Keywords
COVID-19, Hematologic malignancy, Chemotherapy, Lymphopenia, COVID-19 vaccine, Vaccine efficacy
Commentary
The coronavirus disease 2019 (COVID-19) pandemic places the treating hematologist in a quandary: how best to protect patients with hematologic malignancies from potentially deadly COVID-19 infection while also providing the best therapy for their disease and maximizing opportunities for cure. Cancer patients as a whole trend toward more severe infection and increased mortality from COVID-19 infection. This burden, however, is not equally distributed among all cancer patients and outcomes are particularly poor in those with hematologic malignancies [1]. Lymphodepleting treatments have a profound effect on COVID outcomes; we have recently reported that despite proper and even prolonged quarantine after asymptomatic positive screening test for COVID-19, the initiation of rituximab-based chemotherapy resulted in a delayed respiratory failure in three lymphoma patients [2]. In addition to more severe infection and increased mortality, immunocompromised patients shed virus and remain infectious for far longer than the general population, frequently for several months or longer [3,4]. Finally, to add insult to injury, patients with hematologic malignancies have worse clinical and laboratory responses to vaccines, compromising their ability to be protected against infection and severe disease [5,6]. Prolonged viral shedding, decreased ability to form a durable immune response to vaccination or infection, and subsequent increased probability for severe infection pose a problem for those needing treatment due to progressive disease. Treatment delays in some cases can reduce cure fractions and increase likelihood of disease-related complications. With these cases in mind, there is a need to identify those patients who are at greatest risk of severe infection and determine what steps can we take to minimize the morbidity and mortality associated with both COVID-19 and the hematologic malignancy.
Attempts at stratifying risk for severe COVID-19 infection have been made in order to predict and better understand the course of illness in those with hematologic malignancies. All varieties of cancer and their associated treatments have a range of immunomodulatory effects, and the nature of many treatments for hematologic malignancies is to target the host’s immune system and mediate its function when it goes awry. Clinical outcomes data are limited by significant heterogeneity in the actual data that was collected, timing of data collection (outcomes as a whole improved later in the pandemic), and by the difficulty obtaining granular correlation data regarding treatment and cancer histories. A meta-analysis of 3377 patients specifically focusing on those with hematologic malignancies supports the conclusion that recent systemic anti-cancer therapy did not put patients at significantly higher risk of mortality from COVID-19, though further classification based on the type of treatment received was not performed [1]. In this cohort, stratifying by primary diagnosis displayed the further risk of death: highest in acquired bone marrow failure syndromes (53%) followed by acute leukemias (41%), and similar rates among myeloproliferative neoplasms, plasma cell dyscrasias, and lymphomas (34%, 33%, 32% respectively) (Table 1). Solid organ cancers, though still immunocompromising, have different effects on the immune system by alternate mechanisms. Jee et al. investigated how these inherent differences may affect severity of COVID-19 infection in 309 patients [7]. It was found that patients with hematologic malignancies and primary lung cancers had a significantly higher risk of severe COVID-19 infection as compared to other solid malignancies [7]. Other significant risk factors were peri-COVID-19 lymphopenia and baseline neutropenia although, interestingly, peri-COVID-19 neutropenia was not a significant risk factor. The authors concluded that recent cytotoxic chemotherapy or immunotherapy were not associated with more severe cases, whereas targeted therapies such as treatment with a tyrosine kinase inhibitor (TKI) trended toward worse outcomes. However, those conclusions should be interpreted with caution given that patients with hematologic malignancies were overrepresented among TKI patients and so the effect of primary cancer site vs therapy effect is difficult to separate. Another study of 697 COVID-19-positive patients focused specifically on hematologic malignancies and compared infection severity between anti-neoplastic treatments [8]. Again, AML diagnosis was significantly associated with higher risk of mortality. When types of treatment were compared, there was a significantly greater risk of death in those on active antineoplastic therapy with monoclonal antibodies (HR 2.02), but a lower observed mortality in those receiving active therapy with hypomethylating agents (HR 0.47) compared to those not on active treatment. Of note, a majority of patients undergoing therapy with hypomethylating agents had AML, offering hope to those patients in a higher risk category.
| Primary Diagnosis | Risk of Death from COVID-19 (%) |
|---|---|
| Acquired Bone Marrow Failure Syndromes | 53 |
| Acute Leukemia | 41 |
| Myeloproliferative Neoplasms | 34 |
| Plasma Cell Dyscrasias | 33 |
| Lymphomas | 32 |
Why we are seeing these differences may be attributed to a better understanding of the immune response in COVID-19 and how this response varies in different disease states. Cytotoxic T lymphocytes are crucial in the immune system’s fight against viral pathogens, and it is has been proposed that T cell exhaustion is part of the pathogenesis of severe infection [4]. Lymphopenia is increasingly becoming a known risk factor for severe COVID-19 infection and has been reported in several studies to result in as much as a 3-fold increase of severe COVID-19 infection [7,9]. Abdul-Jawad et al. compared immune signatures and their legacies associated with COVID-19 among 41 active cancer patients (23 solid organ and 18 hematologic) and compared them to 35 healthy controls (Figure 1); all patients had confirmed infection by nasopharyngeal PCR [4]. To understand leukocyte dysregulation, the authors analyzed flow cytometry panels to measure eight components of immune response, including lymphocyte quantity, activation, cycling and exhaustion as well as innate immune quantity and response. The results were remarkable. While solid organ patients had a postinfectious immune phenotype indistinguishable from healthy controls, patients with hematologic malignancies demonstrated poorer humoral response and increased T-cell exhaustion. These features were uniform regardless of primary diagnosis or treatment with B cell depleting therapies. Upon recovery from infection, both viral clearance and seroconversion to developing neutralizing antibodies against SARS-CoV-2 spike protein was delayed far more in hematologic compared to solid organ cancers. Finally, unique among patients with hematologic malignancies were large populations of CD56bright NK cells skewing immune activity to inflammatory rather than cytotoxic predominance. What implications can this distinct difference in immune response have on these patients that are beginning to feel hopeful in the face of vaccine availability? 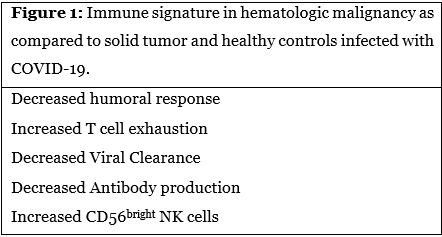
One of the most important contributions to controlling the spread of infection and minimizing morbidity and mortality amidst the COVID-19 pandemic has been the development of a vaccine. Excluding those with immunocompromising conditions or on immunosuppressive therapy, efficacy of the BNT162b2 mRNA vaccine is reported at 95% in preventing COVID-19 [10]. Now with widespread availability of the vaccine, we are able to better study its effects and efficacy in immunocompromised patients. The NCCN COVID-19 Vaccination Advisory Committee recommends vaccination for all hematologic and solid organ cancer patients when available with few exceptions: delaying until 3 months post-HCT/cellular therapy, and delaying until ANC recovery in those undergoing intense cytotoxic chemotherapy in AML [11]. Hematologic malignancies and their treatments are immunocompromising, excluding them from participation in landmark vaccine studies, and consequently there is little high quality prospective data on vaccine efficacy in this population. There is a great deal of evidence that immune response to other vaccines such as influenza, pneumococcal, and haemophilus is blunted in those with hematologic malignancies receiving lymphodepleting therapies [12-14]. So unfortunately, but unsurprisingly, Herishanu et al. shows us that BNT162b2 vaccine efficacy is substantially reduced in a cohort of uniformly vaccinated Israeli patients with chronic lymphocytic leukemia (CLL) [6]. Disease state appeared to be very contributory to the ability to produce an antibody response. Patients in clinical remission post-treatment had the highest antibody response (79.2%), followed by treatment naïve patients (55%), and lastly those undergoing current active treatment with BTK inhibitors or venetoclax +/- anti-CD20 antibodies (16% and 13.6% respectively) (Table 2). Patients that received anti-CD20 treatment within 12 months did not have any antibody response to vaccination at all [6]. Twelve [13] months after exposure to anti-CD20, 45.5% of patients were able to develop antibody response, and time from exposure after this period did not have a significant impact on antibody response. These observations point to the longterm immune deficits associated with lymphodepleting therapy and, importantly, offer the treating physician with a timeframe for offering boosters or re-vaccination among patients who have received anti-CD20 monoclonal antibodies (mAb). Interestingly, there was no difference in vaccine reaction prevalence compared with the general population and, similar to what has been observed in immunocompetent patients, presence of local or systemic reactions had no correlation with titer levels. It has been shown that patients with cancer overall have lower antibody response with COVID-19 infection as compared to those without cancer [15], though there is little data available regarding vaccine efficacy in those with solid organ cancers. No comparative conclusions can be made between hematologic malignancies and solid organ cancers but, armed with the knowledge of the different immune responses against the virus itself, it would be interesting to observe a similar pattern with vaccine response.
| Disease state/treatment type in CLL patients |
Antibody Response Rate to COVID-19 vaccine (%) |
|---|---|
| Clinical remission, post-treatment | 79.2 |
| Treatment naïve | 55 |
| Active treatment with BTK inhibitor | 16 |
| Active treatment with Venetoclax +/- anti-CD20 | 13.6 |
| <12 months after anti-CD20 treatment |
0 |
| 12+ months after anti-CD20 treatment |
45.5 |
In spite of the sobering information above regarding worse COVID infections and poorer serologic conversion following infection and vaccination among patients with hematologic malignancies, we do have some data that can lead to cautious optimism. In spite of very poor rates of serologic conversion and extreme immunodeficiency, vaccination against seasonal influenza is highly effective among allogeneic stem cell transplant recipients. Infections were fewer, less severe and required fewer hospitalizations in the vaccinated compared to unvaccinated cohorts [16]. In other words, antibody response likely does not tell the complete picture, and similar data for vaccination against community-acquired respiratory viruses in highly immunocompromised patients suggests protection in spite of poor serologic markers. It should be noted that the NCCN in their vaccine guidelines do not recommend the use of post-vaccine antibody testing due to the general confusion concerning testing and the unknown correlation between antibody titers and immunity.
Based on the above data and the recommendation against post-vaccine antibody testing, it may be safest to not assume immunity in those with CLL, especially those that are treatment naïve, undergoing active treatment, or those less than 12 months out from anti-CD20 monoclonal antibody treatment. While not all of these conclusions can be extrapolated to all patients with hematologic malignancies, it is likely that the short and long term effects of anti-CD20 mAb has similarly deleterious immune effects and compromises vaccine efficacy. We await further data however, in the meantime, advocate considering that any patient with exposure to anti-CD20 mAb in the past 12 months should be considered to have had sub-optimal vaccine response (including those treated for non-malignant indications). In the context of these findings, the role of booster vaccination should also be explored to possibly increase antibody response rate in those in varying stages of disease and treatment. Vaccination is arguably the most important step that we can take to protect ourselves and those around us from COVID-19, but with concern for limited vaccine efficacy in patients with hematologic malignancies, the importance of vaccination among those closest to these patients in addition to continuing precautions such as masking and distancing again should be maintained. It is reassuring that achieving remission may confer appropriate levels for immunity, but until this stage of disease is reached, immunity cannot be assumed and additional precautions should be taken.
As research clarifies the interaction of the COVID-19 virus and hematologic malignancies, physicians are better equipped to make decisions regarding their recommendations to patients from prevention with vaccination to details pertaining to their therapy. First, COVID-19 outcomes and immune responses in cancer patients are heterogenous. Clinical outcomes, viral clearance, and sustained immune response are demonstrably worse in patients with hematologic malignancies compared to healthy controls. This appears less true in patients with solid organ cancers and, as a consequence, recommendations for these two groups should be different. Second, vaccine response is particularly poor in patients with hematologic malignancies. These patients, even when vaccinated, need to maintain strict social distancing and masking precautions while community transmission of COVID continues. Finally, treatment decisions should take COVID into account. For patients with hematologic malignancies who do not require urgent therapy, such as those on maintenance treatments, or for whom efficacy of therapy is uncertain, treatment should be avoided or delayed both to improve COVID-19 infection outcomes and allow for improved vaccine efficacy.
References
2. Hoffmann MS, Ganguly S. Delayed COVID-19 Respiratory Failure in Patients with Lymphoma on Rituximab-based Chemoimmunotherapy. Clinical Lymphoma Myeloma and Leukemia. 2021 Jun 1;21(6):e548-50.
3. Aydillo T, Gonzalez-Reiche AS, Aslam S, van de Guchte A, Khan Z, Obla A, et al. Shedding of viable SARS-CoV-2 after immunosuppressive therapy for cancer. New England journal of medicine. 2020 Dec 24;383(26):2586-8.
4. Abdul-Jawad S, Baù L, Alaguthurai T, Del Barrio ID, Laing AG, Hayday TS, et al. Acute immune signatures and their legacies in severe acute respiratory syndrome coronavirus-2 infected cancer patients. Cancer Cell. 2021 Feb 8;39(2):257-75.
5. Yasuda H, Tsukune Y, Watanabe N, Sugimoto K, Uchimura A, Tateyama M, et al. Persistent COVID-19 pneumonia and failure to develop anti-SARS-CoV-2 antibodies during rituximab maintenance therapy for follicular lymphoma. Clinical Lymphoma, Myeloma & Leukemia. 2020 Nov;20(11):774.
6. Herishanu Y, Avivi I, Aharon A, Shefer G, Levi S, Bronstein Y, et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood, The Journal of the American Society of Hematology. 2021 Jun 10;137(23):3165-73.
7. Jee J, Foote MB, Lumish M, Stonestrom AJ, Wills B, Narendra V, et al. Chemotherapy and COVID-19 outcomes in patients with cancer. Journal of Clinical Oncology. 2020 Oct 20;38(30):3538-46.
8. García-Suárez J, De La Cruz J, Cedillo Á, Llamas P, Duarte R, Jiménez-Yuste V, et al. Impact of hematologic malignancy and type of cancer therapy on COVID-19 severity and mortality: lessons from a large populationbased registry study. Journal of Hematology & Oncology. 2020 Dec;13(1):1-2.
9. Zhao Q, Meng M, Kumar R, Wu Y, Huang J, Deng Y, et al. Lymphopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a systemic review and meta-analysis. International Journal of Infectious Diseases. 2020 Jul 1;96:131-5.
10. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. New England Journal of Medicine. 2020 Dec 10;383:2603-2615. DOI: 10.1056/ NEJMoa2034577
11. National Comprehensive Cancer Network. (2021). Recommendations of the NCCN COVID-19 Vaccination Advisory Committee [Version 2.0]. https://www.nccn. org/docs/default-source/covid-19/2021_covid-19_ vaccination_guidance_v2-0.pdf
12. Yri OE, Torfoss D, Hungnes O, Tierens A, Waalen K, Nordøy T, et al. Rituximab blocks protective serologic response to influenza A (H1N1) 2009 vaccination in lymphoma patients during or within 6 months after treatment. Blood, The Journal of the American Society of Hematology. 2011 Dec 22;118(26):6769-71.
13. Mauro FR, Giannarelli D, Galluzzo CM, Vitale C, Visentin A, Riemma C, et al. Response to the conjugate pneumococcal vaccine (PCV13) in patients with chronic lymphocytic leukemia (CLL). Leukemia. 2021 Mar;35(3):737-46.
14. Hartkamp A, Mulder AH, Rijkers GT, van Velzen-Blad H, Biesma DH. Antibody responses to pneumococcal and haemophilus vaccinations in patients with B-cell chronic lymphocytic leukaemia. Vaccine. 2001 Feb 8;19(13- 14):1671-7.
15. Shang Y, Liu T, Li J, Wang X, Zhou F. Factors affecting antibody response to SARS-CoV-2 in patients with severe COVID-19. Journal of Medical Virology. 2021 Feb;93(2):612-614.
16. Piñana JL, Pérez A, Montoro J, Giménez E, Gómez MD, Lorenzo I, et al. Clinical effectiveness of influenza vaccination after allogeneic hematopoietic stem cell transplantation: a cross-sectional, prospective, observational study. Clinical Infectious Diseases. 2019 May 17;68(11):1894-903.
