Introduction
Percutaneous coronary angiography and interventions have become essential diagnostic and therapeutic tools in coronary artery disease management. Vascular closure devices were introduced in the early 1990s. They provided a safe, reliable, and efficient method to achieve puncture site hemostasis. Collagen-based vascular closure devices are frequently used modalities. An Angio seal is a medical device that is used to close a puncture in the arterial system after a catheterization procedure [1-3]. Although associated with a lower complication rate, some cases have been reported. Complications include thrombosis, false aneurysm formation, embolization, and infection. Thrombotic complications refer to the formation of a blood clot within a blood vessel as a result of improper placement or malfunction of the device, frequently managed through open surgical repair [3]. In our case, we will highlight the endovascular management of collagen-based thrombotic complications as a viable option.
Case Presentation
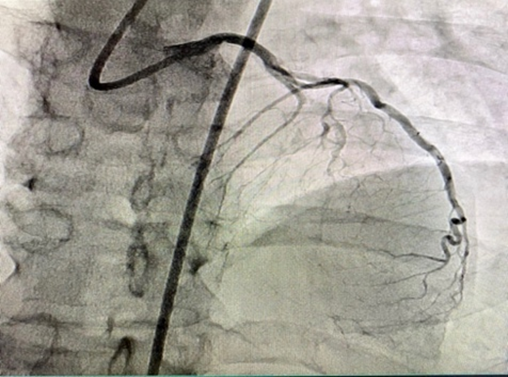
A 66-year-old male with a past medical history of hypertension, diabetes mellites, dyslipidemia, peripheral arterial disease, and ischemic heart disease. He underwent successful percutaneous coronary intervention to chronic totally occluded left anterior descending artery after failed prior attempts (Figures 1 and 2). The coronary intervention was done using an 8 Fr system through the right common femoral artery approach. The arteriotomy site was closed with an 8 Fr Angio-Seal closure device.
Figure 1. CTO LAD.
Figure 2. Recanalized LAD.
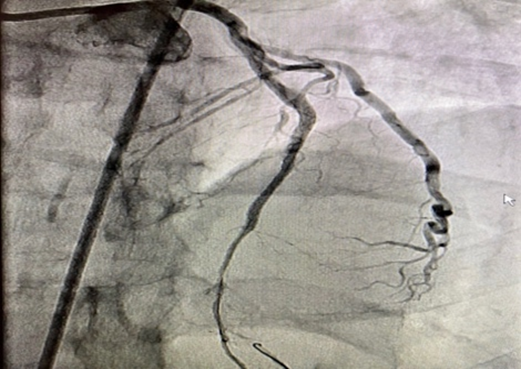
Upon examination, peripheral pulsations were noted to be absent. Right common femoral artery acute thrombosis was confirmed through peripheral angiogram via contralateral approach (Figures 3). A successful endovascular bailout technique was performed utilizing Supera peripheral stent system.
Figure 3. Rt CFA thrombosis.
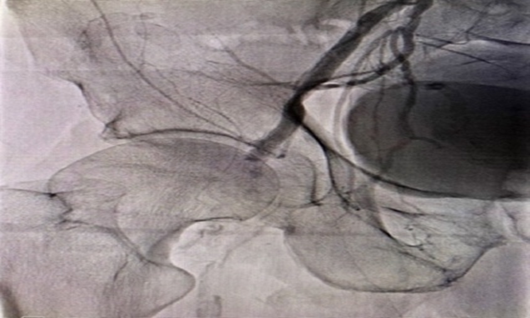
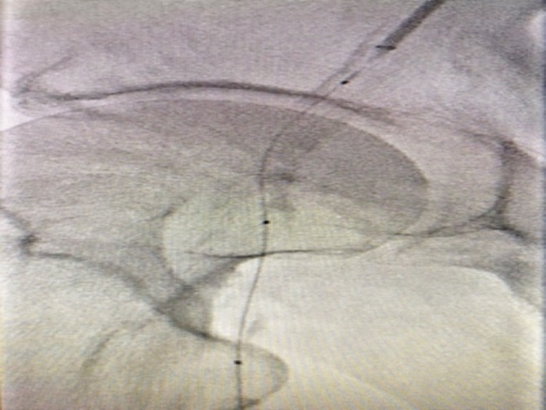
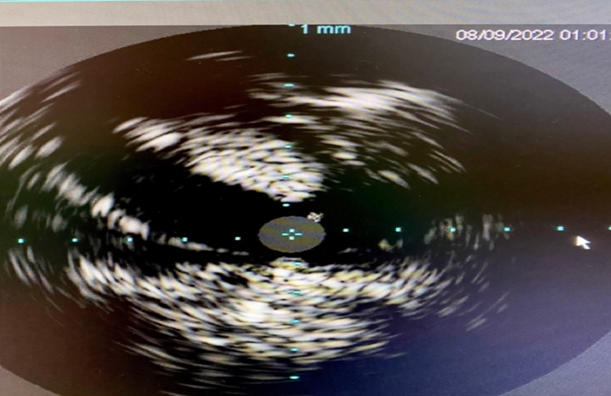
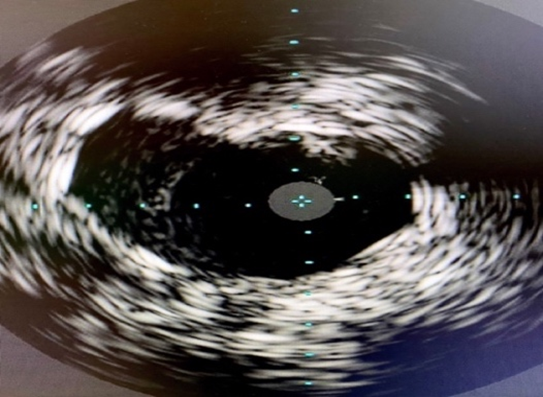
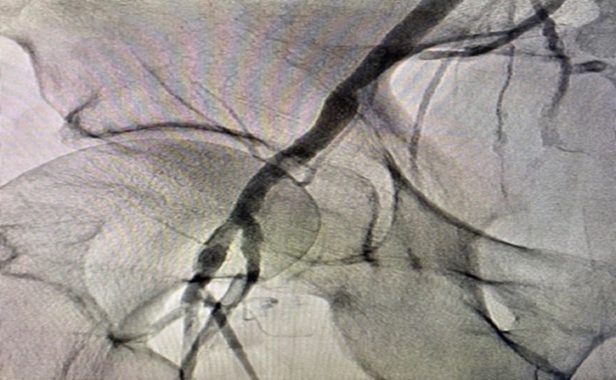
Left CFA access was upgraded to a 6 F system. Attempt to cross occluded Rt CFA through contralateral approach was unsuccessful with multiple wires. Ipsilateral Rt SFA retrograde access was obtained with ultrasound guidance. Micro-puncture sheath placed in the Rt SFA. The occluded Rt CFA was crossed with Fielder XT guide wire and 2.6 F CXI support catheter. The wire was externalized through the contralateral side (Figure 4). Initial balloon angioplasty performed 5 x 40 mm Ultraverse balloon. Intravenous ultrasonography (IVUS) (Figure 5) was utilized for sizing which also showed the Angio-Seal footplate (*). Repeat angioplasty with 7x40mm Ultraverse balloon performed prior to stenting with 6.5 x 40 mm Supera stent. Intravenous ultrasonography (IVUS) (Figure 6) post-intervention confirmed stent well opposition. The right SFA micropuncture sheath was removed and successful balloon tamponade with a 6.0 x 40 mm balloon for hemostasis was performed. The flow was restored post-intervention (Figure 7) and the patient was discharged home the same day.
Figure 4. Wire externalized through the contralateral side.
Figure 5. IVUS showing Angiosele footplate.
Figure 6. IVUS post-intervention.
Figure 7. Post-intervention Angiogram.
Discussion
Vascular closure devices are routinely used to achieve hemostasis in patients undergoing angiography. The Angio-Seal is a type of collagen vascular closure device that consists of an extra-arterial collagen layer, an intra-arterial anchor comprised of polyglycolic and polylactic acids, and a bioabsorbable suture connecting the two layers. They create a mechanical seal by sandwiching the artery between a bio-absorbable anchor and a collagen sponge that dissolve within 60–90 days. They allow early ambulation and subsequent discharge. A reported complication rate of 0.4% is associated with using arterial closure devices. Complications include thrombosis, false aneurysm formation, embolization, and infection. We demonstrate the bailout endovascular strategy in managing Angio-seal related thrombotic complications [1-3]. Our report highlights the importance of thorough examination for any associated vascular complications commencing immediately after device implantation. It is vital that these complications if happened, be managed urgently to avoid subsequent comorbidities.
Conclusion
Endovascular management of thrombotic complications related to collagen-based vascular closure devices is a viable alternative option to the surgical approach. Future follow-ups will illustrate the long-term outcomes of this approach.
Acknowledgment
I would like to thank the Ochsner Cardiology team for helping manage and stabilize this case.
References
2. Georg Y, Thaveau F, Lejay A, Bajcz C, Bakassa S, Chakfe N, et al. Arterial thrombosis after using Angio-Seal. Ann Vasc Surg. 2011 Nov;25(8):1078-93.
3. Lupattelli T, Clerissi J, Clerici G, Minnella DP, Casini A, Losa S, et al. The efficacy and safety of closure of brachial access using the AngioSeal closure device: experience with 161 interventions in diabetic patients with critical limb ischemia. J Vasc Surg. 2008 Apr;47(4):782-8.