Introduction
Alzheimer’s disease (AD) remains a formidable challenge in neuroscience, with its complex pathology still lacking an effective cure. Recent research has highlighted the critical role of epigenetic regulation in AD, demonstrating how environmental factors and genetic predispositions influence disease progression at a molecular level. This commentary examines key advancements in AD-related epigenetic research, with a particular focus on histone deacetylases (HDACs) and adrenergic signaling-two mechanisms that have emerged as promising therapeutic targets for mitigating AD symptoms and slowing neurodegeneration. Drawing insights from our two recent studies [1,2], we provide a systematic overview of the developmental trajectory of epigenetic research in AD, discuss existing challenges, and propose innovative strategies to translate these findings into effective treatments. We conclude by offering specific recommendations for future research directions, including the refinement of epigenetic therapeutics and the integration of precision medicine approaches to optimize patient outcomes.
The Developmental History of Epigenetic Research in AD
Epigenetic modifications, particularly histone acetylation and DNA methylation, are fundamental mechanisms governing gene expression and cellular function. Histone acylation involves the transfer of acyl-CoA groups to specific amino acid residues on histone proteins, modulating chromatin accessibility. This process is tightly regulated by histone acetyltransferases (HATs), which promote transcriptional activation, and histone deacetylases (HDACs), which condense chromatin and repress gene expression Disruptions in this balance can have profound consequences on neuronal function and cognitive processes, particularly in the context of neurodegenerative disorders like AD [3-6].
The involvement of histone modifications in AD pathogenesis was first recognized through studies linking histone acetylation to memory formation [7-9]. Early investigations demonstrated that histone hypoacetylation correlated with cognitive deficits, suggesting that reduced acetylation impairs the expression of genes essential for synaptic plasticity and memory consolidation. Seminal work using AD mouse models confirmed that global reductions in histone acetylation were associated with learning and memory impairments, reinforcing the idea that chromatin modifications directly influence cognitive function. These findings provided a mechanistic link between epigenetic dysregulation and AD pathology, fueling interest in histone-modifying enzymes as therapeutic targets [10].
A breakthrough in this field came with the discovery that pharmacological inhibition of HDACs could counteract cognitive decline in AD models [11,12]. Studies showed that broad-spectrum HDAC inhibitors (HDACis), such as suberoylanilide hydroxamic acid (SAHA) and sodium butyrate, significantly improved memory performance in AD mice by restoring the expression of genes involved in synaptic plasticity. However, the non-selective inhibition of HDACs raised concerns about potential side effects, as HDACs are involved in numerous physiological processes beyond the central nervous system. This prompted researchers to identify specific HDAC isoforms most relevant to AD pathology, paving the way for more targeted therapeutic approaches.
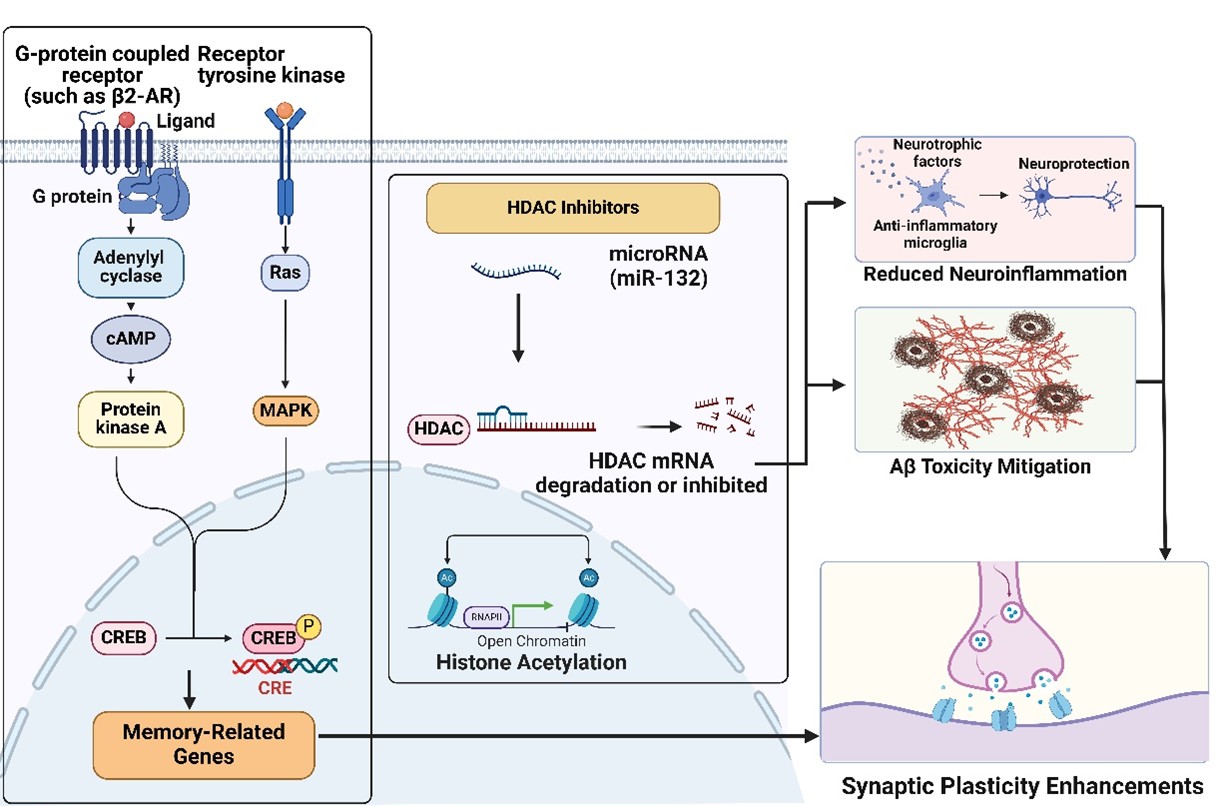
Our work contributed to this effort by focusing on the selective inhibition of HDAC3, a key regulator of synaptic plasticity. We demonstrated that an enriched environment (EE) upregulates miRNA-132, which in turn targets HDAC3 to preserve synaptic function in the presence of Aβ oligomers [1]. This finding highlighted the interplay between environmental factors, epigenetic regulation, and neuroprotection. Expanding on this, our later studies explored the role of β2-adrenergic receptor (β2-AR) activation, revealing that it induces protective epigenetic modifications by reducing HDAC2 levels in hippocampal neurons, thereby mitigating Aβ-induced synaptic dysfunction [2] (Figure 1).
The evolution of epigenetic research in AD has, therefore, progressed from broad investigations into histone modifications to a more refined understanding of how specific HDACs contribute to disease pathology. This shift has led to a growing emphasis on the development of selective HDAC inhibitors and epigenetic-based pharmacological interventions that strike a balance between therapeutic efficacy and safety. As research advances, integrating these findings with precision medicine strategies holds promise for developing personalized epigenetic therapies tailored to individual patient profiles.
Current Key Challenges in Epigenetic AD Research
Aberrant HDAC activity, particularly HDAC2 and HDAC3, contributes to cognitive deficits and synaptic dysfunction in AD [1,2,13,14]. While HDAC inhibitors (HDACis) show promise, challenges remain in developing safe, effective treatments. Key obstacles include HDAC isoform selectivity, differentiating HDAC roles, clinical translation, and environmental influences on epigenetics.
Lack of HDAC selectivity
Current HDACis, such as Vorinostat and Trichostatin A, lack isoform specificity, inhibiting multiple HDACs in diverse cellular functions, including cell cycle regulation, immune responses, and metabolism. This broad inhibition increases the risk of side effects, including hematological toxicity, fatigue, and gastrointestinal issues [15]. To mitigate these risks, efforts are underway to develop isoform-specific inhibitors that selectively target pathological pathways while sparing normal cellular functions. For example, selective HDAC2 inhibitors aim to restore synaptic dysfunction and memory function while avoiding disruptions to other HDAC-dependent processes [2,4,16,17]. Advances in structure-guided drug design and allosteric modulation are further refining next-generation HDACis with improved safety and efficacy.
Differentiating HDAC roles in AD
HDAC2 and HDAC3 play distinct yet overlapping roles in AD pathology. HDAC2 suppresses genes essential for synaptic plasticity, and its overexpression correlates with cognitive decline. Inhibition of HDAC2 enhances synaptic gene expression, alleviates synaptotoxicity, and improves memory function [2]. HDAC3, in contrast, is involved in stress responses and inflammation. Studies show that EE reduces HDAC3 activity, promoting synaptic resilience and counteracting Aβ-induced long-term potentiation (LTP) deficits [1]. The interplay between HDAC2 and HDAC3 suggests both independent and potentially synergistic contributions to AD pathology, underscoring the need for targeted interventions that optimize cognitive benefits while minimizing off-target effects.
Translation epigenetic research to clinic
Despite promising preclinical findings, translating HDACis to clinical applications remains a challenge. Vorinostat, an FDA-approved HDAC inhibitor for cancer treatment, has shown memory-enhancing effects in rodent but exhibits non-selective inhibition, leading to severe side effects such as bone marrow suppression and gastrointestinal toxicity [18-20]. Additionally, hydroxamate-based HDACis raise concerns about potential mutagenicity, highlighting the need for safer alternatives [21]. Another hurdle is the species-specific differences in drug metabolism, target accessibility, and disease progression. While β2-adrenergic receptor (β2-AR) activation or HDAC inhibition enhance neuroprotection in mice [2,22], these findings may not directly translate to humans. Overcoming these challenges requires improved AD models that better mimic human pathology, as along with rigorous testing of isoform-specific inhibitors across diverse populations.
Environmental modulation of epigenetics
Environmental factors such as cognitive stimulation, social interaction, and physical activity influence epigenetic mechanisms, potentially enhancing cognitive resilience against AD [23]. EE, for instance, has been shown to modulate HDAC3 and miRNA-132 promoting neuroprotection [1]. Other studies have linked EE to epigenetic modifications, including DNA methylation, histone acetylation, and non-coding RNA expression [24,25], suggesting broad effects on neuroplasticity. However, individual variability in responses to EE-shaped by genetic, environmental, and lifestyle factors-highlights the need for further studies to determine how these mechanisms can be harnessed effectively across different demographics and disease models.
Epigenetic research has provided critical insights into AD pathology and potential therapies, yet major challenges remain. Addressing issues of HDAC selectivity, clarifying isoform-specific roles, bridging preclinical and clinical findings, and leveraging environmental influences are crucial next steps. Overcoming these hurdles will require interdisciplinary collaboration, innovative drug development, and a commitment to translating basic science into effective AD treatments.
Forecasting Future Trends in Epigenetic AD Research
AD is marked by cognitive decline and synaptic dysfunction, partly driven by epigenetic changes, including histone acetylation dysregulation. HDAC inhibitors, such as sodium butyrate, Vorinostat, Trichostatin A, and valproate, show promise in enhancing memory, improving cognition, and reducing Aβ levels in preclinical studies and early trials [26]. These inhibitors work by promoting histone acetylation, which facilitates gene transcription critical for synaptic function and plasticity. However, clinical translation remains challenging due to off-target effects and the complexity of histone regulation. Emerging evidence suggests that addressing epigenetic dysregulation alone may not be sufficient; combining this approach with drugs that directly enhance synaptic function, such as β2-AR agonists, BDNF, and nootropics, could provide more comprehensive benefits [27,28]. For example, Nosustrophine, a porcine brain extract, modulates HDAC activity, DNA methylation, and Aβ levels, showcasing the potential of multi-target strategies [29]. Safe, specific, and effective therapies remain a key focus for advancing AD treatment. Based on recent findings and emerging insights, several future trends are anticipated in AD-related epigenetic research:
Development of selective HDAC inhibitors
As HDAC isoforms continue to be elucidated in terms of their distinct contributions to cognitive decline, developing selective HDAC inhibitors targeting HDAC2 or HDAC3 could be a pivotal step forward. Selective inhibitors would enable targeted intervention without the broad effects seen with non-specific HDACis, potentially improving therapeutic outcomes. As our findings highlight, HDAC3 and HDAC2 play distinct but significant roles in AD pathology [1,2]. Thus, isoform-specific HDAC inhibitors could serve as precision medicine tools, facilitating tailored treatments based on individual patient profiles.
Harnessing β2-adrenergic receptor activation as a neuroprotective strategy
We presented compelling evidence that β2-AR activation reduces HDAC2 levels, which enhances LTP and counters Aβ-induced synaptotoxicity [2]. With selective β2-AR agonists showing neuroprotective effects, this pathway presents an exciting avenue for intervention in early AD stages, possibly even as a preventive measure. β2-AR agonists could be combined with HDAC inhibitors to achieve synergistic effects, optimizing treatment outcomes by modulating both HDAC levels and cellular receptor responses.
Integration of environmental and pharmacological interventions
The benefits of environmental enrichment in modulating HDAC activity and cognitive resilience are well-documented, as shown by our studies [1]. Future approaches could integrate pharmacological treatments with environmental or lifestyle modifications, such as cognitive training and social engagement, to maximize neuroprotective effects [23]. Such a combined strategy could prove particularly effective in sporadic AD, where genetic predisposition plays a minor role compared to lifestyle and environmental factors.
Personalized epigenetic profiling for tailored therapeutics
With the rise of precision medicine, epigenetic profiling could become a standard tool for identifying individuals at risk for AD or in the early stages of cognitive decline. Personalized profiling could reveal specific epigenetic modifications that correspond to a patient’s unique risk factors or disease progression patterns, allowing for tailored treatments. For example, patients with elevated HDAC2 or HDAC3 levels could be candidates for selective HDAC inhibition or β2-AR agonist therapy, providing a customized and potentially more effective treatment approach.
Unique Insights and Innovative Perspectives
Our group’s studies [1,2] open doors to transformative approaches in AD treatment through their emphasis on the unique roles of HDAC isoforms and β2-AR activation. Building on these findings, several innovative perspectives emerge that could reshape the future of AD therapy:
Targeting HDAC isoforms in combination with receptor activation
The combination of selective HDAC inhibition and β2-AR activation could be explored as a comprehensive treatment strategy. This dual approach could simultaneously address multiple aspects of AD pathology, enhancing LTP, reducing inflammation, and improving overall synaptic plasticity. This strategy has the potential to optimize therapeutic effects by leveraging the complementary mechanisms of HDAC inhibition and receptor-mediated signaling.
Investigating non-neuronal cells in epigenetic regulation
Given AD’s multifactorial nature, it is critical to consider the role of non-neuronal cells, such as microglia and astrocytes, in AD pathology and epigenetic regulation. HDACs and β2-ARs are also expressed in these cells, which play critical roles in neuroinflammation, synaptic pruning, and overall brain health. Targeting epigenetic regulation in non-neuronal cells could provide a broader neuroprotective effect, addressing inflammation and synaptic dysfunction at a cellular network level rather than focusing solely on neurons.
Exploring β2-AR agonists beyond synaptic protection
β2-AR agonists are traditionally used in bronchodilation, but their application in AD could extend beyond synaptic protection. β2-ARs also influence immune responses, and activating these receptors may mitigate the neuroinflammatory cascade associated with AD. Since neuroinflammation is a core component of AD pathology, β2-AR agonists could serve as dual-function therapeutics, offering both synaptic and anti-inflammatory benefits. We demonstrated that β2-AR activation reduces HDAC2 and lowers inflammatory cytokine levels, suggesting a broader application of this receptor in AD therapy [2].
Conclusion
The exploration of epigenetic regulation in Alzheimer’s disease has provided critical insights into novel therapeutic avenues, offering the potential to modify disease progression rather than merely managing symptoms. Targeting specific HDAC isoforms and β2-adrenergic receptors has emerged as a promising strategy for preserving synaptic integrity and mitigating neurodegeneration while minimizing off-target effects. These findings pave the way for precision therapies that integrate pharmacological interventions with personalized epigenetic profiling, ultimately allowing for more effective, individualized treatment approaches.
Looking ahead, future research should prioritize the development of highly selective HDAC inhibitors, the optimization of combination therapies that incorporate lifestyle and environmental modifications, and the translation of preclinical discoveries into clinical trials. Additionally, advancing biomarker-driven strategies to assess epigenetic modifications in patients could enhance early diagnosis and therapeutic monitoring. By bridging the gap between molecular research and clinical application, this evolving field offers hope for transforming Alzheimer’s disease from an inevitable degenerative condition into a manageable, and potentially preventable, disorder.
References
2. Jin M, Wei Z, Ramalingam N, Xiao M, Xu A, Yu X, et al. Activation of β2-adrenergic receptors prevents AD-type synaptotoxicity via epigenetic mechanisms. Mol Psychiatry. 2023 Nov;28(11):4877-88.
3. Marzi SJ, Leung SK, Ribarska T, Hannon E, Smith AR, Pishva E, et al. A histone acetylome-wide association study of Alzheimer's disease identifies disease-associated H3K27ac differences in the entorhinal cortex. Nat Neurosci. 2018 Nov;21(11):1618-27.
4. McClarty BM, Rodriguez G, Dong H. Class 1 histone deacetylases differentially modulate memory and synaptic genes in a spatial and temporal manner in aged and APP/PS1 mice. Brain Res. 2024 Aug 15;1837:148951.
5. Nativio R, Lan Y, Donahue G, Sidoli S, Berson A, Srinivasan AR, et al. An integrated multi-omics approach identifies epigenetic alterations associated with Alzheimer's disease. Nat Genet. 2020 Oct;52(10):1024-35.
6. Schueller E, Paiva I, Blanc F, Wang XL, Cassel JC, Boutillier AL, et al. Dysregulation of histone acetylation pathways in hippocampus and frontal cortex of Alzheimer's disease patients. Eur Neuropsychopharmacol. 2020 Apr;33:101-16.
7. de la Fuente V, Federman N, Zalcman G, Salles A, Freudenthal R, Romano A. NF-κB transcription factor role in consolidation and reconsolidation of persistent memories. Front Mol Neurosci. 2015 Sep 9;8:50.
8. Federman N, de la Fuente V, Zalcman G, Corbi N, Onori A, Passananti C, et al. Nuclear factor κB-dependent histone acetylation is specifically involved in persistent forms of memory. J Neurosci. 2013 Apr 24;33(17):7603-14.
9. Lubin FD, Roth TL, Sweatt JD. Epigenetic regulation of BDNF gene transcription in the consolidation of fear memory. J Neurosci. 2008 Oct 15;28(42):10576-86.
10. Shukla S, Tekwani BL. Histone Deacetylases Inhibitors in Neurodegenerative Diseases, Neuroprotection and Neuronal Differentiation. Front Pharmacol. 2020 Apr 24;11:537.
11. Davis N, Taylor B, Abelleira-Hervas L, Karimian-Marnani N, Aleksynas R, Syed N, et al. Histone deacetylase-3 regulates the expression of the amyloid precursor protein and its inhibition promotes neuroregenerative pathways in Alzheimer's disease models. FASEB J. 2024 May 31;38(10):e23659.
12. Onishi T, Maeda R, Terada M, Sato S, Fujii T, Ito M, et al. A novel orally active HDAC6 inhibitor T-518 shows a therapeutic potential for Alzheimer's disease and tauopathy in mice. Sci Rep. 2021 Jul 29;11(1):15423.
13. Burns AM, Farinelli-Scharly M, Hugues-Ascery S, Sanchez-Mut JV, Santoni G, Gräff J. The HDAC inhibitor CI-994 acts as a molecular memory aid by facilitating synaptic and intracellular communication after learning. Proc Natl Acad Sci U S A. 2022 May 31;119(22):e2116797119.
14. Campbell RR, Kramár EA, Pham L, Beardwood JH, Augustynski AS, López AJ, et al. HDAC3 Activity within the Nucleus Accumbens Regulates Cocaine-Induced Plasticity and Behavior in a Cell-Type-Specific Manner. J Neurosci. 2021 Mar 31;41(13):2814-27.
15. Li W, Fu Y, Wang W. A real-world pharmacovigilance study investigating the toxicities of histone deacetylase inhibitors. Ann Hematol. 2024 Aug;103(8):3207-17.
16. Nakatsuka D, Izumi T, Tsukamoto T, Oyama M, Nishitomi K, Deguchi Y, et al. Histone Deacetylase 2 Knockdown Ameliorates Morphological Abnormalities of Dendritic Branches and Spines to Improve Synaptic Plasticity in an APP/PS1 Transgenic Mouse Model. Front Mol Neurosci. 2021 Nov 24;14:782375.
17. Yamakawa H, Cheng J, Penney J, Gao F, Rueda R, Wang J, et al. The Transcription Factor Sp3 Cooperates with HDAC2 to Regulate Synaptic Function and Plasticity in Neurons. Cell Rep. 2017 Aug 8;20(6):1319-34.
18. Guan JS, Haggarty SJ, Giacometti E, Dannenberg JH, Joseph N, Gao J, et al. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature. 2009 May 7;459(7243):55-60.
19. Gryder BE, Sodji QH, Oyelere AK. Targeted cancer therapy: giving histone deacetylase inhibitors all they need to succeed. Future Med Chem. 2012 Mar;4(4):505-24.
20. Ho TCS, Chan AHY, Ganesan A. Thirty Years of HDAC Inhibitors: 2020 Insight and Hindsight. J Med Chem. 2020 Nov 12;63(21):12460-84.
21. Wang CY, Lee LH. Mutagenicity and antibacterial activity of hydroxamic acids. Antimicrob Agents Chemother. 1977 Apr;11(4):753-5.
22. Li S. The β-adrenergic hypothesis of synaptic and microglial impairment in Alzheimer's disease. J Neurochem. 2023 May;165(3):289-302.
23. Li S, Jin M, Zhang D, Yang T, Koeglsperger T, Fu H, et al. Environmental novelty activates β2-adrenergic signaling to prevent the impairment of hippocampal LTP by Aβ oligomers. Neuron. 2013 Mar 6;77(5):929-41.
24. Córneo E, Michels M, Abatti M, Vieira A, Gonçalves RC, Gabriel FF, et al. Enriched environment causes epigenetic changes in hippocampus and improves long-term cognitive function in sepsis. Sci Rep. 2022 Jul 7;12(1):11529.
25. Yu Z, Wang J, Zhang P, Wang J, Cui J, Wang H. Enriched environment improves sevoflurane-induced cognitive impairment during late-pregnancy via hippocampal histone acetylation. Braz J Med Biol Res. 2020;53(10):e9861.
26. Rodrigues DA, Pinheiro PSM, Sagrillo FS, Bolognesi ML, Fraga CAM. Histone deacetylases as targets for the treatment of neurodegenerative disorders: Challenges and future opportunities. Med Res Rev. 2020 Nov;40(6):2177-211.
27. Azman KF, Zakaria R. Recent Advances on the Role of Brain-Derived Neurotrophic Factor (BDNF) in Neurodegenerative Diseases. Int J Mol Sci. 2022 Jun 19;23(12):6827.
28. Malík M, Tlustoš P. Nootropics as Cognitive Enhancers: Types, Dosage and Side Effects of Smart Drugs. Nutrients. 2022 Aug 17;14(16):3367.
29. Martínez-Iglesias O, Naidoo V, Carrera I, Corzo L, Cacabelos R. Nosustrophine: An Epinutraceutical Bioproduct with Effects on DNA Methylation, Histone Acetylation and Sirtuin Expression in Alzheimer's Disease. Pharmaceutics. 2022 Nov 12;14(11):2447.