Abstract
Environmental enrichment produces beneficial effects in the brain at genetic, molecular, cellular and behavior levels, and has long been studied as a therapeutic intervention for a wide variety of neurological disorders. However, the complexity of applying a robust environmental enrichment paradigm makes clinical use difficult. Accordingly, there has been increased interest in developing environmental enrichment mimetics, also known as enviromimetics. Here we review the benefits of environmental enrichment for migraine treatment, and discuss the potential of using extracellular vesicles derived from interferon gamma-stimulated dendritic cells as an effective mimetic.
Keywords
Environmental enrichment, Enviromimetics, Migraine, Spreading depression, Extracellular vesicles, Exosomes, Oxidative stress, Interferon gamma
A Brief Overview of Migraine and Environmental Enrichment
Migraine is a common neurological disorder characterized by episodic intense and painful headaches that last between 4 and 72 hours. Typically, the headache is unilateral and pulsating, aggravated by physical activity, and often accompanied with nausea, dizziness, photophobia, hyperosmia, or phonophobia [1]. Migraine can be broadly grouped into two major subtypes, migraine without aura and migraine with aura. In migraine with aura, headaches are preceded by focal neurological symptoms that include transient visual, sensory, language or motor symptoms. Visual auras are by far the most common, and typically include scintillating scotomas – an arc of diminished vision edged by shimmering lights that travels slowly across the visual field [2]. Sensory auras are often experienced as numbness or tingling in one hand or on one side of the face. In its episodic form, migraines occur 0 to 14 days per month, whereas in patients with chronic migraine, headaches are experienced 15 or more days per month for three or more months [3].
Migraine and migraine modeled using spreading depression (SD) or systemic nitroglycerin injections are all associated with increased oxidative stress (OS). Agents that reduce OS show protective effects against migraine and migraine modeled in animals. The impact of OS in migraine models extends to nociceptive signaling within the trigeminal system, which is important to pain pathway activation in migraine [4-6]. OS in the trigeminal ganglion can increase expression of calcitonin gene-related peptide (CGRP) [7], a neuropeptide involved in development of migraine pain [8]. OS can also induce CGRP release from dorsal root ganglion neurons [6,9]. Newly developed anti-CGRP agents for migraine include small molecule CGRP receptor antagonists and CGRP monoclonal antibodies which inhibit or absorb circulating CGRP to block nociceptive activation of the trigeminal system [8]. Unfortunately, these existing therapeutic options to prevent migraine or mitigate the transformation of episodic to high-frequency and chronic migraine are only modestly effective [10]. However, environmental enrichment (EE) can reduce migraine frequency [11,12], decrease susceptibility to migraine modeled in rat [13], and alleviate chronic neuropathic pain concomitant with reduced spinal cord levels of CGRP [14].
EE consists of volitionally increased intellectual (i.e, learning and memory), physical and social activity, and has wide ranging physiological and behavioral effects, including enhancing cognition, memory, learning, behavior and motor coordination [15,16]. Preclinical studies have demonstrated the efficacy of EE as an intervention in an impressive number of neurological conditions, including but not limited to: Huntington’s disease [17], Parkinson’s disease [18], Alzheimer’s disease [19,20], traumatic brain injury [21], multiple sclerosis [22] and migraine [11,13]. EE has well-documented effects on immune function as well. In the first study of EE’s effects on immunity, Kingston and Hoffman-Goetz [23] postulated that EE exposure modulates immune reactivity, allowing for better regulation (a quicker response to stimuli, a more vigorous or efficient response, and a faster recovery to prestimulus levels). Much of this work has been conducted in the context of immunosenescence, as age is a critical factor in many neurological diseases (including chronic migraine). EE reverses the microglial dysfunction commonly seen in aging brains [24-27] and dampens pro-inflammatory responses from microglia and astrocytes [28].
While clinical studies of EE are more difficult to conduct, there is significant evidence that EE can produce beneficial effects in human patients as well. EE has been employed as a rehabilitation strategy for stroke survivors [29-30] and to reduce post-operative pain [31]. It is important to note that while prior studies have shown that the effects of voluntary exercise and those from exposure to an enriched environment are separable [32], they provide the greatest benefit when used simultaneously [33]. However, many clinical studies have focused on physical exercise or cognitive stimulation in isolation. Increased voluntary physical exercise has been linked to improved outcome for neurological diseases including depression [34], schizophrenia [35], epilepsy [36] and migraine [37]. Likewise, there have been numerous studies examining the role of intellectual enrichment in creating a ‘cognitive reserve’ that lessens the impact of brain disease on cognitive impairment [38,39]. Reports also show how social engagement and an active lifestyle can protect against dementia [40]. Notably, the Covid-19 pandemic has piqued increased interest in virtual forms of EE to improve cognitive health [41-43]. Taken together, these works provide evidence that EE has a beneficial impact on human health.
Though enrichment paradigms vary, they generally include the following aspects: 1) social enrichment, in which subjects are given increased exposure to conspecifics, 2) cognitive enrichment, which includes exposure to novel stimuli and experiential learning and 3) physical enrichment, consisting of voluntary exercise. An appropriate EE paradigm provides the opportunity to choose to engage in a variety of naturally rewarding activities in a non-stressful setting. Unfortunately, these criteria also present a major limitation. Not only does the complexity of applying a robust EE paradigm makes clinical use difficult, but practical implementation would require patients to have the physical and mental capacity to actively participate, which is not always possible. development of EE mimetics or “enviromimetics” [44,45]. As a result, there has been increased attention to the Conceivably, this approach would provide patients a means to exogenously access the benefits of EE until they are able to engage in an effective EE regimen on their own. Our lab has focused on studying the various mechanisms of EE-based mitigation of migraine (as modeled in rats) in an effort to identify potential mimetics.
Spreading Depression as a Model of Migraine
SD is the most likely cause of migraine auras, and a likely cause of migraine pain through activation of the trigeminal pain pathway [4,46-48]. SD consists of increased synaptic activity followed by a period of electrical silence which slowly propagates through susceptible brain regions [47,49,50]. Following an episode of SD, neuronal excitability is temporarily elevated [51,52]. This increased excitability is accompanied by related increases in production of reactive oxygen species (ROS) [53,54].
SD also activates microglia and stimulates their release of cytokines, including tumor necrosis factor alpha (TNFα) and interferon gamma (IFNγ), suggesting a shift towards a M1-like pro-inflammatory state [55-59]. TNFα enhances synaptic efficacy by increasing membrane expression of excitatory α-amino-3-hydroxy-5-methyl- 4-isoxazolepropionic acid receptors and decreasing membrane expression of inhibitory γ-aminobutyric acid-A receptors [60].
Another mechanism of increased susceptibility to recurrent SD is myelin damage/decompaction. Production of SD increases CNS production of pro-inflammatory cytokines (including IFNγ) and ROS, which converge upon activation of neutral sphingomyelinase 2 (nSMase2)-ceramide pathways. Myelin contains neutral sphingomyelinases [61] whose activity leads to sphingomyelin hydrolysis, ceramide formation and other downstream reactions with deleterious effects on myelin integrity. The net effect of this is a transient demyelination with SD that recovers over the course of a week [62]. Myelin disruption within grey matter may promote SD susceptibility by increasing hyperexcitability via ephaptic transmission (electrical crosstalk). This suggests a pathophysiological link behind the existing clinical evidence correlating disruption of myelin in patients suffering from migraine and multiple sclerosis, as multiple sclerosis also involves damage resulting from production of IFNγ by T cells, increased OS, glutathione depletion, and activation of nSMase2 [63-65].
Thus, SD creates a destructive feed-back cycle in which aberrant neuroexcitability, increased generation of ROS, production of pro-inflammatory cytokines and myelin damage caused by one episode of SD increases susceptibility to subsequent SD [53,54]. Frequent occurrences of SD without sufficient time for recovery may be responsible for the transition from episodic migraine to high-frequency and chronic migraine [66].
In contrast, physiological levels of ROS generated by intermittent increases in neuronal activity (e.g., from engaging in EE) leads to enhancement of antioxidant defenses [67]. This pattern of response is consistent with the hormetic model. The term “hormesis” describes a biphasic dose-response wherein exposure to a low dose of an irritant that would have negative consequences at a higher dose instead induces an adaptive response that is beneficial [68]. It is a well-conserved response pattern that has been observed at the cellular, tissue and organismal level [69], and appears to apply to mechanisms of EEmediated neuroprotection.
Mechanisms Involved in EE-based Mitigation of SD
Insulin-like growth factor-1 (IGF-1) is a hormone that can modulate neuronal plasticity, survival and proliferation [70]. It is a major mediator of the neuroprotective effects of exercise [71], and its expression levels increase with EE [72]. While most circulating IGF-1 is produced by the liver, EE has been shown to increase brain uptake of IGF- 1 from the periphery [73]. IGF-1 is protective against SD in vitro in hippocampal slice cultures [53,54]. This effect involves decreasing post-SD levels of TNFα and decreased production of ROS from microglia; two factors that can contribute to the neuronal hyperexcitability that promotes recurrent SD [53,54]. Furthermore, nasally administered IGF-1 reduces SD susceptibility and OS-induced trigeminal nociceptive activation (i.e., reduces CGRP expression) in two animal models of migraine, SD and systemic injection of nitroglycerin [4,5,74].
Interleukin-11 (IL-11) is an anti-inflammatory cytokine with immunomodulatory and neuroprotective properties [75-78]. Rats exposed to EE have increased levels of neuronal IL-11, perhaps reflective of phasically increased neuronal activity from learning [15]. IL-11 significantly reduces TNFα expression, including that produced following SD. When nasally administered to rats, IL- 11 reduced susceptibility to SD, significantly reduced protein carbonylation (reflective of oxidative damage) and promoted an M2a-skewed microglial phenotype [13]. The M2a phenotype is associated with suppression of inflammation, and has increased surface expression of Arginase-1. This is of note here, as Arg-1 mediates the conversion of arginine to polyamines, proline and orthinines, and outcompetes inducible nitric oxide synthase (iNOS) for access to arginine (also the substrate for nitric oxide production [79]) to reduce production of ROS.
IFNγ is a highly pleotropic cytokine with diverse functions in both innate and adaptive immunity and host defense. While commonly considered a pro-inflammatory cytokine, it is also involved in regulation of antiinflammatory responses, and may be better described as an immunoregulatory effector molecule [80]. IFNγ activates intracellular molecular signaling networks that modulate the transcription of hundreds of genes and mediate numerous biological responses [81]. It is likely involved in the low level inflammatory signaling of EE that acts as a mild stressor to promote adaptive responses [82]. In support of this, studies have shown that regular moderate exercise increases IFNγ levels in human plasma [83], and that IFNγ is involved in spatial learning [84]. Though its role in multiple sclerosis and other degenerative disorders is contested and largely thought to be detrimental, studies have shown that this is disease stage and context dependent [85]. Pre-exposure of healthy brain to IFNγ reduces subsequent demyelination in animal models of multiple sclerosis [86-90]. Likewise, IFNγ plays dual roles in modulation of SD susceptibility. Though elevated IFNγ and associated increases in OS are detrimental in SD, in accordance with physiological conditioning hormesis, phasic exposure to IFNγ instead produces an adaptive response that is protective against SD and prevents the associated myelin damage. Treatment with a single 12 hour pulse of IFNγ (or phasic IFNγ treatment for a week) emulates the phasic changes of EE and likewise increases myelination, reduces susceptibility to SD and reduces OS [91]. This effect is likely mediated by release of extracellular vesicles from IFNγ-stimulated microglia, and is in line with work showing that activation of microglia with low concentrations of IFNγ induces a protective antiinflammatory (M2a-like) phenotype [92].
IFNγ Stimulated Dendritic Cell Extracellular Vesicles as an EE-mimetic
Extracellular vesicles (EVs) are lipid membrane vesicles that are secreted by many cell types and are involved in a multitude of functions, both physiological and pathological [93]. EVs have the potential for targeting specific cell types to deliver their cargo of protein, mRNA and miRNA. This cargo is protected from degradation by proteases and RNases while the vesicle is in the interstitial space, and retains bioactivity once taken up by a recipient cell. In this way, EVs facilitate interactive signaling between cells. EVs are non-toxic and do not provoke adverse immune reactions, and can be easily nasally delivered to the brain - traits that support the potential utility of EV-based therapies. The specific composition of their cargo is influenced by their parent cell type and disease and/or activation state. EVs can be isolated from biofluids or conditioned medium by one of a variety of methods that yield an EV enriched preparation that likely represents a heterogeneous mixture of exosomes (30-150 nm diameter), microvesicles (200-1000 nm diameter), apoptotic bodies and protein complexes [94]. EVs isolated from the serum of rats exposed to EE significantly increase myelin content and oligodendrocyte precursor cell levels, and reduce OS in hippocampal slice cultures and when nasally administered to naive rats [95]. Importantly, exposure of an aged animal to EE restores their ability to produce myelination-promoting EVs, suggesting that EEinduced reversal of age-related immune dysfunction may be involved.
To mimic EE, we stimulated primary dendritic cell cultures with low-level IFNγ. EVs produced in this way (IFNγ-DC-EVs) increase myelination and oxidative tolerance in vitro and in vivo [96]. Treatment with IFNγ- DC-EVs significantly reduces SD susceptibility in vitro and in vivo, reduces microglial M1 product iNOS, and reduces OS-mediated damage [97]. IFNγ-DC-EVs appear to impact all pathways identified above that contribute to EEmediated modulation of SD susceptibility. 1) IFNγ-DC-EVs reduce OS and increase glutathione. This is perhaps due to their containing miRNA species involved in resolution of inflammation and reduction of OS. For example, IFNγ- DC-EVs contain miR-532-5p which reduces microglial expression of pro-inflammatory cytokines in response to lipopolysaccharide [98] and suppresses NADPH oxidase 2 expression to reduce ROS production [99]. They also contain miR-181a, which dampens pro-inflammatory signaling and reduces production of ROS in macrophages/ monocytes [100] and similarly regulates inflammation in the CNS [101]. 2) IFNγ-DC-EVs reduce microglial markers of M1 polarization. Another enriched miRNA, miR-124, increases anti-inflammatory signaling and downregulates M1-associated IL-6, TNFα and iNOS [102,103]. This is in line with our finding that microglial polarization state impacts SD with M1-like pro-inflammatory microglia increasing susceptibility. It is likely that IFNγ-DCEVs produce a similar effect to EE-induced changes in microglial polarization via neuronal-activity-induced increased production of IL-11. 3) IFNγ-DC-EVs promote myelination. Like phasic treatment with low-level IFNγ, IFNγ-DC-EVs promote myelin production, likely through delivery of miR-219 which is necessary for myelination. Taken together, this suggests IFNγ-DC-EVs may be a potent EE-mimetic for use in migraine treatment. It is important to note that EVs are non-toxic and can easily cross the blood brain barrier without use of an additive vehicle.
Although most studies of the benefits of EE on the central nervous system have been conducted in rodents, there is ample evidence that EE is effective in a wide variety of other species including but not limited to fish [104,105], birds [106,107], rabbits [108,109], ferrets [110], pigs [111], dogs [112], octopi [113], and marmosets [114]. As detailed above, there is evidence that EE is likely beneficial for CNS health in humans as well. Additionally, miRNAs show high levels of conservation even between distantly related species [115]. Thus, it is likely that IFNγ-DCExos will function as an EE-mimetic in humans as well. However, while the biological function of EVs can be well-conserved between species [116], xenogenic EVs may initiate the synthesis of foreign proteins in recipient cells [117] and potentially trigger adverse immune reactions. Accordingly, proof of efficacy of human EVs (hIFNγ-DCEVs) is important to development of IFNγ-DC-EVs for use as an enviromimetic.
Exploring the Potential of Human-derived IFNγ-DC-EVs
Fresh human bone marrow aspirates that have been verified to be pathogen-free were obtained from Lonza (Walkersville, MD) for differentiation into dendritic cells. Monocytes were isolated via density centrifugation with Lymphocyte Separation Medium (Lonza), and CD34+ cells were purified by magnetic cell isolation (Miltenyi Biotec). Cells were maintained in RPMI+10% fetal bovine serum (FBS) supplemented with a standard complement of cytokines for 10 days of culture. At day 10, cells were transferred to RPMI+10% exosome-depleted FBS (System Biosciences) containing the same cytokines, with or without IFNγ. At day 13, conditioned medium was harvested from unstimulated or IFNγ-treated dendritic cells for EV isolation.
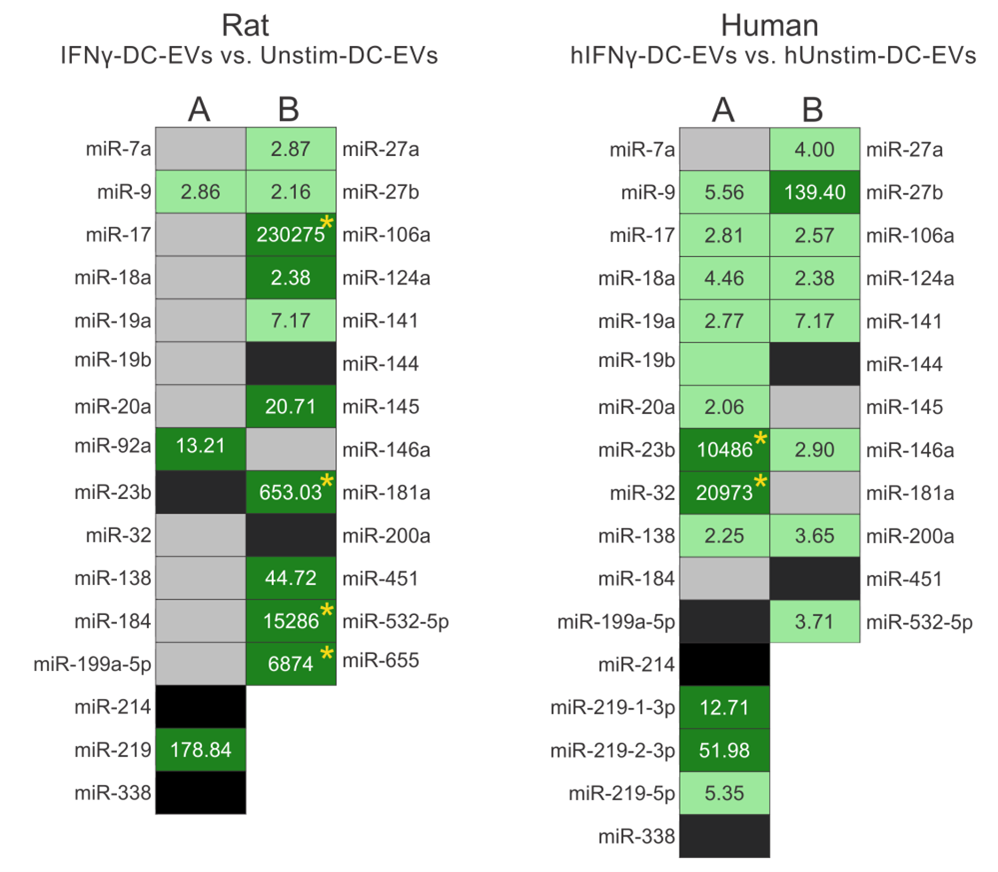
As a first measure of the feasibility of producing human EE-mimetic EVs, their miRNA contents were profiled. Here we show that hIFNγ-DC-EVs contain a similar cohort of miRNA species as rat IFNγ-DC-EVs (Figure 1). Importantly, miRNA species found to play functional roles in rat IFNγ-DC-EVs were similarly upregulated in hIFNγ- DC-EVs. While future studies to explore their functional effects are necessary, this begins to suggest that humanderived dendritic cells respond to IFNγ in a similar way as rat dendritic cells and may indeed produce similarly beneficial EVs.
Results show expression levels of specific miRNAs involved in (A) myelin production/oligodendrocyte differentiation, and (B) anti-inflammatory responses. (Left) miRNA content of rat IFNγ-DC-EVs were compared to that of exosomes from unstimulated rat dendritic cell EVs (Unstim-DC-EVs). (Right) miRNA content of interferon gamma-stimulated human EVs (hIFNγ-DC-EVs) were compared to that of exosomes from unstimulated human dendritic cell EVs (hUnstim-DC-EVs). Black panels indicate mature miRNA species that could not be detected; grey panels indicate miRNAs that were readily detectible but not significantly enriched; light green indicate significantly enriched (i.e., >2 fold) miRNAs; and dark green indicates very highly enriched (i.e., >10 fold) miRNAs. Rat data was adapted from a previous publication [13].
In conclusion, there is a need to harness the beneficial effects of EE for treatment of migraine and we believe hIFNγ-DC-EVs have great potential for development as an effective mimetic that can be easily nasally administered to the brain.
Conflict of Interest
A.D.P., K.M.P. and R.P.K. are co-inventors on multinational patent applications (issued and pending) by The University of Chicago related to this work all dealing with the use of exosomes to reduce oxidative stress in the central nervous system and promote remyelination of damaged neurons. L.W. reports no conflicts.
Author Contributions
A.D.P. and K.M.P. wrote the manuscript. A.D.P., K.M.P., L.W. and R.P.K. performed experiments and analysed data. A.D.P., K.M.P, L.W. and R.P.K read and commented on the final version of the manuscript and all approve of its submission.
Funding
This work was supported by grants from the National Institute of Neurological Disorders and Stroke (NS-019108), National Institute of Health Common Fund, through the Office of Strategic Coordination/Office of the Director; Grant number: 5UH3 TR000918UH-04/05, 3UH3 TR000918-03S1, 3UH3 TR000918-04S1, the Innovation Fund from the Polsky Center for Entrepreneurship and Innovation at the University of Chicago and by the National Center for Advancing Translational Sciences of the National Institutes of Health through Grant Number 5UL1 TR002389-02. In addition, A.D.P. was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number 5KL2 TR002387-02 and a 5UL1 TR002389-02 award.
References
2. Lashley KS. Patterns of cerebral integration indicated by the scotomas of migraine. Archives of Neurology & Psychiatry. 1941 Aug 1;46(2):331-9.
3. Katsarava Z, Buse DC, Manack AN, Lipton RB. Defining the differences between episodic migraine and chronic migraine. Current Pain and Headache Reports. 2012 Feb;16(1):86-92.
4. Won L, Kraig RP. Insulin-like growth factor-1 inhibits spreading depression-induced trigeminal calcitonin gene related peptide, oxidative stress & neuronal activation in rat. Brain Research. 2020 Apr 1; 1732:146673.
5. Won L, Kraig RP. Insulin-like growth factor-1 inhibits nitroglycerin-induced trigeminal activation of oxidative stress, calcitonin gene-related peptide and c-Fos expression. Neuroscience Letters. 2021 Apr 23; 751:135809.
6. Shatillo A, Koroleva K, Giniatullina R, Naumenko N, Slastnikova AA, Aliev RR, et al. Cortical spreading depression induces oxidative stress in the trigeminal nociceptive system. Neuroscience. 2013 Dec 3; 253:341-9.
7. Chen SP, Qin T, Seidel JL, Zheng Y, Eikermann M, Ferrari MD, et al. Inhibition of the P2X7–PANX1 complex suppresses spreading depolarization and neuroinflammation. Brain. 2017 Jun 1;140(6):1643-56.
8. Edvinsson L. CGRP antibodies as prophylaxis in migraine. Cell. 2018 Dec 13;175(7):1719.
9. Durham PL. Calcitonin gene-related peptide (CGRP) and migraine. Headache: The Journal of Head and Face Pain. 2006 Jun;46:S3-8.
10. Mack KJ. Can we help patients with chronic migraine?. Neurology. 2011 Feb 22;76(8):682-3.
11. Darabaneanu S, Overath CH, Rubin D, Lüthje S, Sye W, Niederberger U, et al. Aerobic exercise as a therapy option for migraine: a pilot study. International Journal of Sports Medicine. 2011 Jun;32(06):455-60.
12. Irby MB, Bond DS, Lipton RB, Nicklas B, Houle TT, Penzien DB. Aerobic exercise for reducing migraine burden: mechanisms, markers, and models of change processes. Headache: The Journal of Head and Face Pain. 2016 Feb;56(2):357-69.
13. Pusic KM, Pusic AD, Kemme J, Kraig RP. Spreading depression requires microglia and is decreased by their M2a polarization from environmental enrichment. Glia. 2014 Jul;62(7):1176-94.
14. Vachon-Presseau E, Roy M, Martel MO, Caron E, Marin MF, Chen J, et al. The stress model of chronic pain: evidence from basal cortisol and hippocampal structure and function in humans. Brain. 2013 Mar 1;136(3):815-27.
15. Van Praag H, Kempermann G, Gage FH. Neural consequences of enviromental enrichment. Nature Reviews Neuroscience. 2000 Dec;1(3):191-8.
16. Kempermann G. Environmental enrichment, new neurons and the neurobiology of individuality. Nature Reviews Neuroscience. 2019 Apr;20(4):235-45.
17. Van Dellen A, Blakemore C, Deacon R, York D, Hannan AJ. Delaying the onset of Huntington’s in mice. Nature. 2000 Apr;404(6779):721-2.
18. Jadavji NM, Kolb B, Metz GA. Enriched environment improves motor function in intact and unilateral dopaminedepleted rats. Neuroscience. 2006 Jan 1;140(4):1127-38.
19. Jankowsky JL, Melnikova T, Fadale DJ, Xu GM, Slunt HH, Gonzales V, et al. Environmental enrichment mitigates cognitive deficits in a mouse model of Alzheimer’s disease. Journal of Neuroscience. 2005 May 25;25(21):5217-24.
20. Lima MG, Schimidt HL, Garcia A, Daré LR, Carpes FP, Izquierdo I, et al. Environmental enrichment and exercise are better than social enrichment to reduce memory deficits in amyloid beta neurotoxicity. Proceedings of the National Academy of Sciences. 2018 Mar 6;115(10):E2403-9.
21. Passineau MJ, Green EJ, Dietrich WD. Therapeutic effects of environmental enrichment on cognitive function and tissue integrity following severe traumatic brain injury in rats. Experimental Neurology. 2001 Apr 1;168(2):373- 84.
22. Silva BA, Leal MC, Farías MI, Erhardt B, Galeano P, Pitossi FJ, et al. Environmental enrichment improves cognitive symptoms and pathological features in a focal model of cortical damage of multiple sclerosis. Brain Research. 2020 Jan 15;1727:146520.
23. Kingston SG, Hoffman-Goetz L. Effect of environmental enrichment and housing density on immune system reactivity to acute exercise stress. Physiology & Behavior. 1996 Jul 1;60(1):145-50.
24. Pedersen BK, Hoffman-Goetz L. Exercise and the immune system: regulation, integration, and adaptation. Physiological Reviews. 2000 Jul;80(3):1055-81.
25. Arranz L, De Castro NM, Baeza I, Maté I, Viveros MP, De la Fuente M. Environmental enrichment improves agerelated immune system impairment: long-term exposure since adulthood increases life span in mice. Rejuvenation Research. 2010 Aug 1;13(4):415-28.
26. Ali S, Liu X, Queen NJ, Patel RS, Wilkins RK, Mo X, et al. Long-term environmental enrichment affects microglial morphology in middle age mice. Aging (Albany NY). 2019 Apr 30;11(8):2388.
27. de Oliveira TC, Carvalho-Paulo D, de Lima CM, de Oliveira RB, Santos Filho C, Diniz DG, Bento Torres Neto J, Picanço-Diniz CW. Long-term environmental enrichment reduces microglia morphological diversity of the molecular layer of dentate gyrus. European Journal of Neuroscience. 2020 Nov;52(9):4081-99.
28. Williamson LL, Chao A, Bilbo SD. Environmental enrichment alters glial antigen expression and neuroimmune function in the adult rat hippocampus. Brain, Behavior, and Immunity. 2012 Mar 1;26(3):500-10.
29. Rosbergen IC, Grimley RS, Hayward KS, Walker KC, Rowley D, Campbell AM, et al. The effect of an enriched environment on activity levels in people with stroke in an acute stroke unit: protocol for a before-after pilot study. Pilot and Feasibility Studies. 2016 Dec;2(1):1-6.
30. Rosbergen IC, Grimley RS, Hayward KS, Walker KC, Rowley D, Campbell AM, et al. Embedding an enriched environment in an acute stroke unit increases activity in people with stroke: a controlled before–after pilot study. Clinical Rehabilitation. 2017 Nov;31(11):1516-28.
31. Yeung SC, Irwin MG, Cheung CW. Environmental enrichment in postoperative pain and surgical care: potential synergism with the enhanced recovery after surgery pathway. Annals of Surgery. 2021 Jan 1;273(1):86- 95.
32. Olson AK, Eadie BD, Ernst C, Christie BR. Environmental enrichment and voluntary exercise massively increase neurogenesis in the adult hippocampus via dissociable pathways. Hippocampus. 2006;16(3):250-60.
33. Fabel K, Wolf S, Ehninger D, Babu H, Galicia P, Kempermann G. Additive effects of physical exercise and environmental enrichment on adult hippocampal neurogenesis in mice. Frontiers in Neuroscience. 2009 Nov 10;3:2.
34. Ströhle A, Stoy M, Graetz B, Scheel M, Wittmann A, Gallinat J, et al. Acute exercise ameliorates reduced brainderived neurotrophic factor in patients with panic disorder. Psychoneuroendocrinology. 2010 Apr 1;35(3):364-8.
35. Beebe LH, Tian L, Morris N, Goodwin A, Allen SS, Kuldau J. Effects of exercise on mental and physical health parameters of persons with schizophrenia. Issues in Mental Health Nursing. 2005 Jan 1;26(6):661-76.
36. Arida RM, Scorza FA, Cavalheiro EA. Favorable effects of physical activity for recovery in temporal lobe epilepsy. Epilepsia. 2010 Jul;51:76-9.
37. Varkey E, Cider Å, Carlsson J, Linde M. Exercise as migraine prophylaxis: a randomized study using relaxation and topiramate as controls. Cephalalgia. 2011 Oct;31(14):1428-38.
38. Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. The Lancet Neurology. 2012 Nov 1;11(11):1006-12.
39. Crescentini C, Urgesi C, Fabbro F, Eleopra R. Cognitive and brain reserve for mind-body therapeutic approaches in multiple sclerosis: a review. Restorative Neurology and Neuroscience. 2014 Jan 1;32(5):575-95.
40. Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. The Lancet Neurology. 2004 Jun 1;3(6):343-53.
41. Hampel H, Vergallo A. The Sars-Cov-2 pandemic and the brave new digital World of Environmental Enrichment to prevent brain aging and cognitive decline. The Journal of Prevention of Alzheimer’s Disease. 2020;7(4):294-298.
42. Belchev Z, Boulos ME, Rybkina J, Johns K, Jeffay E, Colella B, et al. Remotely delivered environmental enrichment intervention for traumatic brain injury: Study protocol for a randomised controlled trial. BMJ Open. 2021 Feb 1;11(2):e039767.
43. Davim A, Vieira P. Environmental Enrichment as a Strategy to Confront Social Isolation Under the COVID-19 Pandemic. Frontiers in Behavioral Neuroscience. 2021 Jan 21;14:248.
44. McOmish CE, Hannan AJ. Enviromimetics: exploring gene environment interactions to identify therapeutic targets for brain disorders. Expert Opinion on Therapeutic Targets. 2007 Jul 1;11(7):899-913.
45. Guerrieri D, Moon HY, van Praag H. Exercise in a pill: the latest on exercise-mimetics. Brain Plasticity. 2017 Jan 1;2(2):153-69.
46. Milner PM. Note on a possible correspondence between the scotomas of migraine and spreading depression of Leao. Electroencephalography and Clinical Neurophysiology. 1958 Nov 1;10(4):705.
47. Moskowitz MA, Nozaki K, Kraig RP. Neocortical spreading depression provokes the expression of c-fos protein-like immunoreactivity within trigeminal nucleus caudalis via trigeminovascular mechanisms. Journal of Neuroscience. 1993 Mar 1;13(3):1167-77.
48. Kunkler PE, Kraig RP. Hippocampal spreading depression bilaterally activates the caudal trigeminal nucleus in rodents. Hippocampus. 2003;13(7):835-44.
49. Bures J, Bureš J, Buresova O, Bures?ová O, K?ivánek J, Krivanek J. The mechanism and applications of Leao’s spreading depression of electroencephalographic activity. Academic Press; 1974.
50. Somjen GG. Mechanisms of spreading depression and hypoxic spreading depression-like depolarization. Physiological Reviews. 2001 Jul 1;81(3):1065-96.
51. Krüger H, Luhmann HJ, Heinemann U. Repetitive spreading depression causes selective suppression of GABAergic function. Neuroreport. 1996 Nov 1;7(15- 17):2733-6.
52. Grinberg YY, Milton JG, Kraig RP. Spreading depression sends microglia on Lévy flights. PloS One. 2011 Apr 26;6(4):e19294.
53. Grinberg YY, van Drongelen W, Kraig RP. Insulin-like growth factor-1 lowers spreading depression susceptibility and reduces oxidative stress. Journal of Neurochemistry. 2012 Jul;122(1):221-9.
54. Grinberg YY, Dibbern ME, Levasseur VA, Kraig RP. Insulin-like growth factor-1 abrogates microglial oxidative stress and TNF-α responses to spreading depression. Journal of Neurochemistry. 2013 Sep;126(5):662-72.
55. Gehrmann, J., Mies, G., Bonnekoh, P., Banati, R., Iijima, T., Kreutzberg, G. W., & Hossmann, K. A. (1993). Microglial reaction in the rat cerebral cortex induced by cortical spreading depression. Brain Pathology (Zurich, Switzerland), 3(1), 11–7.
56. Caggiano AO, Kraig RP. Prostaglandin E2 and 4-aminopyridine prevent the lipopolysaccharide-induced outwardly rectifying potassium current and interleukin- 1β production in cultured rat microglia. Journal of Neurochemistry. 1998 Jun;70(6):2357-68.
57. Jander S, Schroeter M, Peters O, Witte OW, Stoll G. Cortical spreading depression induces proinflammatory cytokine gene expression in the rat brain. Journal of Cerebral Blood Flow & Metabolism. 2001 Mar;21(3):218- 25.
58. Kunkler PE, Hulse RE, Kraig RP. Multiplexed cytokine protein expression profiles from spreading depression in hippocampal organotypic cultures. Journal of Cerebral Blood Flow & Metabolism. 2004 Aug;24(8):829-39.
59. Hulse RE, Swenson WG, Kunkler PE, White DM, Kraig RP. Monomeric IgG is neuroprotective via enhancing microglial recycling endocytosis and TNF-α. Journal of Neuroscience. 2008 Nov 19;28(47):12199-211.
60. Stellwagen D, Beattie EC, Seo JY, Malenka RC. Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-α. Journal of Neuroscience. 2005 Mar 23;25(12):3219-28.
61. Chakraborty G, Ziemba S, Drivas A, Ledeen RW. Myelin contains neutral sphingomyelinase activity that is stimulated by tumor necrosis factor-α. Journal of Neuroscience Research. 1997 Nov 1;50(3):466-76.
62. Pusic AD, Mitchell HM, Kunkler PE, Klauer N, Kraig RP. Spreading depression transiently disrupts myelin via interferon-gamma signaling. Experimental Neurology. 2015 Feb 1;264:43-54.
63. Kister I, Caminero AB, Herbert J, Lipton RB. Tensiontype headache and migraine in multiple sclerosis. Current Pain and Headache Reports. 2010 Dec 1;14(6):441-8.
64. Pakpoor J, Handel AE, Giovannoni G, Dobson R, Ramagopalan SV. Meta-analysis of the relationship between multiple sclerosis and migraine. PloS One. 2012;7(9):e45295.
65. Bashir A, Lipton RB, Ashina S, Ashina M. Migraine and structural changes in the brain: a systematic review and meta-analysis. Neurology. 2013 Oct 1;81(14):1260-8.
66. Kraig RP, Mitchell HM, Christie-Pope B, Kunkler PE, White DM, Tang YP, Langan G. TNF-α and microglial hormetic involvement in neurological health & migraine. Dose-response. 2010 Oct 1;8(4):389-413.
67. Papadia S, Soriano FX, Léveillé F, Martel MA, Dakin KA, Hansen HH, et al. Synaptic NMDA receptor activity boosts intrinsic antioxidant defenses. Nature neuroscience. 2008 Apr;11(4):476-87.
68. Calabrese EJ, Bachmann KA, Bailer AJ, Bolger PM, Borak J, Cai L, et al. Biological stress response terminology: integrating the concepts of adaptive response and preconditioning stress within a hormetic dose–response framework. Toxicology and Applied Pharmacology. 2007 Jul 1;222(1):122-8.
69. Mattson MP. Hormesis defined. Ageing Research Reviews. 2008 Jan 1;7(1):1-7.
70. Torres-Aleman I. Toward a comprehensive neurobiology of IGF-I. Developmental Neurobiology. 2010 Apr;70(5):384-96.
71. Carro E, Trejo JL, Busiguina S, Torres-Aleman I. Circulating insulin-like growth factor I mediates the protective effects of physical exercise against brain insults of different etiology and anatomy. Journal of Neuroscience. 2001 Aug 1;21(15):5678-84.
72. Chang HC, Yang YR, Wang PS, Kuo CH, Wang RY. Insulin-like growth factor I signaling for brain recovery and exercise ability in brain ischemic rats. Medicine and Science in Sports and Exercise. 2011 Dec 1;43(12):2274- 80.
73. Nishijima T, Piriz J, Duflot S, Fernandez AM, Gaitan G, Gomez-Pinedo U, et al. Neuronal activity drives localized blood-brain-barrier transport of serum insulin-like growth factor-I into the CNS. Neuron. 2010 Sep 9;67(5):834-46.
74. Grinberg YY, Zitzow LA, Kraig RP. Intranasally administered IGF-1 inhibits spreading depression in vivo. Brain Research. 2017 Dec 15;1677:47-57.
75. Trepicchio WL, Bozza M, Pedneault G, Dorner AJ. Recombinant human IL-11 attenuates the inflammatory response through down-regulation of proinflammatory cytokine release and nitric oxide production. The Journal of Immunology. 1996 Oct 15;157(8):3627-34.
76. Trepicchio WL, Dorner AJ. Interleukin-11: A gp130 Cytokine. Annals of the New York Academy of Sciences. 1998 Sep;856(1):12-21.
77. Zhang Y, Taveggia C, Melendez-Vasquez C, Einheber S, Raine CS, Salzer JL, et al. Interleukin-11 potentiates oligodendrocyte survival and maturation, and myelin formation. Journal of Neuroscience. 2006 Nov 22;26(47):12174-85.
78. Maheshwari A, Janssens K, Bogie J, Van Den Haute C, Struys T, Lambrichts I, et al. Local overexpression of interleukin-11 in the central nervous system limits demyelination and enhances remyelination. Mediators of Inflammation. 2013 Jan 1;2013.
79. Corraliza IM, Soler G, Eichmann K, Modolell M. Arginase induction by suppressors of nitric oxide synthesis (IL-4, IL-10 and PGE2) in murine bone-marrow-derived macrophages. Biochemical and Biophysical Research Communications. 1995 Jan 17;206(2):667-73.
80. Mühl H, Pfeilschifter J. Anti-inflammatory properties of pro-inflammatory interferon-γ. International Immunopharmacology. 2003 Sep 1;3(9):1247-55.
81. Majoros A, Platanitis E, Kernbauer-Hölzl E, Rosebrock F, Müller M, Decker T. Canonical and non-canonical aspects of JAK–STAT signaling: lessons from interferons for cytokine responses. Frontiers in Immunology. 2017 Jan 26;8:29.
82. Magalon K, Cantarella C, Monti G, Cayre M, Durbec P. Enriched environment promotes adult neural progenitor cell mobilization in mouse demyelination models. European Journal of Neuroscience. 2007 Feb;25(3):761-71.
83. Vijayaraghava A, Radhika K. Alteration of Interferon Gamma (IFN-γ) in Human Plasma with Graded Physical Activity. Journal of Cinical and Diagnostic Research: JCDR. 2014 Jun;8(6):BC05.
84. Litteljohn D, Nelson E, Hayley S. IFN-γ differentially modulates memory-related processes under basal and chronic stressor conditions. Frontiers in Cellular Neuroscience. 2014 Nov 20;8:391.
85. Arellano G, Ottum PA, Reyes LI, Burgos PI, Naves R. Stage-specific role of interferon-gamma in experimental autoimmune encephalomyelitis and multiple sclerosis. Frontiers in Immunology. 2015 Sep 29;6:492.
86. Gao X, Gillig TA, Ye P, D’Ercole AJ, Matsushima GK, Popko B. Interferon-γ protects against cuprizone-induced demyelination. Molecular and Cellular Neuroscience. 2000 Oct 1;16(4):338-49.
87. Lin W, Harding HP, Ron D, Popko B. Endoplasmic reticulum stress modulates the response of myelinating oligodendrocytes to the immune cytokine interferon-γ. The Journal of Cell Biology. 2005 May 23;169(4):603-12.
88. Lin W, Bailey SL, Ho H, Harding HP, Ron D, Miller SD, et al. The integrated stress response prevents demyelination by protecting oligodendrocytes against immune-mediated damage. The Journal of Clinical Investigation. 2007 Feb 1;117(2):448-56.
89. Lin W, Kunkler PE, Harding HP, Ron D, Kraig RP, Popko B. Enhanced integrated stress response promotes myelinating oligodendrocyte survival in response to interferon-γ. The American Journal of Pathology. 2008 Nov 1;173(5):1508-17.
90. Balabanov R, Strand K, Goswami R, McMahon E, Begolka W, Miller SD, et al. Interferon-γ-oligodendrocyte interactions in the regulation of experimental autoimmune encephalomyelitis. Journal of Neuroscience. 2007 Feb 21;27(8):2013-24.
91. Pusic AD, Kraig RP. Phasic treatment with interferon gamma stimulates release of exosomes that protect against spreading depression. Journal of Interferon & Cytokine Research. 2015 Oct 1;35(10):795-807.
92. Butovsky O, Talpalar AE, Ben-Yaakov K, Schwartz M. Activation of microglia by aggregated β-amyloid or lipopolysaccharide impairs MHC-II expression and renders them cytotoxic whereas IFN-γ and IL-4 render them protective. Molecular and Cellular Neuroscience. 2005 Jul 1;29(3):381-93.
93. Corrado C, Raimondo S, Chiesi A, Ciccia F, De Leo G, Alessandro R. Exosomes as intercellular signaling organelles involved in health and disease: basic science and clinical applications. International Journal of Molecular Sciences. 2013 Mar;14(3):5338-66.
94. Witwer KW, Théry C. Extracellular vesicles or exosomes? On primacy, precision, and popularity influencing a choice of nomenclature. Journal of Extracellular Vesicles. 2019 Aug 1;8(1):1648167.
95. Pusic AD, Kraig RP. Youth and environmental enrichment generate serum exosomes containing miR-219 that promote CNS myelination. Glia. 2014 Feb;62(2):284- 99.
96. Pusic AD, Pusic KM, Clayton BL, Kraig RP. IFNγ- stimulated dendritic cell exosomes as a potential therapeutic for remyelination. Journal of Neuroimmunology. 2014 Jan 15;266(1-2):12-23.
97. Pusic KM, Won L, Kraig RP, Pusic AD. IFNγ-stimulated dendritic cell exosomes for treatment of migraine modeled using spreading depression. Frontiers in Neuroscience. 2019 Sep 3;13:942.
98. Yan X, Zeng D, Zhu H, Zhang Y, Shi Y, Wu Y, et al. MiRNA-532-5p regulates CUMS-induced depression-like behaviors and modulates LPS-induced proinflammatory cytokine signaling by targeting STAT3. Neuropsychiatric Disease and Treatment. 2020;16:2753.
99. Mao L, Zuo ML, Wang AP, Tian Y, Dong LC, Li TM, et al. Low expression of miR?532?3p contributes to cerebral ischemia/reperfusion oxidative stress injury by directly targeting NOX2. Molecular Medicine Reports. 2020 Sep 1;22(3):2415-23.
100. Xie W, Li M, Xu N, Lv Q, Huang N, He J, et al. MiR- 181a regulates inflammation responses in monocytes and macrophages. PloS One. 2013 Mar 13;8(3):e58639.
101. Hutchison ER, Kawamoto EM, Taub DD, Lal A, Abdelmohsen K, Zhang Y, et al. Evidence for miR- 181 involvement in neuroinflammatory responses of astrocytes. Glia. 2013 Jul;61(7):1018-28.
102. Ponomarev ED, Veremeyko T, Weiner HL. MicroRNAs are universal regulators of differentiation, activation, and polarization of microglia and macrophages in normal and diseased CNS. Glia. 2013 Jan;61(1):91-103.
103. Freilich RW, Woodbury ME, Ikezu T. Integrated expression profiles of mRNA and miRNA in polarized primary murine microglia. PloS One. 2013 Nov 11;8(11):e79416.
104. Volgin AD, Yakovlev OV, Demin KA, Abreu MS, Rosemberg DB, Meshalkina DA, et al. Understanding the role of environmental enrichment in zebrafish neurobehavioral models. Zebrafish. 2018 Oct 1;15(5):425- 32.
105. Reiser S, Pohlmann DM, Blancke T, Koops U, Trautner J. Environmental enrichment during early rearing provokes epigenetic changes in the brain of a salmonid fish. Comparative Biochemistry and Physiology Part D: Genomics and Proteomics. 2021 Sep 1;39:100838.
106. Melleu FF, Pinheiro MV, Lino-de-Oliveira C, Marino-Neto J. Defensive behaviors and prosencephalic neurogenesis in pigeons (Columba livia) are affected by environmental enrichment in adulthood. Brain Structure and Function. 2016 May 1;221(4):2287-301.
107. Armstrong EA, Voelkl B, Voegeli S, Gebhardt- Henrich SG, Guy JH, Sandilands V, Boswell T, Toscano MJ, Smulders TV. Cell proliferation in the adult chicken hippocampus correlates with individual differences in time spent in outdoor areas and tonic immobility. Frontiers in Veterinary Science. 2020;7.
108. Illa M, Brito V, Pla L, Eixarch E, Arbat-Plana A, Batalle D, et al. Early environmental enrichment enhances abnormal brain connectivity in a rabbit model of intrauterine growth restriction. Fetal Diagnosis and Therapy. 2018;44(3):184-93.
109. Coda KA, Fortman JD, García KD. Behavioral Effects of Cage Size and Environmental Enrichment in New Zealand White Rabbits. Journal of the American Association for Laboratory Animal Science. 2020 Jul 1;59(4):356-64.
110. Poessel SA, Biggins DE, Santymire RM, Livieri TM, Crooks KR, Angeloni L. Environmental enrichment affects adrenocortical stress responses in the endangered blackfooted ferret. General and Comparative Endocrinology. 2011 Jul 1;172(3):526-33.
111. Arroyo L, Valent D, Carreras R, Pato R, Sabrià J, Velarde A, et al. Neurobiology of environmental enrichment in pigs: hanges in monoaminergic neurotransmitters in several brain areas and in the hippocampal proteome. Journal of Proteomics. 2020 Oct 30;229:103943.
112. Guelfi G, Casano AB, Menchetti L, Bellicci M, Suvieri C, Moscati L, et al. A cross-talk between bloodcell neuroplasticity-related genes and environmental enrichment in working dogs. Scientific reports. 2019 May 6;9(1):1-1.
113. Bertapelle C, Polese G, Di Cosmo A. Enriched environment increases PCNA and PARP1 levels in Octopus vulgaris central nervous system: first evidence of adult neurogenesis in Lophotrochozoa. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution. 2017 Jun;328(4):347-59.
114. Kozorovitskiy Y, Gross CG, Kopil C, Battaglia L, McBreen M, Stranahan AM, et al. Experience induces structural and biochemical changes in the adult primate brain. Proceedings of the National Academy of Sciences. 2005 Nov 29;102(48):17478-82.
115. Warnefors M, Liechti A, Halbert J, Valloton D, Kaessmann H. Conserved microRNA editing in mammalian evolution, development and disease. Genome Biology. 2014 Jun;15(6):1-4.
116. Zhou Y, Tian T, Zhu Y, Jaffar Ali D, Hu F, Qi Y, et al. Exosomes transfer among different species cells and mediating miRNAs delivery. Journal of Cellular Biochemistry. 2017 Dec;118(12):4267-74.
117. Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature Cell Biology. 2007 Jun;9(6):654-9.