality in a mice model of sub-lethal sepsis. Canadian Journal of Physiology and Pharmacology. 2020:1-9.
6. Yoseph BP, Breed E, Overgaard CE, Ward CJ, Liang Z, Wagener ME, et al. Chronic alcohol ingestion increases mortality and organ injury in a murine model of septic peritonitis. PLoS One. 2013;8(5):e62792.
7. Cruvinel Wde M, Mesquita D, Jr., Araujo JA, Catelan TT, de Souza AW, da Silva NP, et al. Immune system - part I. Fundamentals of innate immunity with emphasis on molecular and cellular mechanisms of inflammatory response. Revista Brasileira de Reumatologia. 2010;50(4):434-61.
8. Tirapelli CR, Fukada SY, Yogi A, Chignalia AZ, Tostes RC, Bonaventura D, et al. Gender-specific vascular effects elicited by chronic ethanol consumption in rats: a role for inducible nitric oxide synthase. British Journal of Pharmacology. 2008;153(3):468-79.
9. Chen L, Lu Y, Zhao L, Hu L, Qiu Q, Zhang Z, et al. Curcumin attenuates sepsis-induced acute organ dysfunction by preventing inflammation and enhancing the suppressive function of Tregs. International Immunopharmacology. 2018;61:1-7.
10. Simplicio JA, Resstel LB, Tirapelli DP, D'Orleans-Juste P, Tirapelli CR. Contribution of oxidative stress and prostanoids in endothelial dysfunction induced by chronic fluoxetine treatment. Vascular Pharmacology. 2015;73:124-37.
11. de Padua Lucio K, Rabelo ACS, Araujo CM, Brandao GC, de Souza GHB, da Silva RG, et al. Anti-Inflammatory and Antioxidant Properties of Black Mulberry (Morus nigra L.) in a Model of LPS-Induced Sepsis. Oxidative Medicine and Cellular Longevity. 2018;2018:5048031.
12. Dogan A, Celik I. Hepatoprotective and antioxidant activities of grapeseeds against ethanol-induced oxidative stress in rats. The British Journal of Nutrition. 2012;107(1):45-51.
13. Moss M, Parsons PE, Steinberg KP, Hudson LD, Guidot DM, Burnham EL, et al. Chronic alcohol abuse is associated with an increased incidence of acute respiratory distress syndrome and severity of multiple organ dysfunction in patients with septic shock. Critical Care Medicine. 2003;31(3):869-77.
14. Santolaria F, Rodriguez-Lopez C, Martin-Hernandez B, Aleman- Valls MR, Gonzalez-Reimers E, Alonso-Socas MD, et al. Similar inflammatory response in alcoholic and non-alcoholic sepsis patients. Eur Cytokine Netw. 2011;22(1):1-4.
15. Castro CL, Aguiar-Nemer AS, Castro-Faria-Neto HC, Barros FR, Rocha EM, Silva-Fonseca VA. Effect of chronic ethanol consumption in female rats subjected to experimental sepsis. Brazilian Journal of Medical and Biological Research = Revista Brasileira de Pesquisas Medicas e Biologicas. 2013;46(12):1033-9.
16. Gonzalez-Reimers E, Santolaria-Fernandez F, Martin-Gonzalez MC, Fernandez-Rodriguez CM, Quintero-Platt G. Alcoholism: a systemic proinflammatory condition. World journal of gastroenterology. 2014;20(40):14660-71.
17. Kobayashi M, Asai A, Ito I, Suzuki S, Higuchi K, Suzuki F. Short- Term Alcohol Abstinence Improves Antibacterial Defenses of Chronic Alcohol-Consuming Mice against Gut Bacteria-Associated Sepsis Caused by Enterococcus faecalis Oral Infection. The American Journal of Pathology. 2017;187(9):1998-2007.
18. Gustot T, Fernandez J, Szabo G, Albillos A, Louvet A, Jalan R, et al. Sepsis in alcohol-related liver disease. Journal of Hepatology. 2017;67(5):1031-50.
19. Mehta AJ. Alcoholism and critical illness: A review. World Journal of Critical Care Medicine. 2016;5(1):27-35.
20. Pruett SB, Fan R, Cheng B, Glover M, Tan W, Deng X. Innate immunity and inflammation in sepsis: mechanisms of suppressed host resistance in mice treated with ethanol in a binge-drinking model. Toxicological Sciences : An Official Journal of the Society of Toxicology. 2010;117(2):314-24.
21. O'Brien JM, Jr., Lu B, Ali NA, Martin GS, Aberegg SK, Marsh CB, et al. Alcohol dependence is independently associated with sepsis, septic shock, and hospital mortality among adult intensive care unit patients. Critical Care Medicine. 2007;35(2):345-50.
22. JAMA patient page. Bullying. JAMA. 2001;285(16):2156.
23. Cavaillon JM, Adib-Conquy M. Monocytes/macrophages and sepsis. Critical Care Medicine. 2005;33(12 Suppl):S506-9.
24. Simplicio JA, do Vale GT, Gonzaga NA, Leite LN, Hipolito UV, Pereira CA, et al. Reactive oxygen species derived from NAD(P)H oxidase play a role on ethanol-induced hypertension and endothelial dysfunction in rat resistance arteries. Journal of Physiology and Biochemistry. 2017;73(1):5-16.
25. Larosa DF, Orange JS. 1. Lymphocytes. The Journal of Allergy and Clinical Immunology. 2008;121(2 Suppl):S364-9; quiz S412.
26. Gandhirajan A, Roychowdhury S, Kibler C, Bauer SR, Nagy LE, Vachharajani V. Ethanol Exposure Attenuates Immune Response in Sepsis via Sirtuin 2 Expression. Alcoholism, Clinical and Experimental Research. 2021;45(2):338-50.
27. Patel S, Behara R, Swanson GR, Forsyth CB, Voigt RM, Keshavarzian A. Alcohol and the Intestine. Biomolecules. 2015;5(4):2573-88.
28. Cnubben NH, Rietjens IM, Wortelboer H, van Zanden J, van Bladeren PJ. The interplay of glutathione-related processes in antioxidant defense. Environmental Toxicology and Pharmacology. 2001;10(4):141-52.
29. Tsutsui H, Kinugawa S, Matsushima S. Oxidative stress and heart failure. American Journal of Physiology Heart and Circulatory Physiology. 2011;301(6):H2181-90.
30. Kumar S, Gupta E, Kaushik S, Kumar Srivastava V, Mehta SK, Jyoti A. Evaluation of oxidative stress and antioxidant status: Correlation with the severity of sepsis. Scandinavian Journal of Immunology. 2018;87(4):e12653.
31. McDonough KH. Antioxidant nutrients and alcohol. Toxicology. 2003;189(1-2):89-97.
32. Muhl D, Nagy B, Woth G, Falusi B, Bogar L, Weber G, et al. Dynamic changes of matrix metalloproteinases and their tissue inhibitors in severe sepsis. Journal of Critical Care. 2011;26(6):550-5.
33. Lee JS. Supplementation of Pueraria radix water extract on changes of antioxidant enzymes and lipid profile in ethanol-treated rats. Clinica Chimica Acta; International Journal of Clinical Chemistry. 2004;347(1-2):121-8.
34. Tirapelli LF, Martins-Oliveira A, Batalhao ME, Tirapelli DP, Carnio EC, Tanus-Santos JE, et al. Ethanol consumption increases the expression of endothelial nitric oxide synthase, inducible nitric oxide synthase and metalloproteinases in the rat kidney. The Journal of Pharmacy and Pharmacology. 2012;64(1):68-76.
35. Hoffmann U, Bertsch T, Dvortsak E, Liebetrau C, Lang S, Liebe V, et al. Matrix-metalloproteinases and their inhibitors are elevated in severe sepsis: prognostic value of TIMP-1 in severe sepsis. Scandinavian Journal of Infectious Diseases. 2006;38(10):867-72.
36. Brown LA, Harris FL, Ping XD, Gauthier TW. Chronic ethanol ingestion and the risk of acute lung injury: a role for glutathione availability? Alcohol. 2004;33(3):191-7.
37. Tan HK, Yates E, Lilly K, Dhanda AD. Oxidative stress in alcoholrelated liver disease. World Journal of Hepatology. 2020;12(7):332-49.
38. Allameh A, Razavi-Azarkhiavi K, Mohsenifar A, Jamali- Zavarei M. Effect of acute ethanol treatment on biochemical and histopathological factors in rat liver in an experimental sepsis model. Pathology, Research and Practice. 2012;208(6):331-7.
39. Matyas C, Varga ZV, Mukhopadhyay P, Paloczi J, Lajtos T, Erdelyi K, et al. Chronic plus binge ethanol feeding induces myocardial oxidative stress, mitochondrial and cardiovascular dysfunction, and steatosis. American Journal of Physiology Heart and Circulatory Physiology. 2016;310(11):H1658-70.
40. Marchi KC, Ceron CS, Muniz JJ, De Martinis BS, Tanus-Santos JE, Tirapelli CR. NADPH Oxidase Plays a Role on Ethanol-Induced Hypertension and Reactive Oxygen Species Generation in the Vasculature. Alcohol Alcohol. 2016;51(5):522-34.
41. Hipolito UV, Callera GE, Simplicio JA, De Martinis BS, Touyz RM, Tirapelli CR. Vitamin C prevents the endothelial dysfunction induced by acute ethanol intake. Life Sciences. 2015;141:99-107.
42. Sonmez MF, Narin F, Balcioglu E. Melatonin and vitamin C attenuates alcohol-induced oxidative stress in aorta. Basic & Clinical Pharmacology & Toxicology. 2009;105(6):410-5.
43. Sonmez MF, Narin F, Akkus D, Turkmen AB. Melatonin and vitamin C ameliorate alcohol-induced oxidative stress and eNOS expression in rat kidney. Renal Failure. 2012;34(4):480-6.
44. Mansouri A, Tarhuni A, Larosche I, Reyl-Desmars F, Demeilliers C, Degoul F, et al. MnSOD overexpression prevents liver mitochondrial DNA depletion after an alcohol binge but worsens this effect after prolonged alcohol consumption in mice. Digestive Diseases. 2010;28(6):756-75.
45. Colunga Biancatelli RML, Berrill M, Mohammed YH, Marik PE. Melatonin for the treatment of sepsis: the scientific rationale. Journal of thoracic disease. 2020;12(Suppl 1):S54-S65.
46. Kashiouris MG, L'Heureux M, Cable CA, Fisher BJ, Leichtle SW, Fowler AA. The Emerging Role of Vitamin C as a Treatment for Sepsis. Nutrients. 2020;12(2).
47. Fowler AA, 3rd, Truwit JD, Hite RD, Morris PE, DeWilde C, Priday A, et al. Effect of Vitamin C Infusion on Organ Failure and Biomarkers of Inflammation and Vascular Injury in Patients With Sepsis and Severe Acute Respiratory Failure: The CITRIS-ALI Randomized Clinical Trial. JAMA. 2019;322(13):1261-70.
48. Li J. Evidence is stronger than you think: a meta-analysis of vitamin C use in patients with sepsis. Critical Care. 2018;22(1):258.
49. Marik PE, Khangoora V, Rivera R, Hooper MH, Catravas J. Hydrocortisone, Vitamin C, and Thiamine for the Treatment of Severe Sepsis and Septic Shock: A Retrospective Before-After Study. Chest. 2017;151(6):1229-38.
50. Swanson GR, Gorenz A, Shaikh M, Desai V, Forsyth C, Fogg L, et al. Decreased melatonin secretion is associated with increased intestinal permeability and marker of endotoxemia in alcoholics. American Journal of Physiology Gastrointestinal and Liver Physiology. 2015;308(12):G1004-11.
51. Kurhaluk N, Tkachenko H, Lukash O, Winklewski PJ, Wszedybyl- Winklewska M. Melatonin maintains the function of the blood redox system at combined ethanol-induced toxicity and subclinical inflammation in mice. Sleep & Breathing = Schlaf & Atmung. 2021;25(2):1045-54.
52. CITRIS-AH. Vitamin C Infusion for TReatment in Sepsis and Alcoholic Hepatitis (CITRIS-AH). ClinicalTrialsgov Identifier: NCT03829683. 2019.
