Abstract
This study was conducted to validate the efficacy of therapeutic plasma exchange (TPE) in reducing the excessive cytokine load complicating a subset of patients with severe COVID-19 and respiratory compromise. Additionally, this trial explored molecular signals of potential benefit by the addition of JAK inhibition to TPE. Findings included an improvement in cytokine excess and oxygenation along with incremental benefit in cytokine reduction with the addition of ruxolitinib. While exploratory in nature, this study adds momentum to the pursuit of larger randomized trials.
Keywords
COVID-19, Therapeutic plasma exchange, Cytokines, Respiratory failure, Clinical trial, JAK inhibition
Introduction
Observations early in the viral pandemic of 2020 noted the resemblance between severe coronavirus disease 2019 (COVID-19) infection and the hypercytokinemic state of secondary hemophagocytic lymphohistiocytosis (sHLH) [1]. Conti and colleagues [2] have outlined the binding of COVID-19 to the Toll-like receptor with subsequent cytokine driven fever and pneumonitis, while in Kenderian’s [3] murine model of CRS, ruxolitinib abrogated the development of cytokine excess. Cao [4] reported faster clinical recovery with JAK inhibition and Capochiani’s RESPIRE trial reported an 89% overall response rate (ORR) with ruxolitinib therapy [5]. Discordant findings regarding JAK inhibition have been reported on the superiority of baricitinib plus remdesivir over remdesivir alone in shortening recovery times in hospitalized patients [6] while the randomized RUXCOVID trial failed to impact severe complications of the disease by adding ruxolitinib to standard of care [7]. Other groups [8,9] have reported inflammatory cytokine profiles along with evidence that elevated IL-6 and TNFα are strong predictors of disease severity and survival. While cytokine excess could be an epiphenomenal surrogate for another process, therapeutic strategies have evolved around addressing the excessive cytokine levels complicating a significant fraction of COVID-19 patients.
Several case reports [10,11] coupled with a small case series [12] reported beneficial intervention with therapeutic plasma exchange (TPE) in COVID-19 patients cytokine release syndrome (CRS). Gluck et al. [8] reported a formal prospective trial of 10 patients treated early in the pandemic before glucocorticoids, antiviral agents or convalescent plasma usage became commonplace. That study mapped daily cytokine levels and demonstrated marked reduction in cytokine load, improvement in oxygenation, and enhanced clinical outcomes with TPE.The FDA approved trial (NCT04374149) reported herein was conducted to validate the previously reported efficacy of TPE in reducing cytokine excess in severely ill COVID-19 patients, to expand the mapping of cytokine signatures throughout the illness, and to evaluate the impact of the addition of ruxolitinib treatment in ameliorating cytokine excess and clinical outcomes.
Materials and Methods
Study population
Eligible patients were COVID-19 positive by polymerase chain reaction (PCR), met criteria for Penn class 3 or 4 CRS, were aged 12 to 80 and required supplemental oxygen or mechanical ventilation. Patients were excluded if pregnant, breastfeeding, categorized as Class 3 or 4 New York Heart Association heart failure, stage 4 obstructive lung disease or interstitial lung disease with chronic hypoxic respiratory failure requiring supplemental oxygen at baseline. Patients were also excluded for current use of disease-modifying anti-rheumatic drugs (with the exception of hydroxychloroquine or IL-6 inhibitors), chronic corticosteroids if in excess of prednisone 10 mg/ day or equivalent, suspected or confirmed clinically significant bacterial infection, history of tuberculosis, HIV, or irritable bowel disease, creatinine clearance of less than 15 mL/min, absolute neutrophil count less than 1000/μL, platelet count less than 50,000/μL and AST or ALT greater than five times the institutional upper limit of normal.
Trial design
The trial, as approved by the FDA and the health system’s Institutional Review Board (IRB), included two single arm cohorts of ten patients each. All patients provided informed consent. Cohort 1A underwent TPE with one plasma volume exchange daily for two consecutive days then every other day three times for a total of five exchanges using the Spectra Optia Apheresis System (Terumo BCT Inc., Lakewood, Colorado, USA) employing centrifugal blood component separation. Patients received isovolemic replacement with 5% albumin. Cohort 1B utilized the same protocol of TPE with the addition of a 14-day course of ruxolitinib at 5 mg po twice daily beginning the day prior to first TPE. These cohorts functioned as two consecutive phase 1, 2 trials performed to explore the potential value of further expansion into subsequent randomized studies. In the absence of a validated COVID-19 specific grading system for CRS, the Penn classification [13] was employed by extrapolation. Patients had to complete at least two procedures of TPE to be considered evaluable.
Study endpoints
The primary endpoint of the trial was to document the efficacy of TPE alone or TPE plus ruxolitinib in decreasing the CRP and cytokine load (IL-6, IL-8, IL-10, TNFα, IFNγ, GM-CSF) by measuring these levels daily and immediately post TPE over a 14-day period. Secondary endpoints included improvement in oxygenation and median time of mechanical ventilation. Supplemental oxygen requirements were tracked daily for non-ventilated patients. The oxygenation index (OI) was calculated daily for ventilated patients.
Laboratory assessments
CRP and fibrinogen levels were analyzed by the hospital laboratory. Cytokine assays were performed in the cancer institute research laboratory. Serum levels of IL-6, IL-8, IL-10, TNFα, GM-CSF, and IFNγ were measured using a Magnetic Luminex Performance Assay (R&D Systems) according to the manufacturer’s protocol [14]. Data were analyzed using Bio-Plex Manager Software and GraphPad Prism. COVID-19 serology was assayed at baseline, day 7 and day 14 utilizing Bio-Rad’s Platelia SARS-CoV-2 total antibody assay.
Safety
Study related adverse events were graded using NCI Common terminology criteria for adverse events (CTCAE Version 5.0). The safety analysis included all patients who underwent at least one TPE. All patients were reviewed by the study Data Safety and Monitoring Board.
Statistical analysis plans
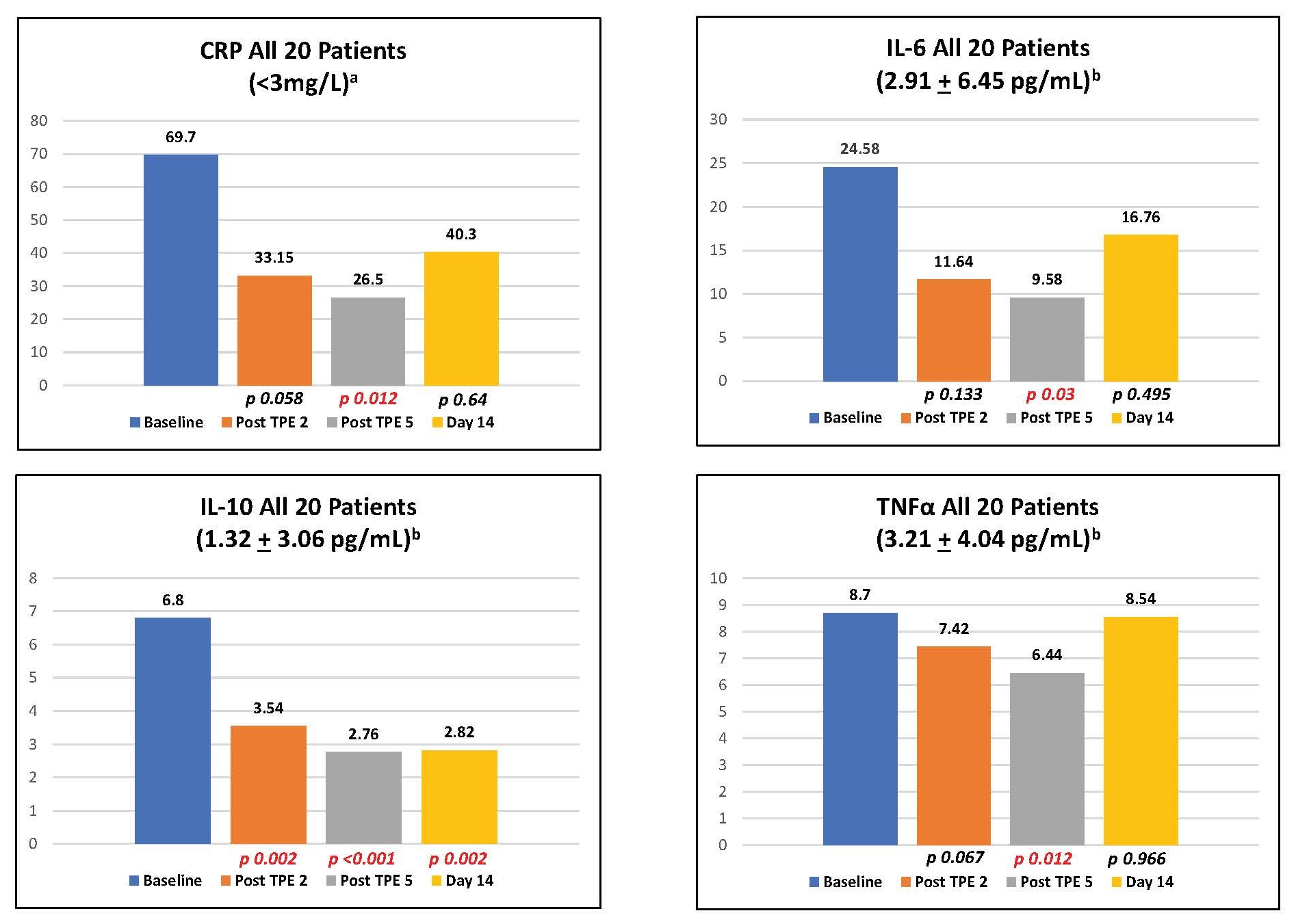
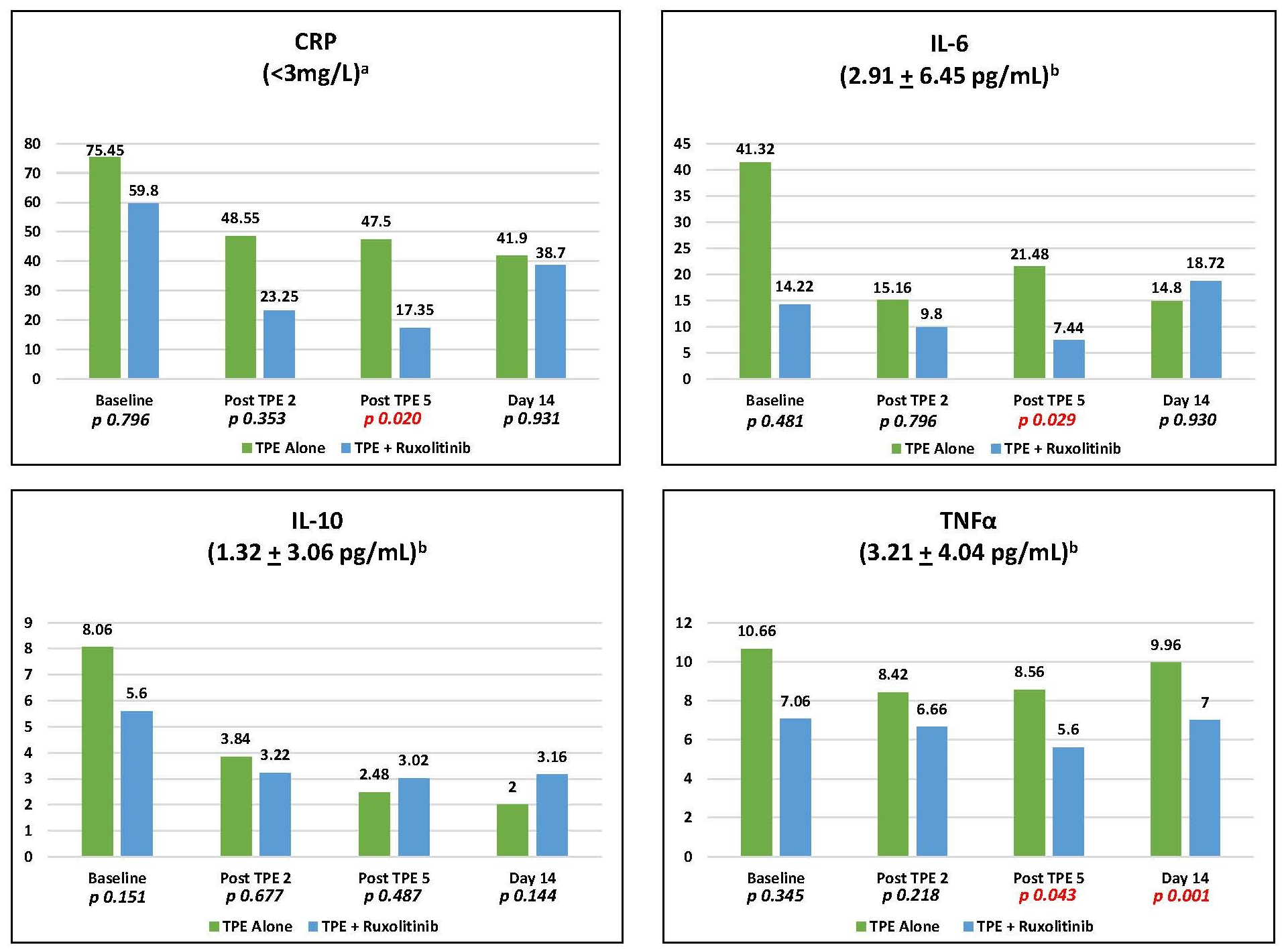
Two-sample median comparisons of CRP, IL-6, IL-10, and TNFα levels at baseline, following the second and fifth (final) plasma exchange and at day 14 for all 20 patients were performed using the Wilcoxon Signed Rank test (Figure 1). In addition, the median of CRP, IL-6, IL-10, and TNFα levels at baseline, following the second and fifth (final) plasma exchange and at day 14 were compared between cohorts 1A and 1B (Figure 2).
Results
Eligible patients were enrolled consecutively as referred from critical care services. Patient demographics and clinical characteristics are outlined in Table 1. There was one death in cohort 1A and two deaths in cohort 1B within the day 28 study window. The cohorts were remarkable for Hispanic female dominance in cohort 1A and Caucasian male dominance in cohort 1B. Additionally, the imbalance between cohorts with respect to previous or concomitant therapy reflects the greater adoption of convalescent plasma and antiviral medication over the time span of the trial. Baseline respiratory status and comorbidities were similar.
| Cohort 1A (TPE Alone) | Cohort 1B (TPE + Rux) | |
|---|---|---|
| N | 10 | 10 |
| Age, Mean ± SD | 51.8 ± 12.6 | 57.4 ± 8.8 |
| Body Mass Index (kg/m2), Mean ± SD | 34.4 ± 10.2 | 36.0 ± 5.6 |
| Gender, N (%) | ||
| Female | 7 (70) | 1 (10) |
| Male | 3 (30) | 9 (90) |
| Race/Ethnicity, N (%) | ||
| Hispanic | 6 (60) | 2 (20) |
| White | 2 (20) | 6 (60) |
| Asian | 1 (10) | 0 |
| Black | 1 (10) | 2 (20) |
| Days from COVID+ test to 1st TPE, Median (IQR) | 4.5 (3, 6) | 9 (4,12) |
| ABO Blood Group, N (%) | ||
| A- | 1 (10) | 1 (10) |
| A+ | 2 (20) | 2 (20) |
| B+ | 2 (20) | 1 (10) |
| O+ | 5 (50) | 6 (60) |
| Respiratory Status, N (%) | ||
| Penn Class 3 Nasal Cannula or High Flow Nasal Cannula |
4 (40) | 4 (40) |
| Penn Class 4 Invasive Mechanical Ventilation |
6 (60) | 6 (60) |
| Previous or Concomitant Therapy | ||
| Glucocorticoids | 9 (90) | 10 (100) |
| Convalescent Plasma | 2 (20) | 7 (70) |
| Remdesivir | 3 (30) | 8 (80) |
| Comorbidities, N (%) | ||
| Diabetes | 3 (30) | 3 (30) |
| Hypertension | 5 (50) | 5 (50) |
| Obesity | 6 (60) | 8 (80) |
TPE resulted in immediate reductions in CRP, IL-6, IL-10, and TNFα. Ruxolitinib treatment incrementally reduced CRP (p = 0.020), IL-6 (p = 0.029), and TNFα (p = 0.043) levels following the fifth TPE compared to TPE alone (Figure 1). Additionally, ruxolitinib substantially reduced day 14 TNFα (p = 0.001) compared to TPE alone (Figure 2). The oxygenation index was improved by 35.3% by day 3 with TPE alone and 30% with TPE plus ruxolitinib reflecting no incremental benefit from the addition of ruxolitinib. The median time on mechanical ventilation was six days for both cohorts. Fifteen of 20 patients were either extubated or returned to room air (7 in cohort 1A, 8 in cohort 1B) within the 28-day study window. COVID-19 seropositivity was present in all 20 patients at baseline with no conversion to seronegativity through study day 14. There were no serious adverse events related to TPE or ruxolitinib treatment. All patients received 5% human albumin as replacement therapy without the need for fresh frozen plasma (FFP).
Discussion
This study is the first to report the combination of two complementary treatments to address the hypercytokinemic state of CRS complicating COVID-19 infection. The improvement in cytokine levels with TPE reproduces the findings of our earlier pilot study [8] as well as findings of others [10-12]. Noted were significant reductions of CRP, IL-6, IL-10, and TNFα with no baseline signal of excess IFNγ or GM-CSF. The addition of ruxolitinib resulted in statistically significant reductions in CRP, IL-6, and TNFα following the fifth TPE as well as day 14 TNFα levels. When this study was initiated in June 2020, it was notably within a different clinical context compared to our pilot trial and other case reports. As previously mentioned, the majority of patients received antecedent and concomitant therapies following a diagnosis of CRS. Some of the enrolled patients had been exposed to these interventions for several days. Therefore, in contrast to our pilot study, which was free of concomitant therapies, patients in this study had decreased baseline cytokine levels as reflected in CRP values which were 56% lower at study entry. This observation likely reflects the immediate impact of glucocorticoids and other therapies. Furthermore, the utilization of high flow oxygen skewed comparisons with previous studies wherein many of these patients would have been mechanically ventilated. This FDA approved trial required the inclusion of emerging therapies deemed of possible merit and thus introduced uncontrolled complexity in the interpretation of the data.
The favorable impact of ruxolitinib on IL-6 and TNFα levels is particularly noteworthy as these cytokines may play a key role in COVID-19 induced CRS. IL-6 has been consistently reported at excessive levels and many have suggested that it is “the kingpin” of the process [15]. The literature also reflects an increasing awareness of the role of TNFα in what is likely a highly multifactorial cytokine load. Del Valle et al. [8] noted that elevated “IL-6 and TNFα are significantly associated with a worse prognosis (page 1637)” and are “independently predictive of patient outcomes in terms of both disease severity and survival (page 1640).” Our findings of incremental benefit of ruxolitinib treatment on IL-6 and TNFα levels post apheresis (p = 0.029 and p = 0.043 respectively) as well as day 14 TNFα (p = 0.001) underscore the need for further evaluation of ruxolitinib in the treatment of CRS. Dose escalation of ruxolitinib is warranted as the 5 mg twice daily dose demonstrated a positive signal of impact without any evidence of toxicity. A year into the global pandemic of 2020, a brisk death toll persists in the face of a paucity of predictably effective therapies. Just reported was a randomized trial noting the failure of convalescent plasma in COVID-19 severe pneumonia to improve clinical status or overall mortality [16] and the failure of tocilizumab to improve clinical outcomes in hospitalized patients [17]. The results of the FDA approved trial presented here lend support to the pursuit of larger, prospective, randomized trials to better define the risks of clinical benefit of TPE alone and in conjunction with JAK inhibitors and other novel agents.
Declaration of Competing Interest
W. Jeffery Edenfield serves as a consultant for Chimerix, Inc., unrelated to the submitted work. The remaining authors declare that there are no competing interests.
Funding Sources
The funders of the study had no role in the study design, data collection, data analysis, or writing of the manuscript. Terumo BCT, Inc. provided the disposable apheresis exchange kits. Incyte Corporation provided the study drug, ruxolitinib, and partial support for study related laboratory tests. Prisma Health Office of Philanthropy and Partnership assisted in securing funding for the laboratory analysis. All authors had full access to all study data and had final responsibility for the decision to submit the manuscript for publication.
CRediT Authorship Contribution Statement
W. Larry Gluck: Conceptualization, Methodology, Formal analysis, Data curation, Writing – Original draft, Funding acquisition. Sean Callahan: Investigation, Supervision, Writing – Review and Editing. Robert Brevetta: Investigation, Supervision, Writing – Review and Editing. Antine Stenbit: Investigation, Supervision, Writing – Review and Editing. Wesley Smith: Investigation, Visualization, Data curation, Writing – Original draft, Formal analysis. Julie Martin: Project administration, Visualization, Data curation, Writing – Original draft, Formal analysis. Anna Blenda: Data curation, Validation, Writing – Review and Editing. Sergio Arce: Data curation, Validation, Writing – Review and Editing. W. Jeffery Edenfield: Visualization, Funding acquisition, Writing – Review and Editing.
Acknowledgments
The authors express their sincere thanks to the patients who participated in this study. We thank the following individuals from Prisma Health for their contributions: Research Manager Jan Kueber for leading the on-site implementation; Jennifer Caldwell and Noreen Denham for patient case management; Erin Hudgins and Katie McKelvey for their expertise and care of the participants undergoing therapeutic plasma exchange; the staff of the Cancer Institute Biorepository and Phase I unit for diligent specimen deidentification, processing, storage and data management; and Alex Ewing for guidance regarding the data analysis.
References
2. Conti P, Ronconi G, Caraffa AL, Gallenga CE, Ross R, Frydas I, et al. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. Journal of Biological Regulators and Homeostatic Agents. 2020 Mar 1;34(2):1.
3. Kenderian SS, Ruella M, Shestova O, Kim M, Klichinsky M, Chen F, et al. Ruxolitinib prevents cytokine release syndrome after car T-cell therapy without impairing the anti-tumor effect in a xenograft model. Biology of Blood and Marrow Transplantation. 2017 Mar 1;23(3):S19-20.
4. Cao Y, Wei J, Zou L, Jiang T, Wang G, Chen L, et al. Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): A multicenter, single-blind, randomized controlled trial. Journal of Allergy and Clinical Immunology. 2020 Jul 1;146(1):137-46.
5. Capochiani E, Frediani B, Iervasi G, Paolicchi A, Sani S, Roncucci P, et al. Ruxolitinib rapidly reduces acute respiratory distress syndrome in COVID-19 disease. analysis of data collection from RESPIRE protocol. Frontiers in Medicine. 2020 Aug 4;7:466.
6. Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghazaryan V, et al. Baricitinib plus remdesivir for hospitalized adults with Covid-19. New England Journal of Medicine. 2021 Mar 4;384(9):795-807.
7. Novartis provides update on RUXCOVID study of ruxolitinib for hospitalized patients with COVID-19. December 14, 2020. Available at: https://www.novartis. com/news/media-releases/novartis-provides-updateruxcovid- study-ruxolitinib-hospitalized-patientscovid- 19. Accessed January 5, 2021.
8. Gluck WL, Callahan SP, Brevetta RA, Stenbit AE, Smith WM, Martin JC, et al. Efficacy of therapeutic plasma exchange in the treatment of penn class 3 and 4 cytokine release syndrome complicating COVID-19. Respiratory Medicine. 2020 Dec 1;175:106188.
9. Del Valle DM, Kim-Schulze S, Hsin-Hui H, Beckmann ND, Nirenberg S, Wang B, et al. An inflammatory cytokine signature helps predict COVID-19 severity and death. Preprint. medRxiv. 2020;2020.05.28.20115758.
10. Shi H, Zhou C, He P, Huang S, Duan Y, Wang X, et al. Successful treatment with plasma exchange followed by intravenous immunoglobulin in a critically ill patient with COVID-19. International Journal of Antimicrobial Agents. 2020 Aug 1;56(2):105974.
11. Bolaman AZ, Turgutkaya A, Yavasoglu I. Can Plasmapheresis be Useful in the Treatment of Patients with Covid-19?. Asian Hematology Research Journal. 2020 Jul 4:1-4.
12. Morath C, Weigand MA, Zeier M, Speer C, Tiwari- Heckler S, Merle U. Plasma exchange in critically ill COVID-19 patients. Critical Care. 2020 Dec;24(1):1-4.
13. Porter D, Frey N, Wood PA, Weng Y, Grupp SA. Grading of cytokine release syndrome associated with the CAR T cell therapy tisagenlecleucel. Journal of Hematology & Oncology. 2018 Dec;11(1):1-2.
14. Magnetic Luminex Performance Assay. Minneapolis, MN: R&D Systems, Inc. (https://www.rndsystems.com/ products/luminex-high-performance-assays)
15. Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. International Journal of Antimicrobial Agents. 2020 May 1;55(5):105954.
16. Simonovich VA, Burgos Pratx LD, Scibona P, Beruto MV, Vallone MG, Vázquez C, et al. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. New England Journal of Medicine. 2021 Feb 18;384(7):619- 29.
17. Rosas IO, Bräu N, Waters M, Go RC, Hunter BD, Bhagani S, et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. New England Journal of Medicine. 2021 Apr 22;384(16):1503-16.
18. Kim HO, Kim HS, Youn JC, Shin EC, Park S. Serum cytokine profiles in healthy young and elderly population assessed using multiplexed bead-based immunoassays. Journal of Translational Medicine. 2011 Jul 20;9:113.