Abstract
Head and neck squamous cell carcinoma (HNSCC) are a group of cancers that affect various parts of the head and neck, such as the lip, oral cavity, oropharynx, hypopharynx, and nasopharynx. In India, it accounts for approximately 30–40% of all cancers, while in the United States, it represents around 4% of all cancer cases. HNSCC is a significant contributor to cancer-related deaths globally. While smoking is linked to HNSCC, recent research has confirmed the importance of the human papillomavirus (HPV) in its development. Additionally, HNSCC is characterized by immune deficiencies, where the tumor microenvironment alters immune cell activity to facilitate carcinogenesis. However, researchers have identified novel immunotherapeutic targets for HNSCC, mainly by studying the involvement of inflammatory cells such as neutrophils, eosinophils, and macrophages. Unfortunately, patients with recurring malignancy and distant metastases have limited treatment options, with a prognosis of less than one year. Platinum-based chemotherapy, cetuximab, and other conventional therapies are used to treat recurrent and metastatic HNSCC. The pro and anti-tumor roles of neutrophils in cancer and immunotherapy are explored in this study, with a focus on HNSCC. The primary objective is to increase our understanding of HNSCC biology and immunobiology to uncover viable therapeutic options that are both valid and less cytotoxic.
Keywords
Pro-inflammatory, Anti-inflammatory, Tumor associated neutrophils, Metastasis, Head and Neck carcinoma
Introduction
According to Global Cancer Observatory (GLOBOCAN), India ranks third worldwide in terms of cancer prevalence, following China and the United States of America. By 2040, cancer cases in India are projected to exceed 2.08 million, indicating a significant increase of 57.5% compared to the current scenario in 2020 [1,2]. As per GLOBOCAN 2020, head and neck cancer (HNC) accounts for 985,514 cases each year, resulting in an average of 467,125 deaths annually [2]. Developing countries like India, Pakistan, Bangladesh, Papua New Guinea, Taiwan, and Sri Lanka have high incidence rates of cancer, with HNCs comprising approximately 30–40% of all cancers in India [3]. The most common malignancies in the HNC are squamous cell carcinomas (SCC), which arise from the mucosa of the lip, oral cavity, larynx, nasopharynx, oropharynx, hypopharynx, and salivary glands (Figure 1). Squamous cell carcinomas (SCC) are the predominant form of malignancies within the head and neck region and are primarily caused by tobacco use and excessive alcohol consumption [4]. Oropharyngeal tumors have been linked to prior human papillomavirus (HPV) infection, particularly with HPV-16, according to the Centers for Disease Control and Prevention (CDC). HPV is believed to cause 70% of oropharyngeal cancers in the United States. Chewing tobacco, gutkha, areca nuts, betel quid, slaked lime, and drinking habits are some of the factors that affect the incidence of HNSCC in India. Gutkha is a form of smokeless tobacco commonly used in India. It is a mixture of crushed tobacco, areca nuts, slaked lime, and various flavorings [5,6]. Tobacco users with oral cancer have a lower survival rate (44%) than non-tobacco users (57%), with a median overall survival rate of 5 years of 43% and 72%, respectively [7]. The coexistence of tobacco and alcohol leads to a poor prognosis, as observed in various studies [7]. However, smoking prevalence has declined in high-income industrialized nations, resulting in a significant reduction in HNSCC incidences attributed to smoking [8]. Conversely, the global number of HPV-related HNSCC malignancies has increased, while HPV-positive patients typically have a better prognosis than HPV-negative cases in HNSCC malignancies [9]. This is because HPV-positive malignancies respond better to therapy and have a higher overall survival rate. The oropharynx is the most common site for HPV-positive HNSCC, accounting for 60–70% of cases in the United States [9,10]. HNSCC of the oral cavity is treated surgically, followed by chemotherapy or adjuvant radiation therapy [11]. In addition to the HPV vaccine Gardasil-9, the Food and Drug Administration (FDA) has approved cetuximab for use in treating individuals with advanced or recurrent HNSCC cancer [12]. For advanced HNC cases, multimodality treatments are required, and multidisciplinary care is essential.
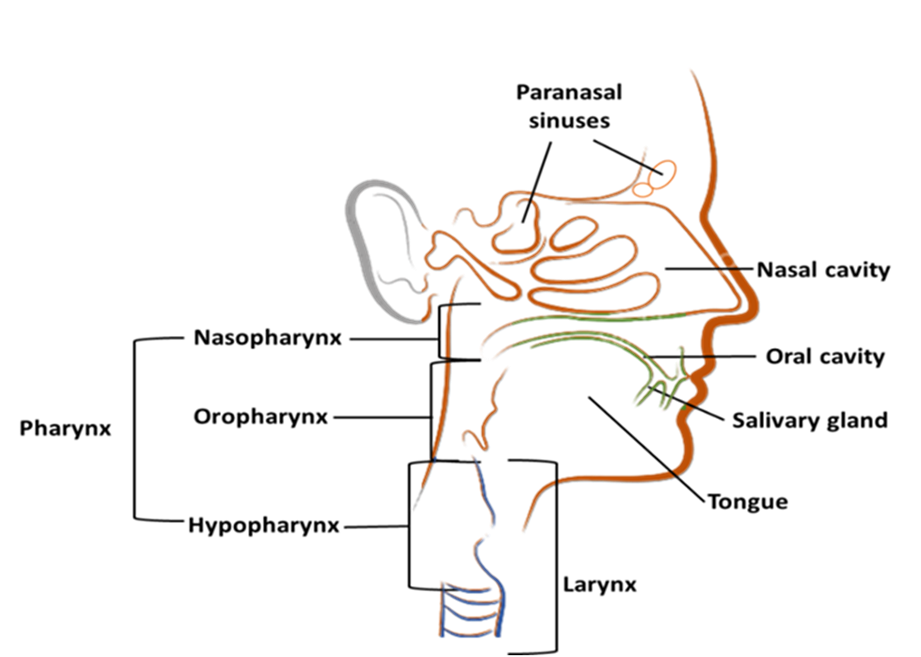
Figure 1. Illustrated site of head and neck cancers: the paranasal sinuses, nasal cavity, oral cavity, tongue, salivary glands, larynx, and throat (including the nasopharynx, oropharynx, and hypopharynx).
The Immune System and Cancer
The immune system is responsible for protecting hosts from infectious organisms. It is composed of several biological components that work together to defend against intruders and illnesses, successfully protecting against millions of bacteria, viruses, and parasites. The human immune system is highly complex, with various cells, organs, and proteins each playing a distinct role in combating foreign particles and aberrant cells [13]. The relationship between malignancy development and the host’s immune system is intricate [14]. The failure of the host’s immune system to recognize and eliminate cancer cells at the outset allows malignant development to begin [15]. The resulting tumor mass proliferation can lead to severe damage to the immune system [16]. Tumor growth is inextricably influenced by a reduction in the host immune response, regardless of tumor location or etiology. The tumor’s expansion has a negative impact on both the adaptive and innate immune systems [17]. Anti-tumor immune cells, including B cells, T cells, macrophages, and (Natural Killer) NK cells, undergo structural and functional changes in the tumor-sustaining host, resulting in a cancer microenvironment that includes tumor-infiltrated immune and non-tumor cells such as fibroblasts, endothelial cells, and various other stromal cells [16]. Within this microenvironment, transformed cancer cells secrete autocrine growth factors such as fibroblast growth factor (FGF), hepatocyte growth factor (HGF), epidermal growth factor (EGF), and transforming growth factor (TGF), which encourage cancer cell proliferation and bind to receptor tyrosine kinases (RTKs) [18,19]. This binding can result in epithelial to mesenchymal transition (EMT) of the most resistant cells, causing them to become invasive and migratory [20,21].
As the tumor expands in size, the core becomes more hypoxic, prompting altered cells to increase the secretion of angiogenic mediators including vascular endothelial growth factor (VEGF), HGF, insulin-like growth factor (IGF), TGF, WNT, neural transcription factor (NOTCH), and FGF, leading to EMT or mesenchymal cell-like alteration in tumor architecture, which leads to metastasis [22-24]. The most resistant cells that survive the hypoxic tumor environment are considered to be cancer stem cells [22-24]. A variety of immune cells, including macrophages, NK cells, B-lymphocytes, and T-lymphocytes, are driven to the tumor site by inflammatory molecules generated by the tumor cells [25]. However, according to research, an individual’s immune response is not only repressed but also altered in a way that promotes cancer growth [26,27]. Molecules released by tumors cells can indeed render immune cells suppressive, which is a phenomenon known as immunosuppression. Tumors release various molecules including interleukin(IL)-4, IL-6, IL-10, MDF, TGF-β, and prostaglandin E2 (PGE2) that manipulate the immune system to evade detection and destruction [16]. These molecules can directly inhibit immune cell function or induce the production of suppressive immune cells, leading to a weakened immune response against the tumor [26]. One such chemokine is CCL2 (also known as monocyte chemoattractant protein-1 or MCP-1). CCL2 is produced by various tumors and has been shown to recruit immunosuppressive cells like tumor-associated macrophages (TAMs) and myeloid-derived suppressor cells (MDSCs) to the tumor microenvironment [27]. Another well-known example of such molecules is the programmed death ligand 1 (PD-L1) protein, which is often overexpressed by tumor cells. PD-L1 interacts with its receptor PD-1 on immune cells, triggering inhibitory signals that dampen the immune response. This interaction is exploited by tumors to suppress immune surveillance and avoid immune attack [28]. Suppressed immune cells, such as regulatory T cells (Tregs) or MDSCs, can release cytokines that promote tumor growth and progression. These cytokines include TNFα, IL-6, and IL-1β [29]. TGF-β is known to have immunosuppressive effects by inhibiting the activation and proliferation of immune cells, such as T cells and NK cells, and by promoting the generation of regulatory T cells (Tregs) that further suppress immune responses [30]. These molecules have been shown to have various effects on cancer cells and cancer stem cells, including promoting cell survival, proliferation, angiogenesis, and immune evasion (Figure 2).
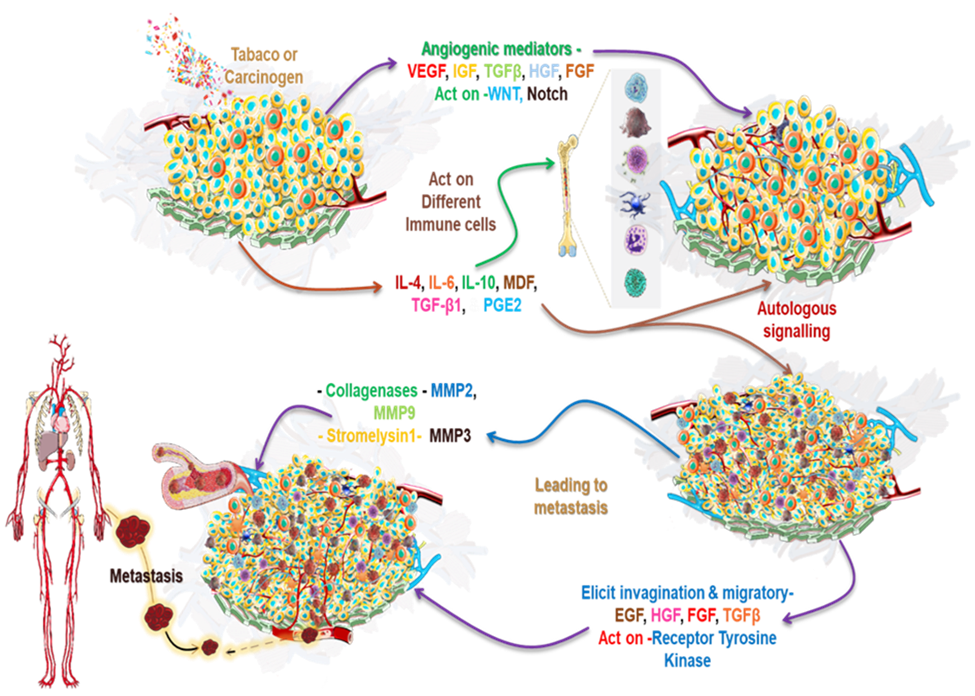
Figure 2. Tumor induction, as well as the processes of angiogenesis and metastasis: Induction of tumor caused by external or internal factors such as tobacco carcinogen transforms cells by causing mutation which leads to tumor formation and results in aberrant cell behaviour. As the tumor expands in size, the tumor core becomes hypoxic, and the altered cells stimulate the upregulation of several angiogenic mediators such as VEGF, HGF, IGF, TGFβ, FGF, Notch, and WNT, which causes cancer cells to undergo EMT. Most resistant tumorigenic cells or CSCs escape the tumor hypoxic environment due to mesenchymal alterations in tumor architecture. Hypoxia and tumor-induced inflammation attracts several immune cells to the tumor site, including macrophages, NK cells, B cells, T cells, and others. Immune cells in the cancer microenvironment are exposed to immunosuppressive and anti-inflammatory cytokines produced by tumor cells, such as interleukin IL-4, IL-6, IL-10, MDF, TGF-β1, and PGE2, which results in antitumor immune dysfunction and immune cell suppression. These suppressed immune cells release cytokines such as TNFα, IL6, and IL1 β, aiding in the development of cancer cells and CSCs.
Abbreviations: HNSCC: Head and Neck Squamous Cell Carcinoma; HPV: Human Papillomavirus; GLOBOCAN: Global Cancer Observatory; HNC: Head and Neck Cancer; SCC: Squamous Cell Carcinoma; FDA: Food and Drug Administration; NK: Natural Killer; FGF: Fibroblast Growth Factor; HGF: Hepatocyte Growth Factor; EGF: Epidermal Growth Factor; TGF: Transforming Growth Factor; RTK: Receptor Tyrosine Kinase; EMT: Epithelial-Mesenchymal Transition; VEGF: Vascular Endothelial Growth Factor; IGF: Insulin-like Growth Factor; NOTCH: Neural Transcription Factor; IL: Interleukin; MDF: Macrophage Derived Factor; PGE: Prostaglandin E; TNF: Tumor Necrosis Factor; IFN: Interferon; CSC: Cancer Stem Cell; TAN: Tumor-Associated Neutrophil; NET: Neutrophil Extracellular Trap; NLR: Neutrophil-to-Lymphocyte Ratio; OM: Overall Mortality; CM: Cancer Mortality; NCM: Non-Cytokine-Mediated; SUV: Standardized Uptake Value; NSCLC: Non-Small Cell Lung Cancer; NAMPT: Nicotinamide Phosphoribosyl Transferase; TDLN: Tumor-Draining Lymph Node; OSCC: Oral Squamous Cell Carcinoma; CD: Cluster of Differentiation; GM-CSF: Granulocyte-macrophage colony-stimulating factor; PDPN: Podoplanin; ROS: Reactive Oxygen Species; NE: Neutrophil Elastase; PI: Protease Inhibitor; IRS: Insulin Receptor Substrate; ELANE: Elastase, Neutrophil Expressed; PR: Pprathogenesis-Related proteins; HLA: Human Leukocyte Antigen; CTSC: Cathepsin C; CCL: Chemokine Ligand; MPO: Myeloperoxidase; MMP: Matrix Metalloproteinase; ECM: Extracellular Matrix; TIMP: Tissue Inhibitor of Metalloproteinases; DNA: Deoxyribonucleic Acid; PMN: Polymorphonuclear Neutrophil; PMA: Phorbol Myristate Acetate; TNM: Tumor Node Metastasis; ARG: Arginase; NO: Nitric Oxide; MDSC: Myeloid-Derived Suppressor Cell; MIF: Macrophage Migration Inhibitory Factor; TLR: Toll-Like Receptor; MET: Mesenchymal-Epithelial Transition; ADCC: Antibody-Dependent Cellular Cytotoxicity; NADPH: Nicotinamide Adenine Dinucleotide Phosphate; HER: Human Epidermal Growth Factor Receptor; NPC: Nasopharyngeal Carcinoma; TME: Tumor Microenvironment; DC: Dendritic Cell; ATP: Adenosine Triphosphate; CCRT: Concurrent Chemoradiotherapy; OS: Overall Survival; EGFR: Epidermal Growth Factor Receptor; CTLA: Cytotoxic T-Lymphocyte Antigen; PD: Programmed Death; NCT: National Clinical Trial; MHC: Major Histocompatibility Complex; TKI: Tyrosine Kinase Inhibitor; CTL: Cytotoxic T Lymphocyte
Neutrophils and Tumor-Associated Neutrophils (TANs)
Neutrophils, the predominant type of polymorphonuclear leukocytes in the innate immune system, act as the primary line of defense against invading pathogens [31]. Generated at a rate of 1011 cells per day in the bone marrow before entering the bloodstream, these cells move to tissues, perform their tasks, and are eventually eliminated by macrophages under normal conditions. Despite the fact that neutrophils do not have discrete varieties, depending on their level of activation and the environment they are exposed to, they exhibit functional heterogeneity. Neutrophils can be categorized into resting and activated states. Resting neutrophils are inactive and present in blood circulation, whereas when they come in contact with infection or inflammation, they are activated and undergo morphological changes. Three key antimicrobial actions of neutrophils include degranulation, phagocytosis, and the release of neutrophil extracellular traps (NETs) [32]. Furthermore, research has shown that neutrophils respond by producing cytokines and inflammatory molecules, which regulate immune cells and inflammation response [33]. Neutrophils display a range of functional phenotypes in different microenvironments, and different tissues usually stimulate neutrophils to perform distinct activities [34]. In cancer, neutrophils count in the circulatory system are increased and their phenotypes change as the tumor progresses [34]. In advanced cancer, a number of subpopulations of circulating neutrophils have been discovered, varying in maturity, immune suppressive characteristics, and tumor cytotoxicity [35].
The neutrophils that enter the cancer microenvironment are known as TANs [36]. Various mechanisms associated with cancer have been identified to increase TANs, including angiogenesis, tumor cell metastasis and invasion, extracellular matrix remodeling, and immunological suppression [36,37]. These TANs can either inhibit or accelerate cancer growth and development in the tumor’s microenvironment [38]. TANs are linked to poor clinical outcomes in various types of cancers such as head & neck cancer, colorectal cancer, hepatocellular carcinoma, melanoma, and pancreatic cancer [38-41]. The neutrophil-to-lymphocyte ratio (NLR) has been used to predict therapy responsiveness and outcomes in different types of cancers, though it has rarely been studied in elderly patients with head & neck squamous cell carcinoma (HNSCC). One study conducted by Ku et al. on HNSCC patients aged 65 and above used cumulative incidence and cause-specific hazard functions to examine risk factors for cancer mortality, overall mortality, and non-cancer mortality [42]. The study included pre-treatment examinations such as the Beck’s depression questionnaire, patient demographics, and circulating biomarkers. NLR, performance scale, age, and nodal stages were independent predictors of overall mortality (OM) and cancer mortality (CM), while body weight loss, age, fragility, and NLR independently predicted Non-Cancer Mortality (NCM) (P <0.05) [42]. Moreover, NLR was closely correlated with all three types of mortality for patients aged 75 and above; an NLR greater than 2.5 was associated with a higher risk of NCM, CM, and OM [42]. Another study by Werner and his colleagues investigated pre-therapeutic NLR ratios in 90 head and neck cancer patients before primary chemo-radiation [43]. The study found that NLR was significantly correlated with primary tumor metabolic markers and disease-specific survival in nodal-positive cancer patients. Higher NLR and SUVs max (Standardized Uptake Values maximum) of lymph node metastases were independently predictive of cancer survival. High NLR was linked to a poor overall survival prognosis and may be suggestive of a nonspecific inflammatory response in the host to increased primary tumor metabolism [43]. Overall, these findings imply that hematological data may predict non-cancer and cancer mortality in elderly individuals with HNSCC, and that NLR can be used as a circulating prognostic marker in this population.
Pro-Tumor Function of Neutrophils
Neutrophils have been shown to exhibit a range of anti-tumor actions, but in most cases, TANs are linked with advanced disease and poor cancer prognosis [36]. This negative correlation has been observed in several solid malignancies, including melanoma, non-small cell lung cancer (NSCLC), head and neck squamous cell carcinoma (HNSCC), and adenocarcinoma [37,44]. It remains unclear what determines the pro-tumorigenic or antitumorigenic phenotype of neutrophils in tumors. Research by Pylaeva et al. using transplantable tumor models demonstrated that the molecule nicotinamide-phosphoribosyl-transferase (NAMPT), implicated in the signaling downstream of CSF3R, is required for the oncogenic transformation of TANs and their pro-angiogenic transition [45]. NAMPT expression is upregulated in TANs from head-and-neck cancer patients and corresponds with the tumor stage, promoting tumor vascularization and proliferation. Inhibiting NAMPT in TANs leads to their conversion into an anti-tumor phenotype, reducing tumor growth and angiogenesis when transferred into mice with tumors [45].
A retrospective study conducted by Lonardi et al. investigated the clinical significance of TANs in oral squamous cell carcinomas (OSCC) by conducting an immunohistochemical examination of tumor-draining lymph nodes (TDLNs) [46]. The results of the study demonstrated a significant proportion of CD 66b+ TANs (59%) in TDLNs. Microscopic examination indicated that TANs migrate through lymphatic vessels and appear as pairs of TANs and cancer cells within the lymphatic system. Similarly, in vitro studies performed using OSCC cancer cells and neutrophils further revealed that OSCC cells promote the survival and activation of neutrophils by releasing granulocyte-macrophage colony-stimulating factor (GM-CSF) [46]. In OSCC patients’ high density of CD 66b+ TANs in TDLNs is associated with poor outcomes where the primary tumor cells of OSCC co-occur with CD 66b+ TANs and proteins called podoplanin (PDPN) and S100A9, leads to the transition of tumor cells from an epithelial to mesenchymal (EMT) state in regions associated with lymph angiogenesis [46]. The findings support the idea that TAN, as a pro-tumorigenic entity, promotes lymphatic nodal metastasis via EMT [46]. TAN pro-tumor activities encompass the same neutrophilic components that are employed to regulate infections and alter inflammatory responses. Molecules that promote tumorigenesis and invasiveness include matrix-degrading proteinases, granule proteins, chemokines, cytokines, and reactive oxygen species (ROS) [47]. These molecules are used by TANs to influence cell proliferation, angiogenesis, metastasis, and immune system surveillance [47].
Neutrophil Elastase Pro-Tumor Function
Neutrophil elastase (NE) are proteases found in neutrophils and have a wide range of substrates, such as antibacterial proteins, extracellular matrix proteins, integrins, cytokines, and cytokine receptors. NE is mainly released during cell degradation to eliminate invasive infections and perform anti-inflammatory functions [48]. While NE is known for its anti-inflammatory and anti-bacterial properties, current research has revealed that NE can have a pro-tumor role in malignancies [49]. In both in vitro and in vivo studies, NE was found to promote the proliferation of lung cancer cells [48]. However, the effect was significantly decreased in the presence of NE inhibitors [48,49]. Similarly, the impact of NE on tumor growth in breast cancer, esophageal cancer, and gastric cancer is dependent on the phosphatidylinositol-3-kinase (PI-3K) pathway and can be diminished by a PI-3K inhibitor [50]. Within the tumor mass, NE affects the insulin receptor substrate-1 (IRS-1), which binds the PI-3K regulatory unit. NE destroys this regulatory unit, allowing for more PI-3K to be available and thus, enhancing proliferation pathways. Interestingly, human neutrophils generate catalytically active neutrophil elastase (ELANE), which can kill many types of cancer cells while sustaining non-cancerous cells [50,52]. The CD-95-death domain, released by ELANE through proteolysis, binds to histone H1 isoforms to destroy cancer cells. This not only restricts primary tumor growth but also affects distant metastases via CD8+ T cells [42,52]. TAN releases the serine proteases NE and P3 into the tumor microenvironment, which influences the antitumor innate immune response. Pathogenesis-related proteins (PR1), a tumor-associated antigen that is derived from NE and P3, is expressed in HNSCC cancer cells [53]. Moreover, NE and P3 upregulate HLA class I molecule expression, resulting in unique, endogenous peptides in the immune-peptidome [53]. Cathepsin C (CTSC), a protease secreted by tumors, has been shown to stimulate metastasis by regulating neutrophil recruitment and the formation of NETs [54]. CTSC promotes PR3 enzymatically to enable nuclear factor B activation, increasing IL-6, IL-1, and C-C motif chemokine ligand 3 (CCL3) processing for neutrophil recruitment [54]. The CTSC-PR3-IL-1 axis is also associated with the generation of ROS and NETs formation in neutrophils, which break down thrombospondin-1 and facilitate cancer cell metastasis [55].
Neutrophil ROS Pro-Tumor function
A multitude of variables, including persistent inflammation, may trigger oxidative stress in cancer patients. Immune cells such as neutrophils and macrophages can generate ROS, which are associated with immunosuppression and tumorigenesis. While ROS generated by neutrophils normally serve to destroy microorganisms, in the case of cancer, these ROS can indirectly promote tumor growth. For example, neutrophils produce hydrogen peroxide (H2O2), which is then converted into hypochlorous acid (HOCl) by myeloperoxidase (MPO) [56]. This HOCl can activate matrix metalloproteinases (MMPs), such as MMP-2, MMP-8, and MMP-9, which break down the extracellular matrix (ECM) [56] and block tissue inhibitor of metalloproteinases (TIMP-1) to intensify the activity of MMPs. MMP activity can facilitate the progression of cancer by increasing angiogenesis and proliferation [57]. Although neutrophil-induced ROS and HOCl can directly damage tumor cells, they can become genotoxic if they fail to do so. Two major pathways through which ROS-induced genotoxicity can occur are MPO-catalyzed chemical carcinogen activation and oxidative DNA damage [58]. Additionally, some studies have revealed that co-culturing different cell types with neutrophils could result in DNA strand breaks and point mutations. Furthermore, HOCl has been found to be mutagenic in the lung epithelial cell line A549 [58]. Trellakis et al. conducted a study to investigate the bioactivities of polymorphonuclear granulocytes (PMN) in individuals with HNSCC and healthy participants [59]. PMNs were observed for their levels of apoptosis, ROS generation, cytokine release, and immune-phenotyping. It was discovered that PMN from HNSCC patients had lower inducible ROS production and spontaneous apoptosis compared to PMN from healthy donors. However, there was no significant difference between the two groups in their levels of inflammatory cytokines. Immuno-phenotyping showed that HNSCC patients had increased frequencies of immature PMN fractions. From these results, it can be concluded that PMN from HNSCC individuals and healthy donors show distinct functional properties [59]. In another study performed by Szuster-Ciesielska et al., neutrophils isolated from the blood of patients with larynx carcinoma and healthy volunteers were compared in terms of their ROS and superoxide anion (O2-) generation, as well as their hydrogen peroxide (H2O2) levels [60]. It was found that the ROS generation and PMA-induced O2 levels in individuals with laryngeal cancer were significantly greater than that of the healthy volunteers, and that it increased in correlation with the tumor stage (node tumors, metastasis-TNM staging) [60]. The concentrations of superoxide dismutase, catalase, and total peroxidase activity in the serum were also determined. It was observed that serum peroxidase activities and superoxide catalase were considerably more elevated in T3 and T4 larynx cancer patients [60]. Post-surgery, individuals with laryngeal cancer (partial or whole laryngectomy) recorded a notable reduction in their serum catalase, ROS generation, and peroxidase activity. In contrast, a significant increase in superoxide dismutase levels in the blood was observed after surgery, particularly in cases of advanced cancer stages (T3-T4) [59]. These findings suggest that people with laryngeal cancer are subject to oxidative stress in their bloodstream, which is notably reduced after undertaking a partial or complete laryngectomy.
Neutrophil Arginase 1 (ARG1) Pro-Tumor Function
Most myeloid cells release Arginase 1 (ARG1), which can convert arginine into ornithine, leading to a decrease in extracellular arginine at infection sites [61,62]. This decrease in arginine levels can suppress the production of nitric oxide (NO), which is an important component of the immune response and wound healing [62-64]. The secretion of ARG1 from neutrophil granules may decrease the efficacy of T cell activation, potentially reducing the immune response in the same manner as Granulocytic-Myeloid-derived suppressor cells (G-MDSCs) do [44,65,66]. Depletion of TANs in tumors is associated with an increase in activated T lymphocytes (CD 8+) and a reduction in tumor growth [67]. Similarly, NSCLC cells stimulated neutrophils to release ARG1 through IL-8, and TANs had lower levels of ARG1 in tumors [67]. ARG1 released from granules of gelatinase was found to be inactive at physiological pH unless activated by azurophil granule-stored factors [48]. By causing simultaneous exocytosis of gelatinase and azurophil granules, TANs can cause ARG1-dependent immunosuppression [48].
Pro-Tumor Neutrophil in Metastasis
In various types of cancer, cancer cells may prompt neutrophils to release pro-inflammatory substances that encourage metastasis. This process involves the dissipation of the basement membrane, allowing tumor cells to infiltrate beyond it. Research has found that neutrophils are particularly involved in promoting metastasis in HNSCC, skin squamous cell carcinoma, and breast cancer [68,69]. Tumor-derived inflammatory cytokine macrophage migration inhibitor (MIF) is responsible for attracting HNSCC neutrophils, which have a pro-migratory effect on tumor cells when responding to them [70]. Additionally, hyaluronan released by tumor cells can activate neutrophils through the phosphoinositide 3-kinase (PI-3K/Akt) and toll-like receptor (TLR4) signaling pathway [71]. In animal models, injecting tumor cell clusters resulted in more metastases than scattering tumor cells. Neutrophils present in the blood promote cancer cell clustering, which helps them to survive and progress. Patients with cancer cell clusters in their blood often have poor survival rates [72].
During mesenchymal-to-epithelial transition (MET) or the exit of circulating cells, neutrophils are often found to be in close association with metastasized cells [73]. These cells also accelerate the MET of metastasized cells at a new location and create favorable conditions for tumor cell growth before they arrive [37,74]. Evaluating the role of neutrophil subsets within a tumor site based on their profile, activation state, and migratory capabilities are necessary to predict cancer prognosis. Studies by Yu, et al., have shown that neutrophils in OSCC tissue infiltrate and express higher levels of TGF-1 and IL-17A, thus taking on pro-tumor characteristics in vitro [75]. Neutrophils activated by TGF-1/IL-17A generate significant cell proliferation, migration, stemness, invasion, and EMT in OSCC cells [75]. Furthermore, individuals with OSCC have higher levels of MMP9 and lower levels of CCL3 in their circulating neutrophils. The combination of neutrophil-associated markers may be used to screen OSCC patients.
Antitumor Function of Neutrophils
Neutrophils have a complex role in tumor progression and metastasis. While they can establish a metastatic niche and promote tumor growth, they also have antitumor functions. Specifically, the N1 phenotype of neutrophils limits metastasis and tumorigenesis through direct cytotoxicity, antibody-dependent cytotoxicity, and activation of other immune cell types such as dendritic cells (DCs) and T cells [75,76]. In fact, research has demonstrated that removing neutrophils from mice leads to an increase in tumor development, highlighting the significance of these cells in anti-tumor responses [77].
In patients with acute systemic inflammation, neutrophils can be divided into three subgroups based on their expression of CD 62 L – CD 16dim CD 62Lhigh, CD 16high CD 62Lhigh, and CD 16high CD 62Ldim markers [78]. A study by Millrud et al. found that patients with HNSCC had higher levels of a specific subgroup of neutrophils, which was associated with a distinct phenotype CD 62Ldim CD 16high and increased migration inside the tumor [79]. Tumor-derived IL-8 was found to facilitate this migration and also stimulate neutrophil transformation [79]. Activated CD 62Ldim CD 16high neutrophils were found to reduce cancer cell proliferation, migration, and induce apoptosis [79,80]. In another study, enhanced CD 16high CD 62Ldim neutrophil circulation in HNSCC was associated with higher HNSCC survival [80]. In several carcinomas, tumor-derived factors such as IL-8 and CCL2 can promote neutrophil recruitment and ROS generation, leading to antitumor activity [81]. However, it is important to note that neutrophils can also indirectly promote tumor growth [82]. Nevertheless, neutrophil-produced ROS can cause tumor cell destruction through an oxidative mechanism, particularly in fast-growing tumors where activated neutrophils can produce enough oxygen radicals [83]. Close contact facilitated by integrins is necessary for the direct release of HOCl to the tumor cell during the oxidative process [83,84].
Neutrophils Mediated Direct Cytotoxicity and Antibody-Dependent Cell-Mediated Cytotoxicity (ADCC)
Neutrophils are highly mobile phagocytic cells that primarily defend against bacterial infections. Their cytotoxicity depends on physical contact mediated by integrins. Neutrophils produce various antibacterial agents that are harmless to eukaryotic cells. However, certain molecules, such as superoxide, H2O2, the NADPH oxidase complex, and HOCl, have been discovered to be directly involved in antitumor cytotoxicity [76,78]. Neutrophils can induce direct cytotoxicity in tumor cells through enzymes such as perforin and granzyme, similar to NK cells [85]. Neutrophils have also been shown to induce apoptosis in certain types of cancers, such as HNSCC and breast cancer cells, when stimulated with anti-HER-2 antibodies [71,77].
The mechanism of neutrophil-mediated cytotoxicity in tumors is not fully understood and appears to be complicated. Another method of neutrophil antitumor cytotoxicity is known as ADCC [86]. In ADCC, antibody-labelled cells are more susceptible to being destroyed by Fc receptors (FcR) expressing immune cells [87]. NK cells are particularly efficient in this response via the Fcγ receptors [87]. Neutrophils also participate in ADCC in several types of cancers, including glioma, squamous cells, breast cancer, and ovarian carcinoma [88,89]. Neutrophil ADCC is thought to be caused by both ROS-dependent and ROS-independent pathways. Several Fc receptors are expressed by neutrophils, including CD 16a (FcγRIIIa), CD 16b (FcγRIIIb), CD 32a (FcγRIIa), and CD 64 (FcγRI), all of which can mediate ADCC [90,91]. When neutrophils’ FcγRIIa are stimulated with IFN-γ and G-CSF, they can upregulate FcRI (CD 64) expression. And when neutrophils engage with FcRIIa, they exhibit more efficient ADCC, particularly in squamous head and neck cancer [91].
Neutrophils in Regulation of T-cell
Nasopharyngeal cancer (NPC) is characterized by a significant infiltration of immune cells in the tumor microenvironment (TME), primarily T-lymphocytes [92]. Neutrophils are a specific type of cells involved in antigen presentation and the recruitment of other immune cells to the site of infection. Neutrophils also enhance anti-tumor immunity in various ways. For example, an experiment involving CD 8+ T cell neutrophils demonstrated that tumor-infiltrating neutrophils can modify DCs and T cell effector functions to effectively eliminate cancer cells [93,94]. While some studies suggest that pro-tumor neutrophils can suppress T cell function, others argue that neutrophil-secreted cytokines such as Cathepsin G, TNF, and neutrophil elastase are crucial for tumor-specific memory and CD 8+ T cell proliferation [95]. In fact, substantial evidence shows that pro-inflammatory N1 neutrophils attract and activate CD 8+ T cells for primary tumor cytotoxicity and anti-tumor activity [96]. Another distinguishing feature of neutrophils is the presence of NETs, which are composed of chromatin fibers loaded with histones and other proteins. NETs are considered an important tool in neutrophil antibacterial properties, and they may also contribute to the stimulation of T cells and the promotion of anti-tumor immune responses and malignancy [86,97].
The impact of MDSCs on T cell-mediated immune responses can greatly affect the success of cancer treatment, infection management, and transplantation procedures. While initially believed to be immature myeloid cells from the bone marrow (monocytic or granulocytic MDSCs), recent studies have shown that mature neutrophils can also exhibit MDSC activity towards T cells, although the mechanisms behind this are still unclear [98]. Research by Millrud et al. and Aarts et al. has shown that neutrophils from both healthy donors and cancer patients do not display MDSC activity unless triggered [79,99]. Additionally, Aarts et al. also found that granule-derived constituents and ROS are necessary for MDSC activity through direct CD 11 b-dependent interaction between neutrophils and T cells [99]. Furthermore, neutrophils have been associated with trogocytosis, the ingestion of T cell membrane components resulting in alterations to mitochondrial function, T cell morphology, and ATP depletion [86,100].
Immunotherapeutic and Clinical Trials in HNSCC
HNSCC has a high recurrence rate and limited treatment options. For locally advanced cancer, surgery followed by adjuvant concurrent chemoradiation (CCRT) is the preferred therapy. Patients with recurrent cancer and distant metastases have few therapeutic choices, with a grim prognosis and overall survival (OS) of less than one year. Immunotherapy has emerged as a promising treatment option for head and neck cancer. Several clinical trials have been conducted to assess the efficacy and safety of multiple immunotherapeutic agents, including immune checkpoint inhibitors, cancer vaccinations, adoptive cell transfer, and oncolytic viruses. Platinum-based chemotherapy and cetuximab, a monoclonal antibody that targets EGFR, have been shown to increase HNSCC response rates [101]. Immunological checkpoints can be bypassed to revitalize immune cells and eradicate cancer cells, as immune escape is associated with the persistence of cancer cells in the tumor microenvironment (TME) [102]. CTLA-4 antibodies, such as ipilimumab and tremelimumab, bind to the CTLA-4 protein on T cells and enhance cytotoxic responses against cancer cells. PD-1 antibodies including nivolumab and pembrolizumab block the PD-1 receptor on immune cells, allowing them to recognize and destroy cancer cells. PD-L1 antibodies, such as avelumab, atezolizumab, cemiplimab, dostarlimab, and durvalumab, also inhibit the PD-L1 proteins on the surface of cancer cells, preventing them from inhibiting the immune response. These therapeutic interventions have shown significant potential in the treatment of various cancers and are now available for end-stage cancers [103,104]. A subgroup of rectal cancer with mismatch repair deficiency has shown a high degree of sensitivity to PD-1 inhibition (NCT04165772). Cercek et al. conducted a six-month trial of dostarlimab and anti-PD-1 monoclonal antibodies on 18 rectal cancer patients with stage II to III mismatch repair deficit, followed by standard chemoradiotherapy [105]. All 12 patients who received treatment showed a complete clinical response, with no evidence of malignancy on evaluation with magnetic resonance imaging, endoscopy, 18 F-fluorodeoxyglucose-positron-emission tomography, biopsy, or digital rectal examination [104]. The EGFR pathway plays a crucial role in the growth signaling of HNSCC. Clinical investigations have revealed that combining platinum-based therapy with cetuximab (EGFR monoclonal antibody) enhances HNSCC patient survival [105]. The EGFR pathway not only inhibits tumor growth but also improves the tumor microenvironment by enhancing DC function, T cell function, and the production of MHC in cancer cells [106]. TGF-β is a cytokine that plays a significant role in TME. Depending on the stage of cancer, it can have both tumor-promoting and tumor-suppressive functions. However, TGF-β has been observed to stimulate angiogenesis, tumorigenesis, and metastasis in cancer by suppressing the immune system [107]. It reduces T cell cytotoxicity while increasing regulatory T cell suppression, leading to immune evasion by cancer cells. Targeting TGF-β signaling has shown promise as a therapeutic strategy in various cancers, including HNSCC. Preclinical studies have demonstrated that blocking TGF-β signaling can enhance the anti-tumor immune response and improve the efficacy of immunotherapy [108]. Clinical trials are currently underway to evaluate the safety and efficacy of TGF-β inhibitors in combination with immunotherapy for HNSCC patients [109]. In addition to immunotherapy, targeted therapies have also shown potential in the treatment of HNSCC. EGFR tyrosine kinase inhibitors (TKIs) have been developed to specifically target the EGFR pathway and inhibit tumor growth. These TKIs, such as erlotinib and gefitinib, have shown modest clinical activity in HNSCC patients, particularly in those with EGFR overexpression or mutations [110]. However, the efficacy of TKIs as monotherapy is limited, and combination strategies are being explored to improve treatment outcomes. One such combination being investigated is the use of EGFR TKIs in combination with immune checkpoint inhibitors. The rationale behind this combination is that EGFR TKIs can induce tumor cell cytolysis and release tumor antigens, which can then stimulate antigen-specific T cells. By combining EGFR TKIs with immune checkpoint inhibitors, it is hoped that the anti-tumor immune response can be further enhanced, leading to improved treatment outcomes. A Phase 1 trial (NCT02088112) evaluated the safety and efficacy of the combination of gefitinib (an EGFR inhibitor) with durvalumab (an immune checkpoint inhibitor) in TKI-naive patients with advanced EGFR-mutant or L858R-mutant NSCLC [111]. The trial involved 56 patients with NSCLC, with dose expansion allowing TKI-naive patients with activating L858R mutation. However, this study did not show any synergistic efficacy of the combination and actually resulted in a higher incidence of adverse events than expected. Liver-related adverse events, such as transaminitis, were particularly high with the combination treatment, leading to treatment discontinuation in more than 25% of patients. This could potentially affect the effectiveness of the EGFR inhibitor [111].
Conclusion
HNSCC is a diverse malignancy with various etiologies, risk factors, and therapeutic options. In this review, we focused on evaluating the pro and antitumor role of neutrophil cells in the HNC microenvironment, including their functions in angiogenesis, metastasis, tumor invagination, and malignancy establishment. Pro-tumor neutrophil cells in HNC facilitate angiogenesis, promote metastasis, assist in tumor invagination and malignancy establishment, and promote MET. Additionally, antitumor neutrophils modify T cells and DC cell effector functions to effectively kill cancer cells, stimulate anti-tumor immunity, and generate tumor-specific memory and CD 8+ T cell proliferation. Besides that, immune surveillance failure is a primary cause of oncogenesis, including HNSCC. To evade the immune system, tumor cells release a range of cytokines and other chemicals. The TEM transforms the nature of immune cells, making them their own helpers in the process of oncogenesis. Immune cells initially constrain tumor growth but subsequently promote carcinogenesis. However, in HNSCC, neutrophil cells play a complex and contradictory role. The pro- and antitumor action of neutrophils is elusive and varies depending on the type of cancer cell. Numerous studies have demonstrated that immunotherapy is the most effective treatment approach for HNSCC. Several immunotherapy medications, including nivolumab (anti-PD1 antibodies), ipilimumab (anti-CTL4 antibodies), cetuximab, and zalutumumab, have been tested in clinical trials for HNSCC and have shown promising outcomes. The success of dostarlimab in rectal cancer has also opened up new treatment options for cancer that can be explored in HNSCC. Therefore, a comprehensive understanding of the pathophysiology of HNSCC and the involvement of inflammatory cells in HNSCC may pave the way for the development of innovative immunotherapeutic medications. Future research should focus on improving outcomes in HNSCC patients by combining frontline therapy with immunotherapy that is effective in relapsed situations while having fewer adverse effects. In conclusion, immunotherapy and targeted therapies have shown promise in the treatment of HNSCC. Immune checkpoint inhibitors, such as PD-1 and PD-L1 antibodies, have demonstrated significant clinical activity in HNSCC patients, leading to improved response rates and survival outcomes. However, challenges remain, including identifying predictive biomarkers to guide patient selection and optimizing combination strategies to enhance treatment efficacy. Further research and clinical trials are needed to fully understand the potential of these therapies and their optimal use in HNSCC treatment.
Conflict of Interest
Authors have no conflicts of interest.
Funding Statement
Council of Scientific & Industrial Research (CSIR), New Delhi, India.
Acknowledgments
Authors would like to thank all lab members at the Department of Biochemistry, All India Institute of Medical Sciences (AIIMS), New Delhi, India for their valuable comments, corrections, and help.
References
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians. 2021 May;71(3):209-49.
3. Bhattacharjee A, Chakraborty A, Purkaystha P. Prevalence of head and neck cancers in the north east—an institutional study. Indian Journal of Otolaryngology and Head and Neck Surgery. 2006 Jan;58:15-9.
4. Johnson DE, Burtness B, Leemans CR, Lui VW, Bauman JE, Grandis JR. Head and neck squamous cell carcinoma. Nature Reviews Disease Primers. 2020 Nov 26;6(1):92.
5. Patel EJ, Oliver JR, Jacobson AS, Li Z, Hu KS, Tam M, et al. Human papillomavirus in patients with hypopharyngeal squamous cell carcinoma. Otolaryngology–Head and Neck Surgery. 2022 Jan;166(1):109-17.
6. Qiu F, Liang CL, Liu H, Zeng YQ, Hou S, Huang S, et al. Impacts of cigarette smoking on immune responsiveness: Up and down or upside down?. Oncotarget. 2017 Jan 1;8(1):268.-284.
7. Jassem J. Tobacco smoking after diagnosis of cancer: clinical aspects. Translational lung cancer research. 2019 May;8(Suppl 1):S50-S58.
8. Lohia N, Bhatnagar S, Singh S, Prashar M, Subramananiam A, Viswanath S, et al. Survival trends in oral cavity cancer patients treated with surgery and adjuvant radiotherapy in a tertiary center of Northern India: Where do we stand compared to the developed world?. SRM Journal of Research in Dental Sciences. 2019 Jan 1;10(1):26.
9. Brouwer AF, Campredon LP, Walline HM, Marinelli BM, Goudsmit CM, Thomas TB, et al. Prevalence and determinants of oral and cervicogenital HPV infection: Baseline analysis of the Michigan HPV and Oropharyngeal Cancer (MHOC) cohort study. Plos One. 2022 May 16;17(5):e0268104.
10. Stein AP, Saha S, Kraninger JL, Swick AD, Yu M, Lambertg PF, et al. Prevalence of human papillomavirus in oropharyngeal cancer: a systematic review. Cancer Journal (Sudbury, Mass.). 2015 May;21(3):138.
11. Venkateshulu S, Br KK. A Study Comparing Acute Toxicities of Cetuximab and Cisplatin in Patients Undergoing Definitive Chemoradiation With Intensity-Modulated Radiotherapy for Locally Advanced Carcinoma Head and Neck. Cureus. 2021 Jul 20;13(7).
12. Pinkbook. 2023 [cited 2023 Jun 17]. Available from: https://www.cdc.gov/vaccines/pubs/pinkbook/hpv.html
13. Iwasaki A, Medzhitov R. Control of adaptive immunity by the innate immune system. Nature Immunology. 2015 Apr;16(4):343-53.
14. Pandya PH, Murray ME, Pollok KE, Renbarger JL. The immune system in cancer pathogenesis: potential therapeutic approaches. Journal of Immunology Research. 2016 Oct; 2016:1-13.
15. Ribatti D. The concept of immune surveillance against tumors: The first theories. Oncotarget. 2017 Jan 1;8(4):7175-80.
16. Gonzalez H, Hagerling C, Werb Z. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes and Development. 2018 Oct 1;32(19-20):1267-84.
17. Sayour EJ, Mitchell DA. Manipulation of innate and adaptive immunity through cancer vaccines. Journal of Immunology Research. 2017 Feb 6;2017.1-7.
18. Witsch E, Sela M, Yarden Y. Roles for growth factors in cancer progression. Physiology. 2010 Apr;25(2):85-101.
19. Du Z, Lovly CM. Mechanisms of receptor tyrosine kinase activation in cancer. Molecular Cancer. 2018 Dec;17:1-3.
20. Zhang X, Nie D, Chakrabarty S. Growth factors in tumor microenvironment. Frontiers in Bioscience:A Journal and Virtual Library. 2010 Jan 1;15:151.
21. Metibemu DS, Akinloye OA, Akamo AJ, Ojo DA, Okeowo OT, Omotuyi IO. Exploring receptor tyrosine kinases-inhibitors in Cancer treatments. Egyptian Journal of Medical Human Genetics. 2019 Dec;20(1):1-6.
22. Sebestyén A, Kopper L, Dankó T, Tímár J. Hypoxia signaling in cancer: from basics to clinical practice. Pathology & Oncology Research. 2021 May 3;27:1609802.
23. Aguilar-Cazares D, Chavez-Dominguez R, Carlos-Reyes A, Lopez-Camarillo C, Hernadez de la Cruz ON, Lopez-Gonzalez JS. Contribution of angiogenesis to inflammation and cancer. Frontiers in Oncology. 2019 Dec 12;9:1399.
24. Emami Nejad A, Najafgholian S, Rostami A, Sistani A, Shojaeifar S, Esparvarinha M, et al. The role of hypoxia in the tumor microenvironment and development of cancer stem cell: a novel approach to developing treatment. Cancer Cell International. 2021 Dec;21(1):1-26.
25. Tan Z, Xue H, Sun Y, Zhang C, Song Y, Qi Y. The role of tumor inflammatory microenvironment in lung cancer. Frontiers in Pharmacology. 2021 May 17;12:688625.
26. Vinay DS, Ryan EP, Pawelec G, Talib WH, Stagg J, Elkord E, Lichtor T, Decker WK, Whelan RL, Kumara HS, Signori E. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Seminars in Cancer Biology. 2015 Dec 1;35:S185-S198.
27. Jin J, Lin J, Xu A, Lou J, Qian C, Li X, et al. CCL2: an important mediator between tumor cells and host cells in tumor microenvironment. Frontiers in Oncology. 2021 Jul 27;11:722916.
28. Han Y, Liu D, Li L. PD-1/PD-L1 pathway: current researches in cancer. American Journal of Cancer Research. 2020;10(3):727.
29. Kartikasari AE, Huertas CS, Mitchell A, Plebanski M. Tumor-induced inflammatory cytokines and the emerging diagnostic devices for cancer detection and prognosis. Frontiers in Oncology. 2021 Jul 7;11:692142.
30. Batlle E, Massagué J. Transforming growth factor-β signaling in immunity and cancer. Immunity. 2019 Apr 16;50(4):924-40.
31. Mayadas TN, Cullere X, Lowell CA. The multifaceted functions of neutrophils. Annual Review of Pathology: Mechanisms of Disease. 2014 Jan 24;9:181-218.
32. Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nature Reviews Immunology. 2018 Feb;18(2):134-47.
33. Scapini P, Cassatella MA. Social networking of human neutrophils within the immune system. Blood, The Journal of the American Society of Hematology. 2014 Jul 31;124(5):710-9.
34. Mishalian I, Granot Z, Fridlender ZG. The diversity of circulating neutrophils in cancer. Immunobiology. 2017 Jan 1;222(1):82-8.
35. Sagiv JY, Michaeli J, Assi S, Mishalian I, Kisos H, Levy L, et al. Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Reports. 2015 Feb 3;10(4):562-73.
36. Masucci MT, Minopoli M, Carriero MV. Tumor associated neutrophils. Their role in tumorigenesis, metastasis, prognosis and therapy. Frontiers in Oncology. 2019 Nov 15;9:1146.
37. Wu CF, Andzinski L, Kasnitz N, Kröger A, Klawonn F, Lienenklaus S, et al. The lack of type I interferon induces neutrophil‐mediated pre‐metastatic niche formation in the mouse lung. International Journal of Cancer. 2015 Aug 15;137(4):837-47.
38. Rao HL, Chen JW, Li M, Xiao YB, Fu J, Zeng YX, et al. Increased intratumoral neutrophil in colorectal carcinomas correlates closely with malignant phenotype and predicts patients' adverse prognosis. PloS One. 2012 Jan 25;7(1):e30806.
39. Li YW, Qiu SJ, Fan J, Zhou J, Gao Q, Xiao YS, et al. Intratumoral neutrophils: a poor prognostic factor for hepatocellular carcinoma following resection. Journal of Hepatology. 2011 Mar 1;54(3):497-505.
40. Ino Y, Yamazaki-Itoh R, Shimada K, Iwasaki M, Kosuge T, Kanai Y, et al. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. British Journal of Cancer. 2013 Mar;108(4):914-23.
41. Trellakis S, Bruderek K, Dumitru CA, Gholaman H, Gu X, Bankfalvi A, et al. Polymorphonuclear granulocytes in human head and neck cancer: enhanced inflammatory activity, modulation by cancer cells and expansion in advanced disease. International Journal of Cancer. 2011 Nov 1;129(9):2183-93.
42. Ku JY, Roh JL, Kim SB, Choi SH, Nam SY, Kim SY. Prognostic value of neutrophil-to-lymphocyte ratio in older patients with head and neck cancer. The Journal of Geriatric Oncology. 2020; 11 (3): 417-22.
43. Werner J, Strobel K, Lehnick D, Rajan GP. Overall neutrophil-to-lymphocyte ratio and SUVmax of nodal metastases predict outcome in head and neck cancer before Chemoradiation. Frontiers in Oncology. 2021 Oct 8;11:679287.
44. Greene S, Robbins Y, Mydlarz WK, Huynh AP, Schmitt NC, Friedman J, et al. Inhibition of MDSC trafficking with SX-682, a CXCR1/2 inhibitor, enhances NK-cell immunotherapy in head and neck cancer models. Clinical Cancer Research. 2020 Mar 15;26(6):1420-31.
45. Pylaeva E, Harati MD, Spyra I, Bordbari S, Strachan S, Thakur BK, et al. NAMPT signaling is critical for the proangiogenic activity of tumor-associated neutrophils. International Journal of Cancer. 2019 Jan 1;144(1):136-49.
46. Lonardi S, Missale F, Calza S, Bugatti M, Vescovi R, Debora B, et al. Tumor‐associated neutrophils (TANs) in human carcinoma‐draining lymph nodes: a novel TAN compartment. Clinical & Translational Immunology. 2021;10(2):e1252.
47. Londero F, Grossi W, Parise O, Cinel J, Parise G, Masullo G, et al. The Impact of Preoperative Inflammatory Markers on the Prognosis of Patients Undergoing Surgical Resection of Pulmonary Oligometastases. Journal of Clinical Medicine. 2020 Oct 21;9(10):3378.
48. Houghton AM, Rzymkiewicz DM, Ji H, Gregory AD, Egea EE, Metz HE, Stolz DB, Land SR, Marconcini LA, Kliment CR, Jenkins KM. Neutrophil elastase–mediated degradation of IRS-1 accelerates lung tumor growth. Nature Medicine. 2010 Feb;16(2):219-23.
49. Lerman I, Hammes SR. Neutrophil elastase in the tumor microenvironment. Steroids. 2018 May 1;133:96-101.
50. Kerros C, Tripathi SC, Zha D, Mehrens JM, Sergeeva A, Philips AV, et al. Neuropilin-1 mediates neutrophil elastase uptake and cross-presentation in breast cancer cells. Journal of Biological Chemistry. 2017 Jun 1;292(24):10295-305.
51. Cully M. A protease with far-reaching anti-cancer effects. Nature reviews. Drug Discovery. 2021 Jul 1;20(7):505.
52. Cui C, Chakraborty K, Tang XA, Zhou G, Schoenfelt KQ, Becker KM, et al. Neutrophil elastase selectively kills cancer cells and attenuates tumorigenesis. Cell. 2021 Jun 10;184(12):3163-77.
53. Peters HL, Tripathi SC, Kerros C, Katayama H, Garber HR, St. John LS, et al. Serine proteases enhance immunogenic antigen presentation on lung cancer cells. Cancer Immunology Research. 2017 Apr 1;5(4):319-29.
54. Xiao Y, Cong M, Li J, He D, Wu Q, Tian P, et al. Cathepsin C promotes breast cancer lung metastasis by modulating neutrophil infiltration and neutrophil extracellular trap formation. Cancer Cell. 2021 Mar 8;39(3):423-37.
55. Weinberg F, Ramnath N, Nagrath D. Reactive oxygen species in the tumor microenvironment: an overview. Cancers. 2019 Aug 16;11(8):1191.
56. Quintero-Fabián S, Arreola R, Becerril-Villanueva E, Torres-Romero JC, Arana-Argáez V, Lara-Riegos J, et al. Role of matrix metalloproteinases in angiogenesis and cancer. Frontiers in Oncology. 2019 Dec 6;9:1370.
57. Güngör N, Knaapen AM, Munnia A, Peluso M, Haenen GR, Chiu RK, et al. Genotoxic effects of neutrophils and hypochlorous acid. Mutagenesis. 2010 Mar 1;25(2):149-54.
58. Güngör N, Knaapen AM, Munnia A, Peluso M, Haenen GR, Chiu RK, et al. Genotoxic effects of neutrophils and hypochlorous acid. Mutagenesis. 2010 Mar 1;25(2):149-54.
59. Trellakis S, Farjah H, Bruderek K, Dumitru CA, Hoffmann TK, Lang S, et al. Peripheral blood neutrophil granulocytes from patients with head and neck squamous cell carcinoma functionally differ from their counterparts in healthy donors. International Journal of Immunopathology and Pharmacology. 2011 Jul;24(3):683-93.
60. Szuster-Ciesielska A, Hryciuk-Umer E, Stepulak A, Kupisz K, Kandefer-Szerszeń M. Reactive oxygen species production by blood neutrophils of patients with laryngeal carcinoma and antioxidative enzyme activity in their blood. Acta Oncologica. 2004 Apr 1;43(3):252-8.
61. Grzywa TM, Sosnowska A, Matryba P, Rydzynska Z, Jasinski M, Nowis D, et al. Myeloid cell-derived arginase in cancer immune response. Frontiers in Immunology. 2020 May 15;11:938.
62. Adua E, Oteng Danso F, Mensah Boa-Amponsem O, Adusei-Mensah F. Effect of neutrophils on nitric oxide production from stimulated macrophages. Iranian Journal of Immunology. 2015;12(2):94-103.
63. Roles of Nitric Oxide and Superoxide in Inflammation | Springer Nature Experiments. Springernature.com. 2022 [cited 2023 Jun 17].
64. Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nature Reviews Immunology. 2005 Aug 1;5(8):641-54.
65. Mishalian I, Bayuh R, Eruslanov E, Michaeli J, Levy L, Zolotarov L, et al. Neutrophils recruit regulatory T‐cells into tumors via secretion of CCL17—a new mechanism of impaired antitumor immunity. International Journal of Cancer. 2014 Sep 1;135(5):1178-86.
66. Bergenfelz C, Leandersson K. The generation and identity of human myeloid-derived suppressor cells. Frontiers in Oncology. 2020 Feb 7;10:109.
67. Korkmaz B, Moreau T, Gauthier F. Neutrophil elastase, proteinase 3 and cathepsin G: physicochemical properties, activity and physiopathological functions. Biochimie. 2008 Feb 1;90(2):227-42.
68. Coffelt SB, Kersten K, Doornebal CW, Weiden J, Vrijland K, Hau CS, et al. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015 Jun 18;522(7556):345-8.
69. Khou S, Popa A, Luci C, Bihl F, Meghraoui-Kheddar A, Bourdely P, et al. Tumor-associated neutrophils dampen adaptive immunity and promote cutaneous squamous cell carcinoma development. Cancers. 2020 Jul 10;12(7):1860.
70. Peltanova B, Raudenska M, Masarik M. Effect of tumor microenvironment on pathogenesis of the head and neck squamous cell carcinoma: a systematic review. Molecular Cancer. 2019 Dec;18(1):1-24.
71. Zhao H, Ma Y, Zhang L. Low‐molecular‐mass hyaluronan induces pulmonary inflammation by up‐regulation of Mcl‐1 to inhibit neutrophil apoptosis via PI 3K/Akt1 pathway. Immunology. 2018 Nov;155(3):387-95.
72. Donato C, Kunz L, Castro-Giner F, Paasinen-Sohns A, Strittmatter K, Szczerba BM, et al. Hypoxia triggers the intravasation of clustered circulating tumor cells. Cell Reports. 2020 Sep 8;32(10).
73. Baj J, Brzozowska K, Forma A, Maani A, Sitarz E, Portincasa P. Immunological aspects of the tumor microenvironment and epithelial-mesenchymal transition in gastric carcinogenesis. International Journal of Molecular Sciences. 2020 Apr 6;21(7):2544.
74. Fridlender ZG, Albelda SM. Tumor-associated neutrophils: friend or foe?. Carcinogenesis. 2012 May 1;33(5):949-55.
75. Yu T, Tang Q, Chen X, Fan W, Zhou Z, Huang W, et al. TGF‐β1 and IL‐17A comediate the protumor phenotype of neutrophils to regulate the epithelial‐mesenchymal transition in oral squamous cell carcinoma. Journal of Oral Pathology & Medicine. 2021 Apr;50(4):353-61.
76. Hsu BE, Shen Y, Siegel PM. Neutrophils: orchestrators of the malignant phenotype. Frontiers in Immunology. 2020 Aug 11;11:1778.
77. Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. The Journal of Clinical Investigation. 2013 Aug 1;123(8):3446-58.
78. Wang Z, Yang C, Li L, Jin X, Zhang Z, Zheng H, et al. Tumor-derived HMGB1 induces CD62Ldim neutrophil polarization and promotes lung metastasis in triple-negative breast cancer. Oncogenesis. 2020 Sep 17;9(9):82.
79. Millrud CR, Kågedal Å, Kumlien Georén S, Winqvist O, Uddman R, Razavi R, et al. NET‐producing CD16high CD62Ldim neutrophils migrate to tumor sites and predict improved survival in patients with HNSCC. International Journal of Cancer. 2017 Jun 1;140(11):2557-67.
80. Peng Z, Liu C, Victor AR, Cao DY, Veiras LC, Bernstein EA, et al. Tumors exploit CXCR4hiCD62Llo aged neutrophils to facilitate metastatic spread. Oncoimmunology. 2021 Jan 1;10(1):1870811.
81. Lavender N, Yang J, Chen SC, Sai J, Johnson CA, Owens P, et al. The Yin/Yan of CCL2: a minor role in neutrophil anti-tumor activity in vitro but a major role on the outgrowth of metastatic breast cancer lesions in the lung in vivo. BMC Cancer. 2017 Dec;17(1):1-5.
82. Kotsafti A, Scarpa M, Castagliuolo I, Scarpa M. Reactive oxygen species and antitumor immunity—from surveillance to evasion. Cancers. 2020 Jul 1;12(7):1748.
83. Kennel KB, Greten FR. Immune cell-produced ROS and their impact on tumor growth and metastasis. Redox Biology. 2021 Jun 1;42:101891.
84. Srinivas US, Tan BW, Vellayappan BA, Jeyasekharan AD. ROS and the DNA damage response in cancer. Redox Biology. 2019 Jul 1;25:101084.
85. Ustyanovska Avtenyuk N, Visser N, Bremer E, Wiersma VR. The neutrophil: The underdog that packs a punch in the fight against cancer. International Journal of Molecular Sciences. 2020 Oct 22;21(21):7820.
86. Tay MZ, Wiehe K, Pollara J. Antibody-dependent cellular phagocytosis in antiviral immune responses. Front Immunol 10: 332.
87. Furumaya C, Martinez-Sanz P, Bouti P, Kuijpers TW, Matlung HL. Plasticity in pro-and anti-tumor activity of neutrophils: shifting the balance. Frontiers in Immunology. 2020 Sep 2;11:2100.
88. Treffers LW, Hiemstra IH, Kuijpers TW, Van den Berg TK, Matlung HL. Neutrophils in cancer. Immunological Reviews. 2016 Sep;273(1):312-28.
89. Wang Y, Jönsson F. Expression, role, and regulation of neutrophil Fcγ receptors. Frontiers in Immunology. 2019 Aug 27;10:1958.
90. Brandsma AM, Bondza S, Evers M, Koutstaal R, Nederend M, Jansen JM, Rösner T, Valerius T, Leusen JH, Ten Broeke T. Potent Fc receptor signaling by IgA leads to superior killing of cancer cells by neutrophils compared to IgG. Frontiers in Immunology. 2019 Apr 11;10:704.
91. Oberg HH, Wesch D, Kalyan S, Kabelitz D. Regulatory interactions between neutrophils, tumor cells and T cells. Frontiers in Immunology. 2019 Jul 18;10:1690.
92. Yang L, Liu G, Li Y, Pan Y. The emergence of tumor-infiltrating lymphocytes in nasopharyngeal carcinoma: Predictive value and immunotherapy implications. Genes & Diseases. 2022 Sep 1;9(5):1208-19.
93. Brostjan C, Oehler R. The role of neutrophil death in chronic inflammation and cancer. Cell Death Discovery. 2020 Apr 22;6(1):26.
94. Raskov H, Orhan A, Christensen JP, Gögenur I. Cytotoxic CD8+ T cells in cancer and cancer immunotherapy. British Journal of Cancer. 2021 Jan 19;124(2):359-67.
95. Herrero-Cervera A, Soehnlein O, Kenne E. Neutrophils in chronic inflammatory diseases. Cellular & Molecular Immunology. 2022 Feb;19(2):177-91.
96. Heemskerk N, van Egmond M. Monoclonal antibody-mediated killing of tumour cells by neutrophils. European Journal of Clinical Investigation. 2018 Nov;48:e12962.
97. Minns D, Smith KJ, Hardisty G, Rossi AG, Gwyer Findlay E. The outcome of neutrophil-T cell contact differs depending on activation status of both cell types. Frontiers in Immunology. 2021 Mar 30;12:633486.
98. Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nature Reviews Immunology. 2009 Mar;9(3):162-74.
99. Aarts CE, Hiemstra IH, Béguin EP, Hoogendijk AJ, Bouchmal S, van Houdt M, et al. Activated neutrophils exert myeloid-derived suppressor cell activity damaging T cells beyond repair. Blood Advances. 2019 Nov 26;3(22):3562-74.
100. Zhao S, Zhang L, Xiang S, Hu Y, Wu Z, Shen J. Gnawing between cells and cells in the immune system: friend or foe? A review of trogocytosis. Frontiers in Immunology. 2022 Feb 3;13:791006.
101. Lee JB, Ha SJ, Kim HR. Clinical insights into novel immune checkpoint inhibitors. Frontiers in Pharmacology. 2021 May 6;12:681320.
102. Darvin P, Toor SM, Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Experimental & Molecular Medicine. 2018 Dec;50(12):1-1.
103. Szturz P, Vermorken JB. Systemic Treatment Sequencing and Prediction of First-line Therapy Outcomes in Recurrent or Metastatic Head and Neck Cancer. InCritical Issues in Head and Neck Oncology: Key Concepts from the Eighth THNO Meeting 2023 Mar 28 (pp. 199-215).
104. Cercek A, Lumish M, Sinopoli J, Weiss J, Shia J, Lamendola-Essel M, et al. PD-1 blockade in mismatch repair–deficient, locally advanced rectal cancer. New England Journal of Medicine. 2022 Jun 23;386(25):2363-76.
105. Kriegs M, Clauditz TS, Hoffer K, Bartels J, Buhs S, Gerull H, et al. Analyzing expression and phosphorylation of the EGF receptor in HNSCC. Scientific Reports. 2019 Sep 19;9(1):13564.
106. Matsumoto Y, Sawa K, Fukui M, Oyanagi J, Izumi M, Ogawa K, et al. Impact of tumor microenvironment on the efficacy of epidermal growth factor receptor‐tyrosine kinase inhibitors in patients with EGFR‐mutant non‐small cell lung cancer. Cancer Science. 2019 Oct;110(10):3244-54.
107. Batlle E, Massagué J. Transforming growth factor-β signaling in immunity and cancer. Immunity. 2019 Apr 16;50(4):924-40.
108. Lan Y, Zhang D, Xu C, Hance KW, Marelli B, Qi J, et al. Enhanced preclinical antitumor activity of M7824, a bifunctional fusion protein simultaneously targeting PD-L1 and TGF-β. Science Translational Medicine. 2018 Jan 17;10(424):eaan5488.
109. Massarelli E, William W, Johnson F, Kies M, Ferrarotto R, Guo M, Feng L, Lee JJ, Tran H, Kim YU, Haymaker C. Combining immune checkpoint blockade and tumor-specific vaccine for patients with incurable human papillomavirus 16–related cancer: a phase 2 clinical trial. JAMA Oncology. 2019 Jan 1;5(1):67-73.
110. Cohen EE, Harrington KJ, Hong DS, Mesia R, Brana I, Segura PP, et al. A phase Ib/II study (SCORES) of durvalumab (D) plus danvatirsen (DAN; AZD9150) or AZD5069 (CX2i) in advanced solid malignancies and recurrent/metastatic head and neck squamous cell carcinoma (RM-HNSCC): Updated results. Annals of Oncology. 2018 Oct 1;29:viii372.
111. Creelan BC, Yeh TC, Kim SW, Nogami N, Kim DW, Chow LQ, et al. A Phase 1 study of gefitinib combined with durvalumab in EGFR TKI-naive patients with EGFR mutation-positive locally advanced/metastatic non-small-cell lung cancer. British Journal of Cancer. 2021 Jan 19;124(2):383-90.
