Abstract
Vitamin C is a part of cell physiology and is utilized in many metabolic reactions. Therapeutically, it was found useful in many ailments including fatal diseases such as cancer. It has ability to induce apoptotic pathway in cancer cells in order to kill them within the body at lower concentrations without affecting the non-cancerous cells. Antioxidant enzymes and reactive oxygen species are involved in the mechanism of initiation of apoptosis mediated by Vitamin C. Current review is highlighting the dose-dependent therapeutic potential of Vitamin C based upon the available literature.
Keywords
Vitamin C, Health benefits, Cancer, Regulation, Chemotherapeutic
Introduction
Vitamin C, also known as ascorbate or ascorbic acid, is an essential water-soluble vitamin required for several physiological mechanisms in plant and animal cells. Humans acquire Vitamin C through diet or supplements. Main sources include fruits and vegetables. An average healthy adult maintains about 1.2 – 2.0 g of Vitamin C. Vitamin C ranges between 50 to 70 μM concentrations in plasma, around 1 mM in tissues and less than 10 mM in lungs, adrenal glands, brain and leukocytes [1]. Uptake of Vitamin C in different parts of body has already been reported such as in bone, eye, skin, kidney, brain, and intestine [1]. Vitamin C has been shown to induce cancer cell death at concentration of 20 mM in various cancer cell lines [17]. Vitamin C serves in various physiological roles by acting as a reducing agent, antioxidant, pro-oxidant and a free radical scavenger [2]. Vitamin C plays fundamental role in growth, healing and repair mechanism of tissues in the body. It is also required for production of collagen, which is a structural component of various body parts [3]. In numerous biological reactions, Vitamin C being an important nutrient serves as a cofactor to many metabolic enzymes. Among all the vitamins, Vitamin C is potentially less toxic and plays an important role in enhancing natural immunity [4].
Initiation of Apoptosis
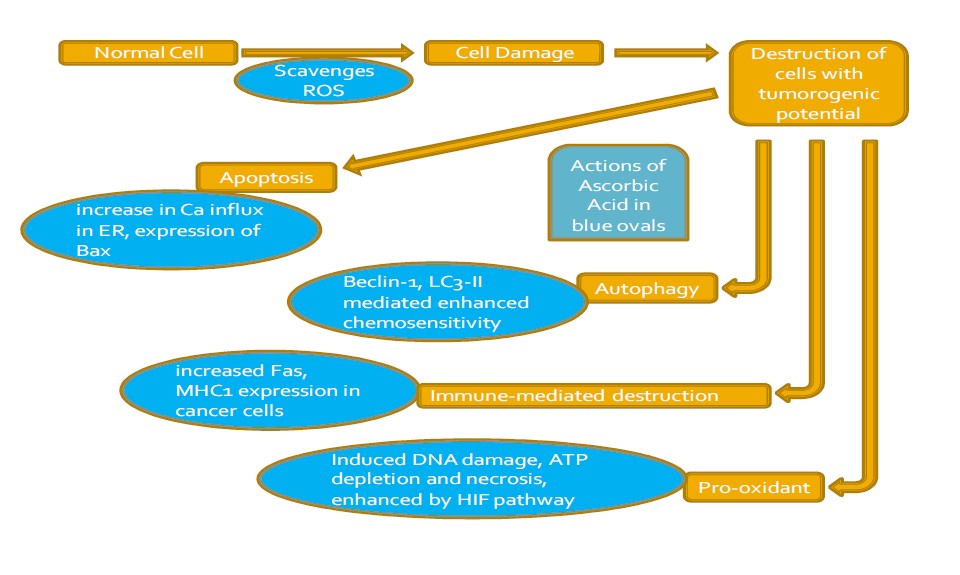
Apoptosis is a programmed cell death, which occurs naturally under some cellular signaling pathways. Sometimes, this cell death is assumed necessary for removing damaged, infected or potentially neoplastic cells. Inside cells, this mechanism is induced by multiple pathways (extrinsic or intrinsic) as shown in Figure 1. Extrinsic pathway is also known as death receptor pathway, which is exclusively controlled by caspases. Intrinsic pathway may affect cellular organelles, including mitochondria, lysosomes, endoplasmic reticulum and nucleus, resulting in death of cells. Some hallmarks of apoptosis include oligonucleosomal DNA degradation, activation of proteases, cytoplasmic blebbing, nuclear fragmentation and nuclear chromatin condensation [5]. Vitamin C acting as an anti- and pro-oxidant agent readily increases levels of glutathione (GSH) and reactive oxygen species (ROS), respectively. Many studies confirmed that Vitamin C provides the dual role of an oxidizing and reducing agent; however, accumulation of GSH & ROS in cells due to Vitamin C does not last long. After 3 hours, presence of GSH has been shown to be involved in defensive mechanisms [6]. Some of the most common ROS produced under the effect of Vitamin C are hydrogen peroxide, hydroxide radical and superoxide anion. ROS formation caused by oxidation of Vitamin C can disturb aerobic respiration process in mitochondria, which leads towards apoptosis. Vitamin C can also induce apoptosis by reducing or oxidizing different ions such as Fe2+, Co2+, Cr2+, Ti2+ and Cu2+. These ions aid in formation of ROS, which in turn cause cell death. Mechanisms involved include blockage of sodium-potassium pump in cell membrane or directly attacking on DNA [7].
Cancer and Immunology
The human immune system mounts natural endogenous response to foreign cells, particularly highly immunogenic cancer cells, through a complex series of steps. Step1 includes presenting of cancer antigens to T-cells via antigen-presenting cells (APCs). Step 2 includes priming and activating T-cells in lymph nodes. Step 3 includes trafficking and infiltration of T-cells into tumor beds (tumor-infiltrating lymphocytes). Step 4 includes recognition of cancer cells by T-cells. Step 5 includes development of antigen-specific systemic effector and memory T-cells. Step 6 includes current treatments such as humoral immunity, allowing effector T-cells, other endogenous immune cells and antibodies to tumor to act in concert in order to eliminate cancer cells [9].
Regulation of Cancer
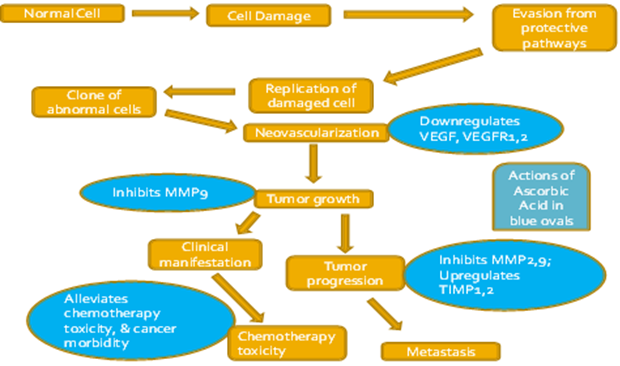
Vitamin C is an essential micronutrient and is included in the list of unorthodox therapy for cancer treatment via various metabolic pathways (Figure 2). Vitamin C is much effective in controlling cancer despite many uncertainties [8]. Distribution of Vitamin C between intracellular and extracellular environments depends upon the absorption of Vitamin C through the transporters in membranes. Vitamin C is a widely accepted, used and controversial alternative cancer treatment [16]. Vitamin C can prevent and treat cancer by multiple mechanisms [3]. Many in vitro studies examined the role of p53 as a sensitizer for ascorbate cytotoxicity in cancer cells by increasing ROS generation and activation of p38 MAPK. Some authors also reported sensitivity of cancer cells to Vitamin C regardless of the involvement of p53. This suggests that cytotoxicity mechanism in cancer cells depends upon more than one pathway. Thus, Vitamin C can potentially kill cancer cells whether expressing p53 or not. Similarly, in vivo studies had also revealed preferential cytotoxicity effect of Vitamin C [8]. The ability to induce apoptosis in cancer cells is a useful strategy in cancer treatment, because apoptosis does not result in inflammation and tissue damage, as happens in necrosis [5].
Pro-Cancer Activity
Vitamin C is well known for its anti-cancer property. But some studies also revealed its pro-cancer property at high doses when co-administered with oxygen and copper. At higher concentrations (more than 20 mM), Vitamin C may increase production of ATP by increasing mitochondrial electron flux, probably via its pro-oxidant action. Anticancer efficacy of Vitamin C is known since several years. Vitamin C principally targets mitochondria in cells and initiates many metabolic reactions which result in development and progression of cancer cells. Vitamin C can act as anti-oxidant as well as pro-oxidant agent on different concentrations. Vitamin C has the ability to conduct both reducing and oxidizing activities dependent on the concentration and environment around it. Low doses of Vitamin C can display antioxidant property and prevent initiation of oxidant-induced apoptosis.
Whereas, high doses can induce cancer cells apoptosis via its pro-oxidant action [3]. It is observed that 0.3-20 mM concentrations of Vitamin C are enough to produce H2O2 and other ascorbate radicals in cancer cells to kill them [2]. Vitamin C has been proved as a pro-oxidant agent at pharmacological concentrations in different cancer studies. From these studies, it is evident that Vitamin C regulates DNA-damaging stress in cancer cells. Cytotoxicity effect of Vitamin C is based upon generation of hydrogen peroxide (H2O2) in affected cell which harms it anyway [8].
Relation with Chemotherapeutic Drugs
For development of cancer, it is necessary for cells to be resistant against apoptotic mechanisms. In certain cases, Vitamin C was proved to be helpful for cancer cells in creation of resistance in them against chemotherapeutic drugs. This notion was confirmed by a study conducted on human colon cancer cells and Vitamin C (at dose of 1 mM) blocks drug-mediated apoptosis induction allowing cancer cells to become insensitive to chemotherapeutics [3]. Similar results have also been reported by Zou & coworkers [1] and Kontek & coworkers [5]. Adversely in certain cases, Vitamin C was found much fruitful and reduced adverse effects of chemotherapeutic drugs in patients when administered in combination. This reduction in toxicity would allow patients to tolerate higher and effective doses of chemotherapy [17].
Activity on Non-Cancerous Cell Lines
Various studies have confirmed that Vitamin C is capable of increasing the generation of induced pluripotent stem cells of both human and mouse [18]. It has been documented that osteoblast cells had a four-fold increase in respiration when treated with Vitamin C and therefore increase in ATP production, which provided the necessary environment for differentiation of cells [3]. A cancer drug is considered as ideal if it kills cancer cells and shows less toxicity on non-cancerous cells [8,19].
Activity on Cancer Cell Lines
Vitamin C can regulate various signaling pathways that regulate microenvironment in cancer cells. That is why, Vitamin C can be proven cytotoxic to cancer cells at optimal doses and is recognized as an important natural antioxidant, but its efficacy differs among different cancer cell lines due to its relative cytotoxicity [8]. At a dose of 1.25 g, Vitamin C is generally recommended as effective in anticancer therapy [1]. In lower concentrations, Vitamin C was found selectively cytotoxic to many cancer cell lines [9]. Kang et al. also mentioned anticancer potential of Vitamin C, wherein it induced apoptosis via caspase- 8-independent pathway in many cancer cell lines in different studies such as B16 murine melanoma cells. Vitamin C can also prevent cells from apoptosis according to a study conducted on monocytes and U937 lymphoma cells [3]. Hela cells were also affected by Vitamin C via apoptotic pathway in a study. Vitamin C induces arrest of cancer cell cycle in higher concentrations at G2/S phase [18]. Additionally, 5-100 mM concentrations of Vitamin C demonstrated enough cytotoxic efficacies against eleven human cancer cell lines including glioblastoma and carcinoma via generation of H2O2. Although noncancerous cell lines remained unaffected at similar doses [7]. At low doses (less than 100 mM), Vitamin C functions as an antioxidant and can prevent cells from injury caused by oxidative stress; however, Vitamin C acts as a pro-oxidant and induces cell death at higher doses more than 300 mM. Cancer cells have generally high metabolic rates and ROS levels as compared to noncancerous cells. As such, Vitamin C induced oxidative stress cannot be ameliorated by internal cellular response, hence cell death occurs. Many studies have confirmed that Vitamin C selectively harms cancer cells without affecting non-cancerous body cells [19]. Another study reported inhibitory effect of Vitamin C on acute myeloid leukemia cell lines at dose of 0.25-1.0 mM. Similarly, dose of 10 nM – 1 mM of Vitamin C induced death in neuroblastoma and melanoma cells. Park, 2013 recommended that 1.0- 10 mM concentration of Vitamin C is enough to kill all types of cancer cell lines. Studies conducted on NB4 and HL-60 cells revealed that Vitamin C induced apoptosis via generation of H2O2 in them when administered for only half to one hour. Additionally, Vitamin C also induced apoptosis in ovarian cell lines (2774, OVCAR-3 & SKOV- 3). Response of Vitamin C was also noted against myeloblast and chronic myelogenous leukemia cell lines. Another study revealed cytotoxic potential of Vitamin C that was evaluated on cell lines obtained from patients of myelodysplastic syndrome or acute myeloid leukemia. Most of the cell lines were sensitive to Vitamin C [6]. Several studies suggested Vitamin C as cytotoxic agent against cancer cells, but its role is controversial in breast cancer. Despite these studies, beneficial effects of Vitamin C in treatment of cancer have been reported in many published reports, which showed inhibitory action of Vitamin C against cancer cells of the stomach, prostate, brain, and breast [4]. Guerriero and coworkers mentioned apoptosis and cell cycle arrest induced by Vitamin C in cells of stomach cancer, prostate cancer, brain tumor, melanoma, leukemia, and lymphoma [17]. It is recommended that anti-tumor property of Vitamin C results from formation of cytotoxic hydrogen peroxide which harms cells [5,7]. Kontek and coworkers also mentioned anticancer property of Vitamin C against A375. S2 melanoma, mesothelioma and colorectal adenocarcinoma cell lines. Vitamin C was believed to modulate the killing of oesophageal cancer cells in vitro by inhibition of translocation of different transcription factors. Breast cancer cell line was also affected by pharmacological concentrations of Vitamin C through apoptosis-inducing factor [5]. At pharmacological concentrations mentioned in a study [20], Vitamin C is able to kill ovarian cancer cells. Chen and coworkers mentioned inhibitory activity of Vitamin C on MCF-7, SKBR3 & Hs578T breast cancer cell lines [21].
Activity in Animals
Vitamin C displayed in vivo anticancer activity when administered alone or together with other agents [16]. Experiments in rodents suggested that administration of Vitamin C increases survival times of host and inhibition of tumor growth [3]. Many other authors also found Vitamin C effective in both in vitro as well in vivo studies [6,18]. High doses of Vitamin C prevented mice from sarcoma [6] and ovarian cancer [19].
Activity in Cancer Patients
Since 30 years, efficacy of Vitamin C in cancer patients of advanced stages has been well known [16]. Some results were obtained in Scottish and Japanese studies in which Vitamin C was administered orally and intravenously [3]. Previous studies have revealed that high concentration of Vitamin C reduced the risk of cervical cancer. However, certain authors discouraged utilization of Vitamin C in some cancers such as cervical intraepithelial neoplasia and minor squamous atypia, as they found Vitamin C not useful in therapy. Intake of diet rich in Vitamin C, has demonstrated a significant protective effect against cervical cancer [18]. Studies conducted at clinical level of acute myeloid patients, Vitamin C administration was shown beneficial. Park, 2013 recommended 0.1 mM concentration of Vitamin C in plasma, is enough to cause toxicity to cancer cells inside the body. Complimentary & alternative medicine practitioners have used Vitamin C to treat different ailments [6]. Several studies revealed a role of Vitamin C in reducing adverse effects of chemotherapeutic drugs during the treatment [17]. 10 g of Vitamin C when administered to cancer patients per day, reduction in pain and overall improvement in the health of patients, were observed [4]. According to epidemiological evidence, it was recommended that daily intake of Vitamin C in diet statistically reduces breast-cancer-specific mortality as well as total mortality [21].
Synergism with Other Anti-Cancer Drugs to Treat Cancer
For prevention and treatment of cancer, Vitamin C supplementation has been reported as beneficial even at large doses as evidenced by many epidemiological studies. At higher doses (100-300 mM), Vitamin C proved safe for the majority of individuals. Use of Vitamin C in several types of cancers yielded promising results. But in most of the cases, alone Vitamin C was not proved fruitful as compared to some combination therapy. Vitamin C is only considered as part of treatment protocol in cancer patients [3]. Based on previous studies, Vitamin C is likely to elicit greater inhibition of p53-expressing tumors when used in combination with second therapeutic drug or gene therapy as compared to Vitamin C alone. Combination of Vitamin C and gene therapy would elevate p53 expression or activation through the tumor body, thereby maximizing the killing mechanism. In this context, Vitamin C with another anticancer drug, would be an effective combination to enhance p53-dependent oxidative stress with fewer side effects [8]. In this instance, administration of Vitamin C along with other oxidants helped chemotherapy in preventing recurrence of ovarian cancer [1]. In a study, Vitamin C combined with arginine to treat different cancer cell lines which proved much cytotoxic as compared to non-cancerous human cells [2]. Emodin and doxorubicin induced anti-proliferative effects in different cancer cell lines in combination with Vitamin C [22]. In a study, Vitamin C was utilized in metastatic pancreatic cancer patients along with gemcitabine and erlotinib. Treatment of diarsenictrioxide (As2O3) with Vitamin C increased accumulation of reactive oxygen species further in cancer cells up to 24 hours [6]. Vitamin C induced apoptosis in non-small cell lung cancer cells in synergism with cytostatic inhibitor of glycolysis [19]. In another study, Vitamin C enhanced the effect of mitoxantrone (synthetic anticancer drug) in inhibition of human breast cancer cell lines such as MCF-7 & MDA-MB231 [17]. In a study conducted on human breast carcinoma cells, Vitamin C potentiated the antineoplastic activity of paclitaxel, cisplatin, and doxorubicin [4]. Use of Vitamin C in combination with radiotherapy or some chemotherapeutic drugs proved much fruitful both in vitro and in vivo studies [5]. Combination of two vitamins such as vitamin B2 & C proved much beneficial in suppressive effect against A459, MCF-7 & MBA-MB-231 cell lines as evidenced by a study [21]. Vitamin C was also found supportive in facilitating ten-eleven translocation activity induced by 5-Azacytidine (DNA methyl transferase inhibitor) [23].
Conclusion with Future Perspectives
Vitamin C is essential for several physiological mechanisms in cells. It may have both pro-oxidant and antioxidant capacity by simultaneously increasing GSH and ROS levels. It can be a useful compound in controlling cancer, is able to inhibit initiation and promotion of cancer via involving different mechanisms. However, Vitamin C may also enhance resistance in cancer cells against chemotherapeutic drugs. On the other hand, Vitamin C may have inhibitory activity against cancer as evidenced by many in vitro and in vivo studies. Previous studies also revealed its protective potential in patients. It may also be used in synergism with other chemotherapeutic drugs to treat cancer. In future, Vitamin C may have an important role in cancer management and there should be clinical trials and case studies relevant to evaluate its efficacy at advanced level. it is also predicted that it may be beneficial individually as well as in combination with other anticancer drugs.
Conflict of Interest
Authors declare no conflicts of interests.
References
2. Hsieh BS, Huang LW, Su SJ, Cheng HL, Hu YC, Hung TC, et al. Combined arginine and ascorbic acid treatment induces apoptosis in the hepatoma cell line HA22T/ VGH and changes in redox status involving the pentose phosphate pathway and reactive oxygen and nitrogen species. The Journal of nutritional biochemistry. 2011 Mar;22(3):234-41.
3. González MJ, Rosario-Pérez G, Guzmán AM, Miranda- Massari JR, Duconge J, Lavergne J, et al. Mitochondria, energy and cancer: the relationship with ascorbic acid. Journal of orthomolecular medicine: official journal of the Academy of Orthomolecular Medicine. 2010;25(1):29.
4. Khurana V, Kwatra D, Pal D, Mitra AK. Molecular expression and functional activity of vitamin C specific transport system (SVCT2) in human breast cancer cells. International Journal of Pharmaceutics. 2014 Oct;474(1- 2):14-24.
5. Kontek R, Jakubczak M, Matlawska-Wasowska K. The antioxidants, vitamin A and E but not vitamin C and melatonin enhance the proapoptotic effects of irinotecan in cancer cells in vitro. Toxicology In Vitro. 2014 Mar;28(2):282-91.
6. Park S. The effects of high concentrations of vitamin C on cancer cells. Nutrients. 2013 Sep;5(9):3496-505.
7. Klingelhoeffer C, Kämmerer U, Koospal M, Mühling B, Schneider M, Kapp M, et al. Natural resistance to ascorbic acid induced oxidative stress is mainly mediated by catalase activity in human cancer cells and catalase-silencing sensitizes to oxidative stress. BMC Complementary and Alternative Medicine. 2012 Dec;12(1):1-0.
8. Kim J, Lee SD, Chang B, Jin DH, Jung SI, Park MY, et al. Enhanced antitumor activity of vitamin C via p53 in cancer cells. Free Radical Biology and Medicine. 2012 Oct;53(8):1607-15.
9. Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013 Jul;39(1):1-0.
10. Slansky JE, Rattis FM, Boyd LF, Fahmy T, Jaffee EM, Schneck JP, et al. Enhanced antigen-specific antitumor immunity with altered peptide ligands that stabilize the MHC-peptide-TCR complex. Immunity. 2000 Oct;13(4):529-538.
11. Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995 Nov;3(5):541-7.
12. Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. Journal of Experimental Medicine. 2009 Dec;206(13):3015-29.
13. Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nature Reviews Immunology. 2009 Mar;9(3):162-74.
14. Dey A, Allen J, Hankey-Giblin PA. Ontogeny and polarization of macrophages in inflammation: blood monocytes versus tissue macrophages. Frontiers in Immunology. 2015 Jan;5:683.
15. Pillay J, Kamp VM, van Hoffen E, Visser T, Tak T, Lammers JW, et al. A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1. The Journal of Clinical Investigation. 2012 Jan;122(1):327-36.
16. Hoffer LJ, Levine M, Assouline S, Melnychuk D, Padayatty SJ, Rosadiuk K, et al. Phase I clinical trial of iv ascorbic acid in advanced malignancy. Annals of Oncology. 2008 Nov;19(11):1969-74.
17. Guerriero E, Sorice A, Capone F, Napolitano V, Colonna G, Storti G, et al. Vitamin C effect on mitoxantrone-induced cytotoxicity in human breast cancer cell lines. PLoS One. 2014 Dec;9(12):e115287.
18. Zhang Z, Liu X, Wu T, Liu J, Zhang X, Yang X, et al. Selective suppression of cervical cancer Hela cells by 2-O-ß-D-glucopyranosyl-L-ascorbic acid isolated from the fruit of Lycium barbarum L. Cell biology and toxicology. 2011 Apr;27(2):107-21.
19. Vuyyuri SB, Rinkinen J, Worden E, Shim H, Lee S, Davis KR. Ascorbic acid and a cytostatic inhibitor of glycolysis synergistically induce apoptosis in non-small cell lung cancer cells. PLoS One. 2013 Jun;8(6):e67081.
20. Li HH, Zhao YJ, Li Y, Dai CF, Jobe SO, Yang XS, et al. Estradiol 17ß and its metabolites stimulate cell proliferation and antagonize ascorbic acid-suppressed cell proliferation in human ovarian cancer cells. Reproductive Sciences. 2014 Jan;21(1):102-11.
21. Chen N, Yin S, Song X, Fan L, Hu H. Vitamin B2 Sensitizes Cancer Cells to Vitamin-C-Induced Cell Death via Modulation of Akt and Bad Phosphorylation. Journal of Agricultural and Food Chemistry. 2015 Aug;63(30):6739- 48.
22. Masaldan S, Iyer VV. Exploration of effects of emodin in selected cancer cell lines: enhanced growth inhibition by ascorbic acid and regulation of LRP1 and AR under hypoxia-like conditions. Journal of Applied Toxicology. 2014 Jan;34(1):95-104.
23. Sajadian SO, Tripura C, Samani FS, Ruoss M, Dooley S, Baharvand H, et al. Vitamin C enhances epigenetic modifications induced by 5-azacytidine and cell cycle arrest in the hepatocellular carcinoma cell lines HLE and Huh7. Clinical Epigenetics. 2016 Dec;8(1):1-3.
24. Roa FJ, Peña E, Gatica M, Escobar-Acuña K, Saavedra P, Maldonado M, et al. Therapeutic Use of Vitamin C in Cancer: Physiological Considerations. Frontiers in Pharmacology. 2020 Mar;11:211.