Abstract
Congenital diaphragmatic hernia (CDH) is a developmental defect of the diaphragm, occurring in approximately 1 in 3,000 live births with a high mortality rate of between 30 and 50%. This narrative review provides an update on antenatal and postnatal management strategies and current outcomes emphasizing where further research is required. Fetal endotracheal obstruction (FETO) has been demonstrated in a large RCT to improve survival in the most severe CDH and is now adopted into clinical practice. Other antenatal interventions such as those aimed at reducing pulmonary hypertension require further study. The results of respiratory function monitoring in the delivery suite have changed practice with avoidance of routinely administering neuromuscular blocking agents, as well as providing predictors of poor outcomes. The proportion of CDH infants benefiting from spontaneously breathing from birth remains to be robustly determined. The delicate lungs of CDH infants require gentile ventilation avoiding volutrauma, conventional ventilation with volume targeting may be the most appropriate option. Although neurally-adjusted ventilatory assist (NAVA) looks promising, it needs further testing. Extracorporeal membrane oxygenation (ECMO) should be offered for infants with severe CDH with a realistic likelihood of a positive outcome; further research is required to identify accurate predictors. Pulmonary vasodilators are commonly used in CDH infants, but supportive evidence is limited. Inhaled nitric oxide (iNO) should be trialled only in the absence of left ventricular systolic dysfunction and stopped if there are no echocardiographic findings of improvement within 24 hours. Milrinone should be used to treat cardiac dysfunction if associated with PH and Sildenafil should be considered in refractory PH or as an adjunct when weaning iNO, but for all such pulmonary vasodilators the evidence needs strengthening. CDH survivors can suffer long term morbidity including chronic lung disease, exertional dyspnea, gastroesophageal reflux disease (GERD), and scoliosis. Well-structured long-term follow-up programs are required to identify morbidities.
Keywords
Pulmonary hypoplasia, Pulmonary hypertension, FETO, Mechanical ventilation, ECMO, Pulmonary vasodilators, Inhaled nitric oxide
Introduction
Congenital diaphragmatic hernia (CDH) is a developmental defect of the diaphragm that occurs in approximately 1 on 3,000 live births. There is maldevelopment of the ipsilateral and contralateral lung as well as abnormal pulmonary vascular growth. There is a high mortality, between 30 and 50%, due to pulmonary hypoplasia and pulmonary hypertension. Infants can suffer ventilator induced injury and oxygen toxicity which can result in prolonged oxygen dependency. Survivors also have other long term morbidities including neurodevelopmental delay, gastroesophageal reflux, pulmonary hypertension, and scoliosis. It is therefore essential that management strategies are optimized in this highly vulnerable population and are shown to improve outcome. As a consequence, this narrative review provides an update on antenatal and postnatal management strategies and current outcomes emphasizing the areas in which further research is required.
Antenatal Care
FETO and surfactant
Fetoscopic Endoluminal Tracheal Occlusion (FETO) is where a balloon is fetoscopically placed in the fetus’ trachea inhibiting the egress of lung fluid hopefully promoting lung growth [1]. In the TOTAL trials fetuses with left sided CDH were randomly assigned either to the FETO or non-FETO surgery group. In fetuses with moderate left CDH there was no significant improvement in survival to discharge [2], but in those with severe left CDH, 40% of infants in the FETO group survived to discharge, as compared to 15% in the expectant management group [3]. There were, however, significant complications, including preterm, prolabor rupture of membranes and preterm birth [2,3]. FETO is therefore now used routinely only in fetuses with severe CDH. Other studies [4,5] have found benefits, including in right sided diaphragmatic hernias [6].
Surfactant administration used as part of neonatal resuscitation in CDH infants has had mixed efficacy and studies have demonstrated that CDH patients do not have inherent surfactant deficiency. FETO, however, has been associated with a reduction in type two pneumocytes and hence surfactant deficiency. Surfactant administration has been shown to be useful in the first 48 hours after balloon removal as lavage for thick airway secretions [7]. In a retrospective cohort study, after controlling for confounders, surfactant recipients who had had FETO tended towards improved survival and had a reduction in extracorporeal membrane oxygenation (ECMO) requirement [8].
Antenatal therapies for CDH associated pulmonary hypertension
There have been a number of preclinical studies assessing antenatal therapies for CDH-induced pulmonary hypertension (PH) [9]. Studies on rats and rabbits with CDH induced by nitrofen or olive oil have shown that intra-amniotic sildenafil normalizes lung development and reduces resistance in the pulmonary arteries, as well as having a good safety profile [10–18]. Antenatal sildenafil treatment in lambs was shown to lower pulmonary arterial pressure and increase pulmonary blood flow [19]. A study protocol for antenatal sildenafil administration to prevent PH in CDH has been published, the main aim is to assess the transfer of sildenafil through the placenta in the second and third trimesters [20]. The STRIDER trial (use of sildenafil in pregnancies with poor intrauterine growth), however, was stopped when at the interim analysis there was increased neonatal mortality following administration of sildenafil [21]. A study in rabbits found that combining FETO surgery and sildenafil resulted in increased bronchiolar density and static elastance [22].
In a nitrofen rat model, prenatal exposure to Treprostinil reduced medial wall thickness as compared to controls, however, pulmonary airway development, lung hypoplasia and pulmonary function were unaffected [23]. Nevertheless, Treprostinil crossed the placenta, attained fetal target concentrations and was well tolerated by mother and baby [23]. Selexipag has been shown to decrease the number of air saccules in rabbits and, when combined with sildenafil, increased mean saccular airspace diameter [22]. A combination of intra-amniotic sildenafil and rosiglitazone, showed decreased pulmonary vascular muscularization and increase in peripheral pulmonary blood flow distribution in controls, but not in CDH rats [24]. Sildenafil and 2(S)-amino-6-boronohexanoic acid (ABH), an arginase inhibitor, increased lung nitrite levels by 1.7 times without changes in eNOS expression, suggesting that it could reverse some pulmonary features in CDH by improving local nitric oxide (NO) synthesis. It also prevented pulmonary vascular smooth muscle cell hyperproliferation [25]. These results are promising, but further data on safety and efficacy are required before there can be changes in routine clinical practice.
Timing of Cord Clamping
Delayed cord clamping (DCC) is now recommended for all healthy newborns and has been found to improve outcomes in infants born preterm. DCC, however, may not be feasible in infants with severe CDH when immediate intervention is required [26]. There is, however, a physiological basis for DCC in CDH infants; allowing placental transfusion during the establishment of a functional residual capacity (FRC) and the pulmonary circulation might increase left ventricular preload and maintain cardiac output during a critical period following birth. Animal studies have shown that intact cord resuscitation markedly improved hemodynamic transition after birth [27] and in a lamb model of CDH there was benefit in oxygenation during transition with intact cord resuscitation [28]. In humans, DCC is feasible [29,30] and there are two ongoing randomized controlled trials to investigate the potential short term benefits of DCC in CDH infants.
Respiratory Support
Delivery room management
The standard management of prenatally diagnosed CDH used to be immediate intubation and ventilation and administration of a neuromuscular blocking agent [31]. Dynamic lung compliance is low at birth in CDH infants and a prospective observational study demonstrated that this was adversely affected by administration of a neuromuscular blocking agent. Indeed, the median lung compliance was reduced from 0.22 to 0.16 ml /cmH2O/kg immediately post administration of pancuronium [32] and hence the recommendation is no longer to routinely administer neuromuscular blocking agents. A prospective observational study found that low expiratory tidal volume and high end-tidal CO2 levels at 10 minutes after birth were risk factors for deterioration requiring extra support, including ECMO [33]. Furthermore, low expiratory tidal volumes and lung compliance during the first minute of recorded resuscitation were lower in non survivors [34]. The achievement of higher maximal preductal oxygen saturation prior to transfer to the NICU was associated with greater survival in CDH infants.
There has been concern that intubation and ventilation even without administration of a neuromuscular blocking agent could increase the risk of adverse effects such as ventilator-induced lung injury. A spontaneous breathing (SB) approach has been considered in order to avoid such issues [35]. A retrospective study reported that SB was successful in 6 of 15 prenatally diagnosed patients with mild CDH. Delayed intubation, if required, did not negatively affect outcome (100% survival rate in both successful and unsuccessful SB patients) [36]. Another single-center retrospective study had success with SBA in six of eight patients and this was associated with shorter length of stay and fewer days of mechanical ventilation and oxygen supplementation [37]. Those studies were carried out in high volume CDH centers with multidisciplinary teams of specialists; therefore, the practice may not be generalizable to all centers. An appropriately sized, randomized controlled trial is required to assess the safety of SB compared to standard treatment.
Modes of respiratory support
Gentle ventilation: Gentle ventilation (GV) prioritizes lung protection, including permissive hypercapnia to avoid increasing tidal volume or ventilator pressures [38]. A retrospective study found that overall survival in a GV-group was better than a non-GV group (95% versus 62.1%) [39]. Another retrospective cohort study also found that survival was higher in a GV group compared to a non-GV group (57% versus 26%) [40].
Conventional mechanical ventilation (CMV) versus high frequency oscillatory ventilation (HFOV): A randomized, multicenter trial demonstrated that infants randomized to HFOV or CMV had similar results regarding death or BPD, but patients initially ventilated by CMV were ventilated for fewer days, less often needed ECMO, inhaled nitric oxide (iNO) or sildenafil and had a shorter duration of vasoactive drugs [41]. The trial did not achieve the calculated sample size, nevertheless it was the largest RCT of CDH patients and recruited from nine high volume centers across Europe [41]. A subsequent retrospective, but smaller study (n=80) also found no significant difference between HFOV and CMV with regard to the duration of supplementary oxygen dependency or death [42]. Furthermore, a multicenter retrospective study performed in 15 centers in Japan, with analysis by propensity score matching, did not show any significant difference in mortality between groups [43]. A French retrospective cohort study also found no significant differences between CMV and HFOV regarding duration of oxygen therapy or one year survival [44]. A systematic review of 15 studies up to 2017 found that there was higher incidence of chronic lung disease and mortality in HFOV patients [45], but stated that the evidence was poor and in some studies HFOV was used as rescue therapy.
If conventional ventilation is to be used, then most centers would use volume targeted ventilation. Assessment of the work of breathing demonstrated that it was higher at a volume targeting level of 4 ml/kg compared to 5 or 6 mls/kg, suggesting the higher targeted volumes within the tidal volume range should be used [46].
ECMO
ECMO is a form of extra corporeal life support (ECLS) typically used in severe cases of CDH (hypoplastic lungs, refractory pulmonary hypertension, ineffective FETO surgery and/or congenital heart disease [47]) and stabilization before surgical repair [31]. CDH is the most common non-cardiac indication for neonatal ECMO [48], however, the benefits are still unclear [49].
Venoarterial versus venovenous ECMO: Venoarterial (VA) ECMO takes blood from the right atrium and returns oxygenated blood to the carotid circulation. It provides both cardiac and respiratory support. Venovenous (VV) ECMO takes from and returns blood to the right atrium via a double lumen catheter. VV ECMO provides only respiratory support [50]. Many patients with CDH who have the most severe disease have associated left ventricular disfunction rendering VV ECMO unsuitable [51]. A review of the ECLS registry results suggests that VV ECMO is suitable in select cases, such as respiratory failure due to pulmonary hypoplasia [52]. In comparison to VA ECMO, VV ECMO delivers more oxygenated blood to the myocardium. In addition, VA ECMO returns blood to the systemic circulation, making embolic events more likely than in VV ECMO [50].
Analysis of the ECLS registry results suggests that the ECMO mode does not significantly affect mortality or severe neurologic injury, however, mortality was higher for VV compared to VA if pre-ECMO repair was required [53]. If pre-ECMO repair was not required, VV ECMO was associated with a lower risk of severe neurological injury [53]. A questionnaire directed at healthcare professionals of the ELSO CDH interest group highlighted that over 60% said that VV ECMO is optimal for average-risk neonates, but there was little agreement regarding the criteria to choose either VA or VV ECMO with wide variation in practice between centers and practitioners [54].
Timing of surgical repair and ECMO
Repair on ECMO may have advantages in the most severely-affected infants. A retrospective review of results from six severely compromised neonates found no intra-operative bleeding or clotting complications, five of whom survived [55]. Early repair in patients from the CDH study group was associated with 87.1% survival compared to 78.4% in those who were not repaired on ECMO (p=0.002) [56]. In one retrospective review, patients with “super early" repair (within 24 hours of ECMO initiation) had 71.4% survival compared to 59.7% in the early repair group [57]. There was also a lower mortality rate in early repair compared to delayed repair on ECMO [58]. Another study reported a lower bleeding incidence (5% versus 36%) [59]. A meta-analysis of non RCTs found that early repair reduced mortality [60]. Other studies, however, have reported that repair post-decannulation has better results. In a retrospective chart review, survival was 100% in patients who underwent repair after decannulation with no significant bleeding [61]. Other studies found 85% survival in patients who underwent repair post-decannulation, as opposed to 36% of patients still on ECLS [62]. In another study, there was 94.4% survival rate for those decannulated before repair, whereas early repair was associated with a prolonged ECMO duration and bleeding complications [63]. Data from the CDH study group database and ECLS registry support repair after decannulation in patients who are stable enough [64,65]. A meta-analysis also suggests a reduced mortality and lower post-operative bleeding rates in patients who underwent repair after being weaned from ECMO as compared to repair on ECMO [60].
There is currently no evidence to promote the prophylactic use of ECMO in infants with CDH with small studies showing no benefit to ‘EXIT ECMO’ in those with the highest risk of mortality. There are no randomized studies determining the most appropriate timing of repair related to ECMO and clearly the timing must be influenced by the acuity of the baby. The results of the above, often retrospective, studies suggest post-decannulation repair is associated with good outcomes. In high-risk patients, repair within 72 hours of cannulation may be better than delayed surgery. Accurate predictors of delayed ECMO weaning would be important to inform decision making.
Mortality and morbidity: The benefit of ECMO on CDH mortality is not clear and guidance on which infants are offered ECMO vary by center [66]. An early trial randomized infants with multiple pathological causes for poor oxygenation and included 35 infants with CDH [67]. In that trial, all CDH infants in the conventional treatment arm died (17 of 17) compared to 14 of 18 patients in the ECMO arm. In one study of 120 centers providing ECMO for infants with CDH, mortality was reported as 50%, the average number of infants who had ECMO per center was 3.8±2.4 [68]. Centers with the highest volume of cases had the lowest mortality [68].
On ECMO, several factors have been associated with an increased mortality. A prospective observation study found 100% mortality in CDH neonates on ECMO with hemolysis [69]. A further prospective study assessing Brain Natriuretic Peptide (proBNP, a marker of heart strain) values between days two and seven of ECMO demonstrated an overall mortality rate of 63% compared to 30.6% for patients without a proBNP increase [70]. A multi-center cohort study showed that best preductal PaO2 was significantly lower in non-survivors than in survivors on ECMO [71]. One national study examined the one year mortality of forty six infants with CDH who were successfully weaned from ECMO, 19 who died before a year of life [72]. The causes of death were reported as sepsis, cardiac arrest, cardiac failure, respiratory failure, and 30% as unknown [72]. In a single-center retrospective study, guideline changes focusing on minimizing stimulation, using preductal saturation and less aggressive ventilator/inotrope support were associated with decreased ECMO use from 37% to 13% [73]. A cohort study from the CDH study group found prostacyclin (PGI2) use during the first weeks of life reduced ECLS use (adjusted odds ratio (aOR 0.39)), as well as ECLS duration (8.6 versus12.6 days, p<0.001) [74].
ECMO is associated with many complications. In a prospective study, 48% of patients had an occluded right common carotid artery after ECMO and hence reduced perfusion, however, there was still sufficient blood flow due to collateral supply [75]. An analysis of a children’s hospital database indicated that bloodstream infection is rare in CDH infants on ECMO in the first week (0.6%), but increases with the duration of ECMO (8.6% after three weeks, p=0.002) [76].
Those on ECMO require anticoagulation which increases the risk of bleeding intraoperatively and postoperatively. In a study of 53 CDH infants who required ECMO, post operative bleeding requiring reoperation was identified in 11 patients [77]. The addition of anti-fibrinolytic therapy has helped mitigate risk of bleeding and significantly improved outcomes of patients repaired on ECMO [78]. A review found anticoagulation protocols usually include perioperative aminocaproic acid or tranexamic acid, as well as close monitoring of coagulation parameters [79]. In a single-center retrospective chart review, a standardized perioperative bivalirudin (anti-thrombin) protocol achieved target anticoagulation within the first 12 hours [80]. Another found anticoagulation with bivalirudin reduced surgical bleeding complications and halved blood product requirement when compared to patients on heparin [81].
An institutional standardization of anti-coagulation management of CDH neonates on ECMO, including use of goal-directed thromboelastography (TEG) and a point of care blood test that gave real time information on clotting function, led to a reduction in hemothoraces and reduced requirement for cryoprecipitate [82].
The level of support required before ECMO is significantly associated with level of multi-organ dysfunction, the largest contributors being cardiac arrest and hand bagging. This suggests initiation of ECMO prior to these events is critical to avoid complications [83]. A single-center review suggested that metabolic complications were common (48.1%) followed by mechanical complications (38.9%) and hemorrhage (22.2%) [84]. In a retrospective case series, nearly one in five neonates with CDH developed seizures during their ECMO course and this was associated with lower survival to discharge (OR 0.10, p=0.0006) [85].
Long term neurodevelopmental outcomes: ECMO use in CDH infants appears to be associated with negative effects on neurodevelopmental outcomes. A prospective study of 178 infants requiring ECMO showed that at two years of age, patients with CDH (36 patients) previously treated on ECMO scored significantly lower on IQ testing and selective attention assessment compared to patients treated with ECMO for other indications [86]. Another study of 162 CDH infants who did not require ECMO versus 50 who did showed that at a mean of 22 months, the need for ECMO was associated with a score of 4.6 points lower on cognitive composite and 9.2 points lower on motor composite scores [87]. A further study across an 11-year period at a single center showed that of their 142 patients, 38 required ECMO. In that study, ECMO was also associated with a higher chance of deficit using the ‘functional status scale’ assessment [88]. One study showed that multiple runs were associated with lower motor composite scores as compared to neonates with a single ECMO run [89]. Although ECMO has been associated with adverse neurodevelopmental outcomes those who are offered ECMO are the sicker and have the highest risk of mortality.
Predictors for ECMO requirement: The observed to expected lung head ratio (O/E LHR) is associated with the need for ECMO in infants with right or left sided CDH (O/E LHR less than 32.8% and 47.0% for left and right sided CDH, respectively) [90]. In those with an LHR of less than 25% and poor intrauterine growth, ECMO was less likely to be required compared to infants where growth was adequate [91]. In addition to the lung head ratio, a systematic review found that liver herniation increased the risk for ECMO in left sided diaphragmatic hernias [92].
One study which included 2,985 CDH infants found that including 1 and 5 minute Apgar scores, as well as the highest and lowest post-ductal pCO2 during the first 24 hours of life allowed the authors to stratify infants risk of ECMO in to 10 distinct groups [93]. Another study found that the ‘SNAP-II score’, which consists of arterial blood pressure, pH , PaO2:FiO2, temperature, diuresis and seizure activity at 12 hours, was strongly associated with need for ECMO with a sensitivity and specificity for predicting ECMO therapy of 91% and 64% [94].
The compensatory reserve index is derived from advanced signal processing of an arterial pulse wave measured non-invasively via an infrared sensor [95]. A prospective single center study found that the compensatory reserve index was significantly lower in newborns requiring ECMO compared to those who did not [96].
Some cardiac biomarkers and physiological measurements have shown to be strong predictors of the requirement for ECMO for infants with CDH. Plasma BNP was shown in a retrospective chart review to be significantly increased in patients with PH and those requiring ECMO [97]. A retrospective review found an increased right ventricular eccentricity index in ECMO patients as compared to non-ECMO patients [98]. Another retrospective chart review found that a decrease in LV cardiac index was more strongly associated with need for ECLS compared to non-ECLS patients [99]. Another retrospective review found biventricular dysfunction had the strongest association with ECMO use [100]. Elevated endothelin levels during the first 48 hours of life in CDH neonates were also significantly associated with increased rates of ECMO in a prospective study [101].
Emerging methods of respiratory support
Neurally adjusted ventilator assist (NAVA) uses the electrical activity of the diaphragm (Eadi) to trigger and deliver ventilatory support, hence it has been thought NAVA may not be feasible in CDH infants. The Eadi signal is measured with an Eadi catheter which has nine electrodes and is positioned in the esophagus at the level of the diaphragm [102]. Nevertheless, in a retrospective study of 16 CDH neonates NAVA demonstrated improvements in short term respiratory outcomes after 72 hours [103]. Another retrospective study found that NAVA was successfully used in patients to transition after surgery from to SIMV to NAVA and then NIV (non-invasive ventilation)—NAVA with a reduction in peak inspiratory pressures and mean airway pressures [104]. Furthermore, in one study NAVA was used successfully post-operatively to wean ventilatory support, but of the infants required a patch [105]. There is a crossover study underway comparing NAVA’s safety and efficacy including in infants who had undergone FETO, which will determine if NAVA is feasible in the most high risk patients [106].
Inhalation of a helium-oxygen mixture (heliox therapy), in addition to permissive hypercapnia, was shown to improve gas exchange including a decrease PaCO2 levels in 27 neonates with CDH [107].
A randomized crossover trial found that closed-loop automated oxygen control (CLAC) in CDH infants was associated with an increased time spent in target oxygen range by 34% and the median duration of desaturations was reduced [108].
Flexible bronchoscopy may facilitate ECMO and ventilator weaning through diagnosing airway anomalies and removal of mucous plugs. A study review found it was safe, with high diagnostic and potentially therapeutic value. A trend showing higher tidal volumes post-flexible bronchoscopy was noted [109].
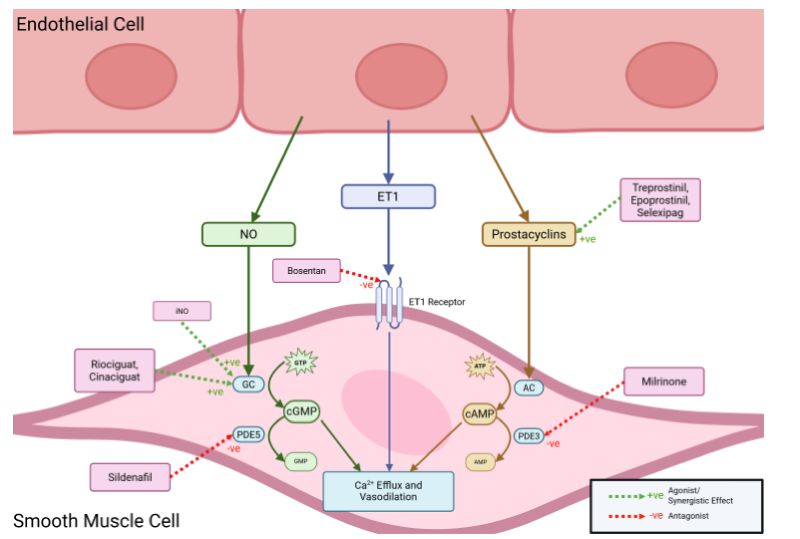
Pulmonary Vasodilators
Pulmonary hypertension (PH) is a common complication of CDH which can result in the preload on the heart increasing and ventricular dysfunction [110]. Hence, use of pulmonary hypertension medications is common in CDH infants. In one series, 68% of neonates with CDH were exposed to at least one PH medication and 11.6% were discharged home with PH medications [111]. At one year in another series, only 9% of infants who had echocardiogram assessments, however, had raised pulmonary pressures; this was particularly found in those who had required prolonged invasive ventilation [112]. There is increasing evidence that fetuses with severe CDH are at risk of left ventricle (LV) hypoplasia and decreased systolic and diastolic function. Infants with CDH then should have an early echocardiography assessment to determine the relative contributions of pulmonary vascular disease and LV dysfunction to PH. Pharmacological recruitment of the arterial duct should be considered if there is ductal dependent systemic blood flow and severe LV dysfunction. In such infants milrinone should be considered, although there is no evidence of efficacy from randomized trials [113].
Inhaled Nitric Oxide (iNO)
iNO is commonly used in CDH infants [35]; it activates the cGMP pathway, which ultimately results in smooth muscle vasodilation [114]. An observational, retrospective study found that iNO use is common amongst different centers (mean 62.3%, range 0-100%) but iNO dosage ranged anywhere from 0.1 to 80 ppm (median 20 ppm) [115]. Not all infants, however, respond to iNO. In a retrospective cohort study only 30.9% of patients showed a significant reduction in the oxygenation index (OI). Among the iNO responders, there was a reduced need for ECMO and reduced mortality [116]. Another retrospective review found 40% of patients in whom iNO was administered had significant improvements in oxygenation: non responders were more likely to have left ventricular systolic dysfunction [117]. An observational study found that 39% of iNO patients had an improved median PaO2/FiO2 ratio, the percentage change post iNO initiation was higher in survivors than non survivors [118]. Other studies have had more negative findings. The NINOS trial found that iNO failed to reduce mortality and led to higher usage of ECMO [119]. The CDH Study Group reported their experience from 70 centers; of 3,367 CDH infants, 2,047 received iNO including 343 infants who did not have PH. Using propensity score matching analysis, iNO use was associated with significantly increased mortality [115]. Retrospective analysis of the results of CDH patients on ECLS who also received iNO did not show a reduced mortality and a significantly higher risk of renal complications [120]. A multi-center cohort study found that use of iNO in the first three days after birth was associated with increased mortality and ECLS use [121].
The American and Canadian professional guidelines now only recommend a trial iNO in the absence of LV systolic dysfunction and to stop use if no echocardiographic findings of improvement are seen within 24 hours [114]. A more personalized approach to iNO initiation should be taken and not started unless there is echocardiographic evidence that it might be efficacious, such as right ventricular dysfunction promoting use and not if there is left ventricular dysfunction [122].
Sildenafil
Sildenafil is a phosphodiesterase-5 inhibitor stopping the breakdown of cGMP [123]. It is being increasingly used in CDH-PH, especially in severe cases [31]. A retrospective case review found that nine infants who received intravenous sildenafil had improved oxygenation at 72 to 96 hours post-infusion, including a significantly decreased supplementary oxygen requirement and an improved OI [124]. Another retrospective chart review demonstrated improved oxygenation in 42.3% of patients during sildenafil administration during the first days of life, but a significant increased requirement for vasopressors [125]. Another retrospective cohort showed 50% of CDH patients responded to sildenafil but the results were not statistically significant [126]. Sildenafil weaned after discharge was not associated with a rebound effect on PH [127].
Milrinone
Milrinone is a phosphodiesterase-3 inhibitor and stops the breakdown of cAMP. and enhances myocardial contractility [114]. It has been shown to improve RV function and relax pulmonary arteries [110]. A retrospective analysis of OI matched neonates with mild to moderate CDH demonstrated that administration of milrinone did not improve OI, pulmonary artery pressures, or left ventricular results [128].
Prostacyclins
Prostacyclins are synthesized from arachidonic acid. They activate the cAMP pathway, which activates protein kinase A causing vasodilation and preventing remodeling of smooth muscle [114]. Prostaglandin E (PGE) has been used to maintain ductus arteriosus patency and unload the suprasystemic right ventricle in neonates with CDH and severe PH. In 57 infants with CDH treated with PGE at a mean of seventeen days, there was improvement in echocardiographic estimates of severe PH. Treatment was not associated with post-ductal hypoxemia or systemic hypotension [129]. Epoprostenol and Treprostinil are synthetic prostacyclins that act to augment this pathway. In a retrospective analysis of the CDH study group registry results demonstrated that prostacyclin analogues were used at some time in 14% of patients with significant variation in timing of initiation and duration of use [130]. Infants, however, who received PGI2 in the first week after birth were less likely to receive ECLS and if they did, there was a shorter mean duration of ECLS [74]. Epoprostenol, also called prostaglandin PGI, in a retrospective analysis of the CDH study group registry results did not significantly increase survival, however that may have been due to limited statistical power [131]. A more recent, retrospective review showed that CDH patients who were started on both ECLS and epoprostenol and survived had shorter time on ECLS [132].
A prospective follow-up study found that 14 patients with severe CDH and life-threatening PH treated with subcutaneous treprostinil had improved pulmonary hemodynamics [133]. In infants with 48 hours of persistent clinical instability, treprostinil administration was associated with a decrease in the OI [134]. A retrospective cohort study found that infants treated with treprostinil, had a significant reduction in serum B-type natriuretic peptide (BNP) at one week and a reduction in severity of PH at one month. Despite those improvements, mortality remained high (35%) [135]. Another retrospective single-center review again showed decrease in BNP levels after one month as well as improved RV and LV function [136]. A systematic review of 136 articles of use of treprostinil in infants younger than one year of age demonstrated it reduced the severity of PH, but had side effects which included hypotension and hematoma [137].
Emerging vasodilators
Bosentan is an enterally administered endothelin-1 receptor antagonist which reduces pulmonary vascular resistance as the endothelin pathway leads to potent pulmonary vasoconstriction. In a case series of 50 patients, bosentan administered as an adjunct therapy was associated with improvements in PH in 72% of patients two weeks after initiation [138].
Differences have been seen in the NO-cGMP pathway in CDH patients, including arteries dilating less to sodium nitroprusside, a NO donor [139]. Furthermore, mRNA suppression of a guanylate cyclase subunit and a protein kinase cGMP subunit were reduced as compared to seen in healthy placentae [139]. Riociguat and Cinaciguat are stimulators of soluble guanylate cyclase, which can restore the NO pathway and exert a vasodilatory effect. They are currently only approved for adults [114].
Selexipag, an oral prostacyclin agonist, is a highly selective vasodilator currently in phase 3 trials for adult PH [110]. Nilotinib may be considered to reverse vascular remodeling [110]. Calcium sensitizers, for example levosimendan, have shown to improve PH and LV dysfunction [114].
The rho-kinase pathway plays an important role in pulmonary vasoconstriction—fasudil, a rho-kinase inhibitor, may be useful but must be studied in clinical trials [114]. Tyrosine kinase inhibition, anti-inflammation, anti-oxidation, transcription factor modulation, and epigenetic modulation are other areas to be considered for future treatment [114]. Use of regenerative stem cells or versatile nanoparticles are currently being investigated in other types of PH, they may be of use in CDH-PH [9,110,114].
Figure 1. Illustration of the mode of action of the pulmonary vasodilators.
Summary
Multiple reviews and consensus reports suggest that iNO should only be used in infants where there is no evidence of left ventricular dysfunction and continued only if an improvement is seen within 24 hours of administration [114,123,140,141]. Use of Milrinone should be used to treat cardiac dysfunction if associated with PH [141]. It has been shown to improve oxygenation in iNO-resistant PH [113]. Sildenafil should be considered with refractory PH or as an adjunct when weaning iNO [114,141,142]. Prostacyclins may be considered as rescue therapy prior, during, or after ECLS in infants with severe or refractory PH [141–143]. They can also be used to maintain patency of ductus arteriosus in infants with severe CDH-PH [114] and offload the RV [113]. Bosentan has limited evidence [143] but can cause vasorelaxation [110].
Long Term Outcomes
Although the overall physical health of CDH survivors is often reported as good, there are a range of morbidities which are significantly higher than in the general population [144–146], with chronic lung disease, exertional dyspnea, gastroesophageal reflux disease GERD, and scoliosis being the most prominent [147]. Given the higher rate of morbidities, yet survivors may report few symptoms, well-structured long-term follow-up programs are required to identify common morbidities at the earliest possible stage [148].
Recurrence
Multiple factors contributing to recurring diaphragmatic hernia have been identified. A multi-center retrospective study identified liver herniation, patch repair and severe defect size as the strongest predictors [149]. Database analyses since then have suggested that a larger defect size, ECMO use and minimally invasive surgical repair, such as thoracoscopy, were associated with higher recurrence rates [150–152]. Others have identified biological patches, such as porcine dermis patches, used during repair increase the risk of recurrence compared to synthetic patches such as Gore-tex [153]. A meta-analysis suggests that overall recurrence rate was higher with biological patches [154].
Neurodevelopmental outcomes
Developmental delays, particularly in motor and cognitive skills, have been linked to CDH. In a prospective observational study most children had normal cognitive and motor function at two years of age [155]. Significantly more CDH survivors may have borderline and low scores in at least one domain compared to controls [156]. Another found 51% of survivors had at least a mild delay in one or more domain [157].
Several factors relating to both the disease itself and its management have been shown to worsen neurodevelopmental outcomes [158], with multiple deficits occurring in patients requiring ECLS, supplemental oxygen at 30 days and patch repair [159]. A prospective longitudinal study showed that increased ventilatory time is inversely correlated to neurodevelopment at 6 and 12 months [160]. Prolonged NICU stay, intubation and supplementary oxygen requirement, fundoplication, abnormal brainstem auditory evoked responses and tracheostomy were predictive of lower scores on the Bayley Scales of Infant and Toddler Development 3 (BSID-III), with right-sided CDH, male gender, lower 5 min APGAR scores, pulmonary hypertension, and delayed start of enteral feeding being particularly predictive of lower cognitive and/or language scores [156,161]. Children born before 39 weeks of gestation were at higher risk for neurodevelopmental delays [162]. CDH has also been associated with hearing loss, found in almost a fifth of CDH survivors in a follow-up study [163].
Autism Spectrum Disorder (ASD) was diagnosed in 11% of CDH survivors in a follow-up study, which is significantly higher than the general population [156]. Another study found a nine times higher rate of diagnosis for ASD amongst CDH children compared to the general population [164]. A nationwide case-control study found a higher incidence of ASD and intellectual disorders compared to controls [165].
Gastrointestinal, nutrition and growth outcomes
A single-center prospective follow-up at one year demonstrated CDH patients had a lower median weight z-score (-1.50); failure to thrive and GI issues were mainly attributed to gastroesophageal reflux (GER) and a higher requirement for tube feeding [166]. GER is a common long-term morbidity of CDH, affecting 20 to 84% of CDH survivors [167,168]. Various factors may contribute to this including increased intra-abdominal pressure after repair, intolerance of feeds, prolonged hospitalization, duration of ventilatory support, size of defect and use of a prosthetic patch [167]. GER may be underdiagnosed if systematic esophageal monitoring is not performed [168], regardless of the presence of symptoms [169]. A retrospective review suggested that a symptom-driven approach to diagnosis is warranted and that those who are diagnosed with GER should attend regular long-term follow-up due to early onset of Barret’s Oesophagus. This is a change in the distal squamous epithelial lining to metaplastic columnar epithelium, predisposing patients to esophageal adenocarcinoma (diagnosed in 7% before 18 years of age in CDH survivors) [170].
The frequency of aspiration is high, with 68% of outpatient studies identifying it in CDH patients [171]. A video fluoroscopic swallow study led to changed management in 8 of 10 patients, often altering the consistency of oral feeds [171]. Small bowel obstruction is another potential complication which may be lower following minimally-invasive repair as opposed to open repair [172]. Studies have shown that between 15% and 25% of patients require feeding tubes [173,174]. Affected patients were lighter and shorter [173]; 69% required fortified feedings due to inadequate growth at discharge [174]. High risk CDH patients, such as those requiring patch repair, ECMO support, prolonged ventilation and an initial pH<7.25, were likely to require tube feeding and 33.3% of patients in the same study were still tube feeding at their last follow-up appointment [175].
Failure to thrive and growth stunting is a recognized complication of CDH survivors, found in 14% of clinic visits in a single center [173]. It could be due to increased energy expenditure, as seen in 58% of patients [173]. Growth retardation was found in 22.7% of CDH survivors in a multicenter retrospective study, however there was body weight improvement between 1.5 and 3 years. Birth weight under 2 kg and home oxygen treatment were significant risk factors [176]. Another retrospective study suggested patients with open repair and prolonged intubation or hospitalization were at highest risk of poor nutritional outcomes [174]. Poor linear growth has shown to persist even at 12 years of age, therefore nutritional assessment and intervention should be started early and continued during childhood [177]. Identifying high-risk patients early and enacting interventions such as minimizing steroid exposure and optimizing nutritional support might improve outcome [178].
Pulmonary outcomes
Respiratory problems accounted for half of the readmissions in the first year of life in a single center [166]. There is a significant correlation between FRC and weight at 12 and 24 months, suggesting aggressive nutritional support may reduce respiratory difficulties as well as failure to thrive. CDH survivors have significantly reduced muscle strength at birth, but this increases in early life [179]. A randomized controlled trial showed respiratory muscle training improved respiratory function, maximal exercise capacity and quality of life in children [180]. The results of a non-randomized trial suggested that a personalized rehabilitation program improved respiratory muscle strength and submaximal effort capacity in adolescents and adults with repaired CDH [181].
Most CDH patients who survive beyond infancy have PH which resolves by five years of age, no matter the initial severity [182]. On exercise stress echocardiography, however, 10% had moderate or severe exercise-induced PH, suggesting some ongoing pulmonary vascular abnormalities [183]. Indications for persistent pulmonary hypertension may be preoperative pulmonary hypertension, associated malformations, and longer invasive ventilation [112].
Almost a quarter of patients were on chronic inhaled medication at a one year follow-up clinic [166]. A history of CDH is associated with higher rates of inhaled bronchodilator prescriptions, inhaled steroids, and asthma related physician visits up to 10 years of age [184].
The results of a multicenter retrospective survey suggested that a quarter of CDH survivors develop musculoskeletal abnormalities. Reduction in OI within 24 hours of birth, large diaphragmatic defects and patch repair were predictive for scoliosis [185]. A low 5 minute Apgar score was predictive of pectus excavatum and large defects predictive of chest asymmetry [185]. Fifteen (10.3%) of 145 CDH survivors who underwent CDH repair developed scoliosis, with over half attributable to multisystem abnormalities [186]. In another follow-up study, pectus excavatum was present in 34% of patients and thoracic asymmetry towards the right hemithorax was present in 45.9% of patients, showing pronounced age-related progression. Severity of chest wall deformities have been linked to prenatal diagnosis, liver herniation, lower LHR, fetal relative lung volume, ECMO use, iNO and surgical repair [187].
Quality of life
CDH survivors overall may have reassuring quality of life scores with a PedsQoL total score of 81 in one series [188]. Nevertheless, several factors, including duration of oxygen therapy, hospitalization for respiratory disease, exercise limitation, inhaled corticosteroid treatment, chest deformity, abnormal cardiopulmonary exercise test, lower FEV1, bronchopulmonary dysplasia, longer initial hospitalization, severe cognitive impairment and orthopedic symptoms were associated with lower QoL scores [188,189]. A cross-sectional study again found reassuring PedsQoL scores but found a significant association between respiratory morbidity and lower PedsQoL score [190]. Severe CDH was associated with significantly with lower PedsQL scores (86 versus 68%) [191]. Furthermore, the quality of life, as assessed by PedsQoL score, was significantly lower in in CDH patients at eight years of age compared to healthy controls [192].
Predictors of Outcomes
Historically, observed to expected lung area to head circumference ratio (O/E LHR) has been used to predict survival in fetuses. A recent study found that observed to expected contralateral lung volume measured with fetal MRI was a more accurate in predicting survival to discharge and one year survival compared to O/E LHR [193].
There are several measures that have been developed in the postnatal period to predict mortality. In a retrospective study from a tertiary neonatal unit, non-survivors had a greater higher mean and highest OI in the first 24 hours after birth [194]. A single-center retrospective cohort study also found the maximum OI in the first 24 hours was significantly associated with mortality [195]. A single center retrospective study also showed that chest radiographic thoracic area on day one was significantly reduced in severe CDH non-survivors as compared to survivors [196]. Respiratory support at 30 days after birth was significantly associated with future pulmonary morbidities: increased use of inhalers and steroids and increased severe V/Q mismatch at one year as well as an increased risk of asthma at five years [197].
Conclusions
Despite advances in antenatal and neonatal care, infants with CDH suffer a high mortality and can experience lifelong morbidity. There are promising new antenatal therapies and forms of postnatal respiratory support, but research is hampered by the relatively small number of patients at any one center. To improve the care of these vulnerable patients, it is essential that multicenter studies are performed and international guidelines regularly reviewed.
Conflicts of Interest
None of the authors declare a conflict of interest.
Funding Statement
None relevant to this review.
Authors Contributions
AN undertook the literature review and wrote the first draft; AG and CH critically reviewed the manuscript. All authors were involved in production and approved the final version of the manuscript.
Acknowledgments
The Lochlan and Greer Foundation supports CH.
References
2. Deprest JA, Benachi A, Gratacos E, Nicolaides KH, Berg C, Persico N, et al. TOTAL Trial for Moderate Hypoplasia Investigators. Randomized Trial of Fetal Surgery for Moderate Left Diaphragmatic Hernia. N Engl J Med. 2021 Jul 8;385(2):119–29.
3. Deprest JA, Nicolaides KH, Benachi A, Gratacos E, Ryan G, Persico N, et al. TOTAL Trial for Severe Hypoplasia Investigators. Randomized Trial of Fetal Surgery for Severe Left Diaphragmatic Hernia. N Engl J Med. 2021 Jul 8;385(2):107–18.
4. Kunpalin Y, Otvodenko A, Van Mieghem T, Chiu PPL, Campisi P, Shinar S, et al. Fetal Endoscopic Tracheal Occlusion (FETO) for Left and Right Congenital Diaphragmatic Hernia in Canada. Prenat Diagn. 2025 Jun;45(6):778–86.
5. Li Q, Liu S, Ma X, Yu J. Fetal endoscopic tracheal occlusion for moderate and severe congenital diaphragmatic hernia: a systematic review and meta-analysis of randomized controlled trials. Pediatr Surg Int. 2022 Sep;38(9):1217–26.
6. Russo FM, Cordier AG, Basurto D, Salazar L, Litwinska E, et al. Fetal endoscopic tracheal occlusion reverses the natural history of right-sided congenital diaphragmatic hernia: European multicenter experience. Ultrasound Obstet Gynecol. 2021 Mar;57(3):378–85.
7. Wild KT, Rintoul NE, Ades AM, Gebb JS, Moldenhauer JS ,et al. The Delivery Room Resuscitation of Infants with Congenital Diaphragmatic Hernia Treated with Fetoscopic Endoluminal Tracheal Occlusion: Beyond the Balloon. Fetal Diagn Ther. 2024;51(2):184–90.
8. Sevilmis YD, Olutoye OO 2nd, Peiffer S, Mehl SC, Belfort MA, Rhee CJ, et al. Surfactant Therapy in Congenital Diaphragmatic Hernia and Fetoscopic Endoluminal Tracheal Occlusion. J Surg Res. 2024 Apr; 296:239–48.
9. Marulanda K, Tsihlis ND, McLean SE, Kibbe MR. Emerging antenatal therapies for congenital diaphragmatic hernia-induced pulmonary hypertension in preclinical models. Pediatr Res. 2021 May;89(7):1641–9.
10. Russo FM, Toelen J, Eastwood MP, Jimenez J, Miyague AH, Vande Velde G, et al. Transplacental sildenafil rescues lung abnormalities in the rabbit model of diaphragmatic hernia. Thorax. 2016 Jun;71(6):517–25.
11. Burgos CM, Pearson EG, Davey M, Riley J, Jia H, Laje P, et al. Flake AW, Peranteau WH. Improved pulmonary function in the nitrofen model of congenital diaphragmatic hernia following prenatal maternal dexamethasone and/or sildenafil. Pediatr Res. 2016 Oct;80(4):577–85.
12. Mous DS, Kool HM, Buscop-van Kempen MJ, Koning AH, Dzyubachyk O, et al. Rottier RJ. Clinically relevant timing of antenatal sildenafil treatment reduces pulmonary vascular remodeling in congenital diaphragmatic hernia. Am J Physiol Lung Cell Mol Physiol. 2016 Oct 1;311(4): L734–42.
13. Verla MA, Style CC, Olutoye OO. Prenatal intervention for the management of congenital diaphragmatic hernia. Pediatr Surg Int. 2018 Jun;34(6):579–87.
14. Mous DS, Kool HM, Burgisser PE, Buscop-van Kempen MJ, Nagata K, Boerema-de Munck A, et al. Treatment of rat congenital diaphragmatic hernia with sildenafil and NS-304, selexipag's active compound, at the pseudoglandular stage improves lung vasculature. Am J Physiol Lung Cell Mol Physiol. 2018 Aug 1;315(2): L276–85.
15. Okolo FC, Zhang G, Rhodes J, Potoka DA. Intra-amniotic Sildenafil Treatment Modulates Vascular Smooth Muscle Cell Phenotype in the Nitrofen Model of Congenital Diaphragmatic Hernia. Sci Rep. 2018 Dec 5;8(1):17668.
16. Russo FM, De Bie F, Hodges R, Flake A, Deprest J. Sildenafil for Antenatal Treatment of Congenital Diaphragmatic Hernia: From Bench to Bedside. Curr Pharm Des. 2019;25(5):601–8.
17. De Bie FR, Basurto D, Kumar S, Deprest J, Russo FM. Sildenafil during the 2nd and 3rd Trimester of Pregnancy: Trials and Tribulations. Int J Environ Res Public Health. 2022 Sep 6;19(18):11207.
18. Yoshida S, Kreger AM, Shaik IH, West RE 3rd, Venkataramanan R, Gittes GK. Intra-amniotic sildenafil administration in rabbits: Safety, pharmacokinetics, organ distribution and histologic evaluation. Toxicol Appl Pharmacol. 2023 Jun 15; 469:116527.
19. Kashyap AJ, Dekoninck PLJ, Rodgers KA, Thio M, Mcgillick EV, Amberg BJ, et al. Antenatal sildenafil treatment improves neonatal pulmonary hemodynamics and gas exchange in lambs with diaphragmatic hernia. Ultrasound Obstet Gynecol. 2019 Oct;54(4):506–16.
20. Russo FM, Benachi A, Van Mieghem T, De Hoon J, Van Calsteren K, Annaert P et al. Antenatal sildenafil administration to prevent pulmonary hypertension in congenital diaphragmatic hernia (SToP-PH): study protocol for a phase I/IIb placenta transfer and safety study. Trials. 2018 Sep 27;19(1):24.
21. Patel S. Sildenafil (Revatio and Viagra) should not be used to treat intrauterine growth restriction. Pfizer; 2018.
22. Russo FM, Da Cunha MGMCM, Jimenez J, Lesage F, Eastwood MP, Toelen J, et al. Complementary Effect of Maternal Sildenafil and Fetal Tracheal Occlusion Improves Lung Development in the Rabbit Model of Congenital Diaphragmatic Hernia. Ann Surg. 2022 Mar 1;275(3):e586–95.
23. De Bie FR, Halline CG, Kotzur T, Hayes K, Rouse CC, Chang J, et al. Prenatal treprostinil reduces the pulmonary hypertension phenotype in the rat model of congenital diaphragmatic hernia. EBioMedicine. 2022 Jul;81:104106.
24. Yoshida S, Eichelberger O, Ulis M, Kreger AM, Gittes GK, Church JT. Intra-Amniotic Sildenafil and Rosiglitazone Late in Gestation Ameliorate the Pulmonary Hypertension Phenotype in Congenital Diaphragmatic Hernia. J Pediatr Surg. 2024 Aug;59(8):1515–25.
25. Toso A, Aránguiz O, Céspedes C, Navarrete O, Hernández C, Vio CP, et al. Congenital diaphragmatic hernia: phosphodiesterase-5 and Arginase inhibitors prevent pulmonary vascular hypoplasia in rat lungs. Pediatr Res. 2024 Mar;95(4):941–48.
26. Gien J. Timing of umbilical cord clamping in infants with congenital diaphragmatic hernia. Semin Perinatol. 2023 Jun;47(4):151746.
27. Bhatt S, Alison BJ, Wallace EM, Crossley KJ, Gill AW, Kluckow M, et al. Delaying cord clamping until ventilation onset improves cardiovascular function at birth in preterm lambs. J Physiol. 2013 Apr 15;591(8):2113–26.
28. Kashyap AJ, Hodges RJ, Thio M, Rodgers KA, Amberg BJ, McGillick EV, et al. Physiologically based cord clamping improves cardiopulmonary haemodynamics in lambs with a diaphragmatic hernia. Arch Dis Child Fetal Neonatal Ed. 2020 Jan;105(1):18–25.
29. Lefebvre C, Rakza T, Weslinck N, Vaast P, Houfflin-Debarge V, Mur S, et al. French CDH Study Group. Feasibility and safety of intact cord resuscitation in newborn infants with congenital diaphragmatic hernia (CDH). Resuscitation. 2017 Nov; 120:20–5.
30. Foglia EE, Ades A, Hedrick HL, Rintoul N, Munson DA, Moldenhauer J, et al. Initiating resuscitation before umbilical cord clamping in infants with congenital diaphragmatic hernia: a pilot feasibility trial. Arch Dis Child Fetal Neonatal Ed. 2020 May;105(3):322–6.
31. Snoek KG, Reiss IK, Greenough A, Capolupo I, Urlesberger B, Wessel L, et al. CDH EURO Consortium. Standardized Postnatal Management of Infants with Congenital Diaphragmatic Hernia in Europe: The CDH EURO Consortium Consensus - 2015 Update. Neonatology. 2016;110(1):66–74.
32. Murthy V, D'Costa W, Nicolaides K, Davenport M, Fox G, Milner AD, et al. Neuromuscular blockade and lung function during resuscitation of infants with congenital diaphragmatic hernia. Neonatology. 2013;103(2):112-7.
33. O'Rourke-Potocki A, Ali K, Murthy V, Milner A, Greenough A. Resuscitation of infants with congenital diaphragmatic hernia. Arch Dis Child Fetal Neonatal Ed. 2017 Jul;102(4): F320–3.
34. Mank A, Carrasco Carrasco C, Thio M, Clotet J, Pauws SC, DeKoninck P, et al. Tidal volumes at birth as predictor for adverse outcome in congenital diaphragmatic hernia. Arch Dis Child Fetal Neonatal Ed. 2020 May;105(3):248–52.
35. Williams E, Greenough A. Respiratory Support of Infants with Congenital Diaphragmatic Hernia. Front Pediatr. 2021 Dec 24; 9:808317.
36. Cochius-den Otter SCM, Horn-Oudshoorn EJJ, Allegaert K, DeKoninck PLJ, Peters NCJ, Cohen-Overbeek TE, et al. Routine Intubation in Newborns with Congenital Diaphragmatic Hernia. Pediatrics. 2020 Oct;146(4): e20201258.
37. Kipfmueller F, Leyens J, Pugnaloni F, Bo B, Grass T, Lemloh L, et al. Mueller A. Spontaneous breathing in selected neonates with very mild congenital diaphragmatic hernia. Pediatr Pulmonol. 2024 Mar;59(3):617–24.
38. Kunisaki SM, Desiraju S, Yang MJ, Lakshminrusimha S, Yoder BA. Ventilator strategies in congenital diaphragmatic hernia. Semin Pediatr Surg. 2024 Aug;33(4):151439.
39. Takayasu H, Masumoto K, Jimbo T, Sakamoto N, Sasaki T, Uesugi T et al. Analysis of risk factors of long-term complications in congenital diaphragmatic hernia: A single institution's experience. Asian J Surg. 2017 Jan;40(1):1–5.
40. Bojanić K, Pritišanac E, Luetić T, Vuković J, Sprung J, Weingarten TN, et al. Survival of outborns with congenital diaphragmatic hernia: the role of protective ventilation, early presentation and transport distance: a retrospective cohort study. BMC Pediatr. 2015 Oct 12; 15:155.
41. Snoek KG, Capolupo I, van Rosmalen J, Hout Lde J, Vijfhuize S, Greenough A, et al CDH EURO Consortium. Conventional Mechanical Ventilation Versus High-frequency Oscillatory Ventilation for Congenital Diaphragmatic Hernia: A Randomized Clinical Trial (The VICI-trial). Ann Surg. 2016 May;263(5):867–74.
42. Derraugh G, Levesque M, Schantz D, Sesha M, Minski J, Baier J, et al. High-frequency vs. conventional ventilation at the time of CDH repair is not associated with higher mortality and oxygen dependency: a retrospective cohort study. Pediatr Surg Int. 2020 Nov;36(11):1275–80.
43. Fuyuki M, Usui N, Taguchi T, Hayakawa M, Masumoto K, Kanamori Y, et al. Japanese Congenital Diaphragmatic Hernia Study Group. Prognosis of conventional vs. high-frequency ventilation for congenital diaphragmatic hernia: a retrospective cohort study. J Perinatol. 2021 Apr;41(4):814–23.
44. Semama C, Vu S, Kyheng M, Le Duc K, Plaisant F, Storme L, Claris O, et al. High-frequency oscillatory ventilation versus conventional ventilation in the respiratory management of term neonates with a congenital diaphragmatic hernia: a retrospective cohort study. Eur J Pediatr. 2022 Nov;181(11):3899–906.
45. Yang HB, Pierro A, Kim HY. Comparison of conventional mechanical ventilation and high-frequency oscillatory ventilation in congenital diaphragmatic hernias: a systematic review and meta-analysis. Sci Rep. 2023 Sep 26;13(1):16136.
46. Lee R, Hunt KA, Williams EE, Dassios T, Greenough A. Work of breathing at different tidal volume targets in newborn infants with congenital diaphragmatic hernia. Eur J Pediatr. 2022 Jun;181(6):2453–8.
47. Yu PT, Jen HC, Rice-Townsend S, Guner YS. The role of ECMO in the management of congenital diaphragmatic hernia. Semin Perinatol. 2020 Feb;44(1):151166.
48. Grover TR, Rintoul NE, Hedrick HL. Extracorporeal membrane oxygenation in infants with congenital diaphragmatic hernia. Semin Perinatol. 2018 Mar;42(2):96–103.
49. McHoney M, Hammond P. Role of ECMO in congenital diaphragmatic hernia. Arch Dis Child Fetal Neonatal Ed. 2018 Mar;103(2): F178–81.
50. Chernoguz A, Monteagudo J. Neonatal venoarterial and venovenous ECMO. Semin Pediatr Surg. 2023 Aug;32(4):151326.
51. Patel N, Massolo AC, Paria A, Stenhouse EJ, Hunter L, Finlay E, et al. Early Postnatal Ventricular Dysfunction Is Associated with Disease Severity in Patients with Congenital Diaphragmatic Hernia. J Pediatr. 2018 Dec; 203:400–7.e1.
52. Ham PB, Wise LJ, Wang EJ, Stansfield B, Hatley RM, Walters KC, et al. Venovenous Extracorporeal Membrane Oxygenation for Cardiorespiratory Failure due to Congenital Diaphragmatic Hernia and Ebstein's Anomaly. Am Surg. 2015 Sep;81(9): e322–4.
53. Guner YS, Harting MT, Fairbairn K, Delaplain PT, Zhang L, Chen Y, et al. Outcomes of infants with congenital diaphragmatic hernia treated with venovenous versus venoarterial extracorporeal membrane oxygenation: A propensity score approach. J Pediatr Surg. 2018 Nov;53(11):2092–9.
54. Delaplain PT, Jancelewicz T, Di Nardo M, Zhang L, Yu PT, Cleary JP, et al. Study by ELSO CDH Interest Group. Management preferences in ECMO mode for congenital diaphragmatic hernia. J Pediatr Surg. 2019 May;54(5):903–8.
55. Prabhu S, Mattke AC, Anderson B, McBride C, Cooke L, Karl T, et al. Repair of congenital diaphragmatic hernia during extracorporeal life support: experience with six neonates. ANZ J Surg. 2016 Sep;86(9):711–6.
56. Glenn IC, Abdulhai S, Lally PA, Schlager A. Congenital Diaphragmatic Hernia Study Group. Early CDH repair on ECMO: Improved survival but no decrease in ECMO duration (A CDH Study Group Investigation). J Pediatr Surg. 2019 Oct;54(10):2038–43.
57. Steen EH, Lee TC, Vogel AM, Fallon SC, Fernandes CJ, Style CC, et al. Congenital diaphragmatic hernia repair in patients on extracorporeal membrane oxygenation: How early can we repair? J Pediatr Surg. 2019 Jan;54(1):50–4.
58. Dao DT, Burgos CM, Harting MT, Lally KP, Lally PA, Nguyen HT, et al. Surgical Repair of Congenital Diaphragmatic Hernia After Extracorporeal Membrane Oxygenation Cannulation: Early Repair Improves Survival. Ann Surg. 2021 Jul 1;274(1):186–94.
59. Smithers CJ, Zalieckas JM, Rice-Townsend SE, Kamran A, Zurakowski D, Buchmiller TL. The Timing of Congenital Diaphragmatic Hernia Repair on Extracorporeal Membrane Oxygenation Impacts Surgical Bleeding Risk. J Pediatr Surg. 2023 Sep;58(9):1656–62.
60. Lin M, Liao J, Li L. The Timing of Surgery for Congenital Diaphragmatic Hernia in Infants, on or after Weaning from Extracorporeal Membrane Oxygenation: A Meta-Analysis. Eur J Pediatr Surg. 2024 Oct;34(5):435–43.
61. Partridge EA, Peranteau WH, Rintoul NE, Herkert LM, Flake AW, Adzick NS, et al. Timing of repair of congenital diaphragmatic hernia in patients supported by extracorporeal membrane oxygenation (ECMO). J Pediatr Surg. 2015 Feb;50(2):260–2.
62. Golden J, Jones N, Zagory J, Castle S, Bliss D. Outcomes of congenital diaphragmatic hernia repair on extracorporeal life support. Pediatr Surg Int. 2017 Feb;33(2):125–31.
63. Robertson JO, Criss CN, Hsieh LB, Matsuko N, Gish JS, Mon RA, et al. Comparison of early versus delayed strategies for repair of congenital diaphragmatic hernia on extracorporeal membrane oxygenation. J Pediatr Surg. 2018 Apr;53(4):629–34.
64. Glenn IC, Abdulhai S, McNinch NL, Lally PA, Ponsky TA, Schlager A. Congenital Diaphragmatic Hernia Study Group. Evaluating the utility of the "late ECMO repair": a congenital diaphragmatic hernia study group investigation. Pediatr Surg Int. 2018 Jul;34(7):721–6.
65. Delaplain PT, Harting MT, Jancelewicz T, Zhang L, Yu PT, Di Nardo M, et al. Potential survival benefit with repair of congenital diaphragmatic hernia (CDH) after extracorporeal membrane oxygenation (ECMO) in select patients: Study by ELSO CDH Interest Group. J Pediatr Surg. 2019 Jun;54(6):1132–7.
66. Martino A, Lista G, Guner YS. Management of the CDH patient on ECLS. Semin Fetal Neonatal Med. 2022 Dec;27(6):101407.
67. UK collaborative randomised trial of neonatal extracorporeal membrane oxygenation. UK Collaborative ECMO Trail Group. Lancet. 1996 Jul 13;348(9020):75–82.
68. Martino AM, Nguyen DV, Delaplain PT, Dinh P, Jancelewicz T, Harting MT, et al. Center Volume and Survival Relationship for Neonates With Congenital Diaphragmatic Hernia Treated With Extracorporeal Life Support. Pediatr Crit Care Med. 2023 Dec 1;24(12):987–97.
69. Lemloh L, Bo B, Ploeger H, Dolscheid-Pommerich R, Mueller A, Kipfmueller F. Hemolysis during Venovenous Extracorporeal Membrane Oxygenation in Neonates with Congenital Diaphragmatic Hernia: A Prospective Observational Study. J Pediatr. 2023 Dec; 263:113713.
70. Bo B, Balks J, Gries K, Holdenrieder S, Mueller A, Kipfmueller F. Increased N-terminal Pro-B-Type Natriuretic Peptide during Extracorporeal Life Support Is Associated with Poor Outcome in Neonates with Congenital Diaphragmatic Hernia. J Pediatr. 2022 Feb; 241:83–9.e2.
71. Terui K, Furukawa T, Nagata K, Hayakawa M, Okuyama H, Amari S, et al. Best pre-ductal PaO2 prior to extracorporeal membrane oxygenation as predictor of mortality in patients with congenital diaphragmatic hernia: a retrospective analysis of a Japanese database. Pediatr Surg Int. 2021 Dec;37(12):1667–73.
72. Davis PJ, Firmin RK, Manktelow B, Goldman AP, Davis CF, Smith JH, et al. Long-term outcome following extracorporeal membrane oxygenation for congenital diaphragmatic hernia: the UK experience. J Pediatr. 2004 Mar;144(3):309–15.
73. Yang MJ, Fenton S, Russell K, Yost CC, Yoder BA. Left-sided congenital diaphragmatic hernia: can we improve survival while decreasing ECMO? J Perinatol. 2020 Jun;40(6):935-42.
74. Ramaraj AB, Rice-Townsend SE, Foster CL, Yung D, Jackson EO, Ebanks AH, et al. Congenital Diaphragmatic Hernia Study Group. Association Between Early Prostacyclin Therapy and Extracorporeal Life Support Use in Patients With Congenital Diaphragmatic Hernia. JAMA Pediatr. 2023 Jun 1;177(6):582–9.
75. Henzler C, Zöllner FG, Weis M, Zimmer F, Schoenberg SO, Zahn K, et al. Cerebral Perfusion After Repair of Congenital Diaphragmatic Hernia with Common Carotid Artery Occlusion After ECMO Therapy. In Vivo. 2017 Jul-Aug;31(4):557–64.
76. Keene S, Grover TR, Murthy K, Pallotto EK, Brozanski B, Gien J, et al. Children’s Hospitals Neonatal Consortium’s (CHNC) Congenital Diaphragmatic Hernia Focus Group. Extracorporeal membrane oxygenation and bloodstream infection in congenital diaphragmatic hernia. J Perinatol. 2019 Oct;39(10):1384–91.
77. Schmoke N, Rose A, Nemeh C, Wu YS, Wang P, Kurlansky P, et al. Optimizing Congenital Diaphragmatic Hernia Repair on ECMO: Evaluating the Risk of Bleeding. J Pediatr Surg. 2024 Dec;59(12):161766.
78. Desai AA, Ostlie DJ, Juang D. Optimal timing of congenital diaphragmatic hernia repair in infants on extracorporeal membrane oxygenation. Semin Pediatr Surg. 2015 Feb;24(1):17–9.
79. Low ZK, Tan ASM, Nakao M, Yap KH. Congenital diaphragmatic hernia repair in patients requiring extracorporeal membrane oxygenation: are outcomes better with repair on ECMO or after decannulation? Interact Cardiovasc Thorac Surg. 2021 Apr 19;32(4):632–7.
80. Snyder CW, Goldenberg NA, Nguyen ATH, Smithers CJ, Kays DW. A perioperative bivalirudin anticoagulation protocol for neonates with congenital diaphragmatic hernia on extracorporeal membrane oxygenation. Thromb Res. 2020 Sep;193:198–203.
81. Credille C, Eason CR, Evans LL, Bothwell S, Gien J, Vaughn AE, et al. Bleeding Complications between Bivalirudin and Heparin for Extracorporeal Membrane Oxygenation in Neonates with Congenital Diaphragmatic Hernia. Fetal Diagn Ther. 2025;52(2):133–8.
82. Phillips RC, Shahi N, Leopold D, Levek C, Shirek G, Hilton S, et al. Thromboelastography-guided management of coagulopathy in neonates with congenital diaphragmatic hernia supported by extracorporeal membrane oxygenation. Pediatr Surg Int. 2020 Sep;36(9):1027–33.
83. Delaplain PT, Ehwerhemuepha L, Nguyen DV, Di Nardo M, Jancelewicz T, Awan S, et al. The development of multiorgan dysfunction in CDH-ECMO neonates is associated with the level of pre-ECMO support. J Pediatr Surg. 2020 May;55(5):830–4.
84. Stewart LA, Klein-Cloud R, Gerall C, Fan W, Price J, Hernan RR, et al. Extracorporeal Membrane Oxygenation (ECMO) and its complications in newborns with congenital diaphragmatic hernia. J Pediatr Surg. 2022 Aug;57(8):1642–8.
85. Danzer E, Massey SL, Flohr SJ, Mathew L, Hoffman C, Abramson A, et al. Extracorporeal Membrane Oxygenation for Neonates With Congenital Diaphragmatic Hernia: Prevalence of Seizures and Outcomes. Pediatr Crit Care Med. 2023 May 1;24(5):e224–35.
86. Schiller RM, Madderom MJ, Reuser JJ, Steiner K, Gischler SJ, Tibboel D, et al. Neuropsychological Follow-up After Neonatal ECMO. Pediatrics. 2016 Nov;138(5):e20161313.
87. Danzer E, Hoffman C, D'Agostino JA, Connelly JT, Waqar LN, Gerdes M, et al. Short-Term Neurodevelopmental Outcome in Congenital Diaphragmatic Hernia: The Impact of Extracorporeal Membrane Oxygenation and Timing of Repair. Pediatr Crit Care Med. 2018 Jan;19(1):64–74.
88. O'Hara JE, Buchmiller TL, Bechard LJ, Akhondi-Asl A, Visner G, Sheils C, et al. Long-Term Functional Outcomes at 1-Year After Hospital Discharge in Critically Ill Neonates With Congenital Diaphragmatic Hernia. Pediatr Crit Care Med. 2023 Aug 1;24(8):e372–81.
89. Herco M, Sloan P, Vogel A, Vrecenak J, Najaf T. Survival and Neurodevelopmental Outcomes in Congenital Diaphragmatic Hernia Patients with Single versus Repeat Extracorporeal Membrane Oxygenation Runs. Am J Perinatol. 2024 May;41(S 01):e305–11.
90. Gebb J, Flohr S, Mathew L, Oliver ER, Barr K, Gallagher T, et al. Observed/Expected Lung-To-Head Ratio and Total Lung Volumes That Identify Fetuses With Severe Congenital Diaphragmatic Hernia in a North American Fetal Center. Prenat Diagn. 2025 May;45(5):676–85.
91. Aydın E, Khanmammadova N, Burns P, Lim FY, Habli MA, Peiró JL. Bias in the prenatal lung measurements in fetal congenital diaphragmatic hernia with intrauterine growth restriction. J Perinat Med. 2024 Apr 19;52(5):546–51.
92. Russo FM, Eastwood MP, Keijzer R, Al-Maary J, Toelen J, Van Mieghem T, et al. Lung size and liver herniation predict need for extracorporeal membrane oxygenation but not pulmonary hypertension in isolated congenital diaphragmatic hernia: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2017 Jun;49(6):704–13.
93. Jancelewicz T, Brindle ME, Harting MT, Tolley EA, Langham MR Jr, Lally PA, et al. Extracorporeal Membrane Oxygenation (ECMO) Risk Stratification in Newborns with Congenital Diaphragmatic Hernia (CDH). J Pediatr Surg. 2018 Oct;53(10):1890–5.
94. Kipfmueller F, Schroeder L, Melaku T, Geipel A, Berg C, Gembruch U, et al. Prediction of ECMO and Mortality in Neonates with Congenital Diaphragmatic Hernia Using the SNAP-II Score. Klin Padiatr. 2019 Nov;231(6):297–303.
95. Latimer AJ, Counts CR, Van Dyke M, Bulger N, Maynard C, Rea TD et al. THE COMPENSATORY RESERVE INDEX FOR PREDICTING HEMORRHAGIC SHOCK IN PREHOSPITAL TRAUMA. Shock. 2023 Oct 1;60(4):496–502.
96. Leopold DK, Phillips RC, Shahi N, Gien J, Marwan AI, Kinsella JP, et al. Low postnatal CRI values are associated with the need for ECMO in newborns with CDH. J Pediatr Surg. 2020 Jan;55(1):39–44.
97. Partridge EA, Hanna BD, Rintoul NE, Herkert L, Flake AW, Adzick NS, et al. Brain-type natriuretic peptide levels correlate with pulmonary hypertension and requirement for extracorporeal membrane oxygenation in congenital diaphragmatic hernia. J Pediatr Surg. 2015 Feb;50(2):263–6.
98. Altit G, Bhombal S, Van Meurs K, Tacy TA. Ventricular Performance is Associated with Need for Extracorporeal Membrane Oxygenation in Newborns with Congenital Diaphragmatic Hernia. J Pediatr. 2017 Dec; 191:28-34. e1.
99. Gaffar S, Ellini AR, Ahmad I, Chen Y, Ashrafi AH. Left ventricular cardiac output is a reliable predictor of extracorporeal life support in neonates with congenital diaphragmatic hernia. J Perinatol. 2019 May;39(5):648–53.
100. Le LS, Kinsella JP, Gien J, Frank BS. Failure to Normalize Biventricular Function Is Associated with Extracorporeal Membrane Oxygenation Use in Neonates with Congenital Diaphragmatic Hernia. J Pediatr. 2023 Sep;260:113490.
101. Lemloh L, de Vadder A, Melaku T, Bo B, Patel N, Holdenrieder S, et al. Increased circulating Endothelin-1 is a risk factor for ECMO use and mortality in neonates with congenital diaphragmatic hernia: a prospective observational study. Respir Res. 2025 Mar 21;26(1):110.
102. Amin R, Arca MJ. Feasibility of Non-invasive Neurally Adjusted Ventilator Assist After Congenital Diaphragmatic Hernia Repair. J Pediatr Surg. 2019 Mar;54(3):434–8.
103. Kurland Y, Gurung K, Pallotto EK, Manimtim W, Feldman K, Staggs VS, et al. Neurally adjusted ventilatory assist in neonates with congenital diaphragmatic hernia. J Perinatol. 2021 Aug;41(8):1910–5.
104. Meinen RD, Alali YI, Al-Subu A, Wilhelm M, Wraight CL, McAdams RM, et al. Neurally-Adjusted Ventilatory Assist Can Facilitate Extubation in Neonates With Congenital Diaphragmatic Hernia. Respir Care. 2021 Jan;66(1):41–9.
105. Oda A, Lehtonen L, Soukka H. Neurally adjusted ventilatory assist can be used to wean infants with congenital diaphragmatic hernias off respiratory support. Acta Paediatr. 2018 Apr;107(4):718–9.
106. Poole G, Harris C, Shetty S, Dassios T, Jenkinson A, Greenough A. Study protocol for a randomised cross-over trial of Neurally adjusted ventilatory Assist for Neonates with Congenital diaphragmatic hernias: the NAN-C study. Trials. 2024 Jan 20;25(1):72.
107. Wise AC, Boutin MA, Knodel EM, Proudfoot JA, Lane BP, Evans ML, et al. Heliox Adjunct Therapy for Neonates With Congenital Diaphragmatic Hernia. Respir Care. 2018 Sep;63(9):1147–53.
108. Kaltsogianni O, Dassios T, Harris C, Jenkinson A, Lee RA, Sugino M, et al. Closed-loop oxygen system in late preterm/term, ventilated infants with different severities of respiratory disease. Acta Paediatr. 2023 Jun;112(6):1185–9.
109. Leyens J, Schroeder L, Salatsch C, Schmitt J, Sabir H, Mueller A, et al. Flexible Bronchoscopy in Neonates With Congenital Diaphragmatic Hernia. Pediatr Pulmonol. 2025 May;60(5):e71128.
110. Harting MT. Congenital diaphragmatic hernia-associated pulmonary hypertension. Semin Pediatr Surg. 2017 Jun;26(3):147–53.
111. Seabrook RB, Grover TR, Rintoul N, Weems M, Keene S, Brozanski B, et al. Children’s Hospitals Neonatal Consortium Congenital Diaphragmatic Hernia Focus Group. Treatment of pulmonary hypertension during initial hospitalization in a multicenter cohort of infants with congenital diaphragmatic hernia (CDH). J Perinatol. 2021 Apr;41(4):803–13.
112. Dirickx A, Levy M, Mellul K, Coignard M, Khen-Dunlop N, Lapillonne A, et al. Infants With a Congenital Diaphragmatic Hernia Had Favourable Pulmonary Hypertension Outcomes at 1-Year of Age. Acta Paediatr. 2025 Jul;114(7):1680–90.
113. Kinsella JP, Steinhorn RH, Mullen MP, Hopper RK, Keller RL, Ivy DD, et al. Pediatric Pulmonary Hypertension Network (PPHNet). The Left Ventricle in Congenital Diaphragmatic Hernia: Implications for the Management of Pulmonary Hypertension. J Pediatr. 2018 Jun;197:17–22.
114. De Bie FR, Avitabile CM, Joyeux L, Hedrick HL, Russo FM, Basurto D, et al. Neonatal and fetal therapy of congenital diaphragmatic hernia-related pulmonary hypertension. Arch Dis Child Fetal Neonatal Ed. 2022 Sep;107(5):458–66.
115. Putnam LR, Tsao K, Morini F, Lally PA, Miller CC, Lally KP, et al. Congenital Diaphragmatic Hernia Study Group. Evaluation of Variability in Inhaled Nitric Oxide Use and Pulmonary Hypertension in Patients With Congenital Diaphragmatic Hernia. JAMA Pediatr. 2016 Dec 1;170(12):1188–94.
116. Herich K, Schaible T, Reinhard J, Rafat N, Otto C, Schleef R, et al. iNO Therapy in Patients with Congenital Diaphragmatic Hernia - Discrepancy between Widespread Use and Therapeutic Effects. Klin Padiatr. 2019 Nov;231(6):320–5.
117. Lawrence KM, Monos S, Adams S, Herkert L, Peranteau WH, Munson DA, et al. Inhaled Nitric Oxide Is Associated with Improved Oxygenation in a Subpopulation of Infants with Congenital Diaphragmatic Hernia and Pulmonary Hypertension. J Pediatr. 2020 Apr; 219:167–72.
118. Thodika FMSA, Dimitrova S, Nanjundappa M, Davenport M, Nicolaides K, Dassios T, et al. Prediction of survival in infants with congenital diaphragmatic hernia and the response to inhaled nitric oxide. Eur J Pediatr. 2022 Oct;181(10):3683–9.
119. Novotny AM. The Use of Inhaled Nitric Oxide in Congenital Diaphragmatic Hernia. Adv Neonatal Care. 2020 Dec;20(6):479–86.
120. Gowda SH, Almaazmi A, Hagan J, Niemyjski E, Vogel AM, Jancelewicz T, et al. Inhaled Nitric Oxide Utilization in Congenital Diaphragmatic Hernia Treated With Extracorporeal Life Support: A Propensity Score Analysis. ASAIO J. 2023 May 1;69(5):504–10.
121. Noh CY, Chock VY, Bhombal S, Danzer E, Patel N, Dahlen A, et al. Congenital Diaphragmatic Hernia Study Group. Early nitric oxide is not associated with improved outcomes in congenital diaphragmatic hernia. Pediatr Res. 2023 Jun;93(7):1899–906.
122. Greenough A. Management of infants with congenital diaphragmatic hernia and pulmonary hypertension-one size does not fit all. Pediatr Res. 2023 Jun;93(7):1795–96.
123. Holden KI, Rintoul NE, McNamara PJ, Harting MT. Congenital diaphragmatic hernia-associated pulmonary hypertension. Semin Pediatr Surg. 2024 Aug;33(4):151437.
124. Bialkowski A, Moenkemeyer F, Patel N. Intravenous sildenafil in the management of pulmonary hypertension associated with congenital diaphragmatic hernia. Eur J Pediatr Surg. 2015 Apr;25(2):171–6.
125. Kipfmueller F, Schroeder L, Berg C, Heindel K, Bartmann P, Mueller A. Continuous intravenous sildenafil as an early treatment in neonates with congenital diaphragmatic hernia. Pediatr Pulmonol. 2018 Apr;53(4):452–60.
126. Cohen JL, Nees SN, Valencia GA, Rosenzweig EB, Krishnan US. Sildenafil Use in Children with Pulmonary Hypertension. J Pediatr. 2019 Feb; 205:29–34. e1.
127. Falcão MC, Telles APA, Aguiar MLMD, Bigio JZD. Use of sildenafil in late postoperative period of congenital diaphragmatic hernia. J Bras Pneumol. 2021 Sep 6;47(4): e20210143.
128. Mears M, Yang M, Yoder BA. Is Milrinone Effective for Infants with Mild-to-Moderate Congenital Diaphragmatic Hernia? Am J Perinatol. 2020 Feb;37(3):258–63.
129. Lawrence KM, Berger K, Herkert L, Franciscovich C, O'Dea CLH, Waqar LN, et al. Use of prostaglandin E1 to treat pulmonary hypertension in congenital diaphragmatic hernia. J Pediatr Surg. 2019 Jan;54(1):55–9.
130. Ramaraj AB, Rice-Townsend SE, Foster CL, Yung D, Jackson EO, Ebanks AH, et al. Congenital Diaphragmatic Hernia Study Group. Trends in use of prostacyclin analogs for management of CDH-associated pulmonary hypertension. Pediatr Surg Int. 2022 Sep;38(9):1241–7.
131. Skarda DE, Yoder BA, Anstadt EE, Lally PA, Greene T, McFadden M, et al. Epoprostenol Does Not Affect Mortality in Neonates with Congenital Diaphragmatic Hernia. Eur J Pediatr Surg. 2015 Oct;25(5):454–9.
132. Shah NR, Burgi K, Lotakis DM, Olive MK, McCormick AD, Perrone EE, et al. Patterns and Outcomes of Epoprostenol Use in Infants with Congenital Diaphragmatic Hernia Requiring Extracorporeal Life Support. J Pediatr. 2025 Jan;276:114286.
133. Carpentier E, Mur S, Aubry E, Pognon L, Rakza T, Flamein F, et al. Safety and tolerability of subcutaneous treprostinil in newborns with congenital diaphragmatic hernia and life-threatening pulmonary hypertension. J Pediatr Surg. 2017 Sep;52(9):1480–3.
134. Jozefkowicz M, Haag DF, Mazzucchelli MT, Salgado G, Fariña D. Neonates Effects and Tolerability of Treprostinil in Hypertension with Persistent Pulmonary. Am J Perinatol. 2020 Jul;37(9):939–46.
135. Lawrence KM, Hedrick HL, Monk HM, Herkert L, Waqar LN, Hanna BD, et al. Treprostinil Improves Persistent Pulmonary Hypertension Associated with Congenital Diaphragmatic Hernia. J Pediatr. 2018 Sep;200:44–9.
136. De Bie FR, Avitabile CM, Flohr S, Land S, Mathew L, Wang Y, et al. Treprostinil in Neonates with Congenital Diaphragmatic Hernia-Related Pulmonary Hypertension. J Pediatr. 2023 Aug;259:113420.
137. Lee D. Treprostinil Use in the NICU. Adv Neonatal Care. 2024 Dec 1;24(6):554–60.
138. De Vadder A, Lemloh L, Bo B, Hale L, Patel N, Mueller A, et al. Bosentan as adjunctive therapy in neonates with congenital diaphragmatic hernia-associated pulmonary hypertension: a case series. Eur J Pediatr. 2025 Feb 13;184(3):198.
139. Horn-Oudshoorn EJJ, Broekhuizen M, Harhangi MS, Simons SHP, Eggink AJ, Danser AHJ, et al. Vascular reactivity is altered in the placentas of fetuses with congenital diaphragmatic hernia. Placenta. 2024 Jan;145:51–9.
140. Gien J, Kinsella JP. Management of pulmonary hypertension in infants with congenital diaphragmatic hernia. J Perinatol. 2016 Jun;36 Suppl 2:S28–31.
141. Puligandla P, Skarsgard E, Baird R, Guadagno E, Dimmer A, Ganescu O, et al. Canadian Congenital Diaphragmatic Hernia Collaborative. Diagnosis and management of congenital diaphragmatic hernia: a 2023 update from the Canadian Congenital Diaphragmatic Hernia Collaborative. Arch Dis Child Fetal Neonatal Ed. 2024 Apr 18;109(3):239–52.
142. Mous DS, Kool HM, Wijnen R, Tibboel D, Rottier RJ. Pulmonary vascular development in congenital diaphragmatic hernia. Eur Respir Rev. 2018 Jan 24;27(147):170104.
143. Puligandla PS, Grabowski J, Austin M, Hedrick H, Renaud E, Arnold M, et al. Management of congenital diaphragmatic hernia: A systematic review from the APSA outcomes and evidence based practice committee. J Pediatr Surg. 2015 Nov;50(11):1958–70.
144. Öst E, Joelsson MÖ, Burgos CM, Frenckner B. Self-assessed physical health among children with congenital diaphragmatic hernia. Pediatr Surg Int. 2016 May;32(5):493–503.
145. Gerall CD, Stewart LA, Price J, Kabagambe S, Sferra SR, Schmaedick MJ, et al. Long-term outcomes of congenital diaphragmatic hernia: A single institution experience. J Pediatr Surg. 2022 Apr;57(4):563–9.
146. Pollack JC, Hollinger LE, Buchmiller TL, Jancelewicz T. Long-term follow-up in congenital diaphragmatic hernia. Semin Pediatr Surg. 2024 Aug;33(4):151443.
147. Obed M, Doktor F, Bercovitch R, Zani A, Pederiva F. Long-term outcomes beyond childhood in patients treated for congenital diaphragmatic hernia- a systematic review. Pediatr Surg Int. 2025 Mar 18;41(1):96.
148. Morini F, Valfrè L, Bagolan P. Long-term morbidity of congenital diaphragmatic hernia: A plea for standardization. Semin Pediatr Surg. 2017 Oct;26(5):301–10.
149. Nagata K, Usui N, Terui K, Takayasu H, Goishi K, Hayakawa M, et al. Risk factors for the recurrence of the congenital diaphragmatic hernia-report from the long-term follow-up study of Japanese CDH study group. Eur J Pediatr Surg. 2015 Feb;25(1):9–14.
150. Putnam LR, Gupta V, Tsao K, Davis CF, Lally PA, Lally KP, et al. Congenital Diaphragmatic Hernia Study Group. Factors associated with early recurrence after congenital diaphragmatic hernia repair. J Pediatr Surg. 2017 Jun;52(6):928–32.
151. Cioci AC, Urrechaga EM, Parreco J, Remer LF, Cowan M, Perez EA, et al. One-year outcomes of congenital diaphragmatic hernia repair: Factors associated with recurrence and complications. J Pediatr Surg. 2021 Sep;56(9):1542–6.
152. Gupta VS, Holden KI, Chiu PP, Ramaraj AB, Miller CM, Popp EC, et al. Recurrence in congenital diaphragmatic hernia: A multicenter, postdischarge pilot study. Surgery. 2025 May;181:109209.
153. de Haro Jorge I, Prat Ortells J, Martín-Solé O, Muñoz Fernandez E, Pertierra A, Martin-Lluis A, et al. Porcine dermal patches as a risk factor for recurrence after congenital diaphragmatic hernia repair. Pediatr Surg Int. 2021 Jan;37(1):59–65.
154. Kamal TR, Tyraskis A, Ghattaura H, Fitchie A, Lakhoo K. Synthetic versus Biological Patches for CDH: A Comparison of Recurrence Rates and Adverse Events, Systematic Review, and Meta-Analysis. Eur J Pediatr Surg. 2023 Jun;33(3):198–209.
155. Snoek KG, Capolupo I, Braguglia A, Aite L, van Rosmalen J, Valfrè L, et al. Neurodevelopmental Outcome in High-Risk Congenital Diaphragmatic Hernia Patients: An Appeal for International Standardization. Neonatology. 2016;109(1):14–21.
156. Danzer E, Hoffman C, D'Agostino JA, Gerdes M, Bernbaum J, Antiel RM, et al. Neurodevelopmental outcomes at 5years of age in congenital diaphragmatic hernia. J Pediatr Surg. 2017 Mar;52(3):437–43.
157. Antiel RM, Lin N, Licht DJ, Hoffman C, Waqar L, Xiao R, et al. Growth trajectory and neurodevelopmental outcome in infants with congenital diaphragmatic hernia. J Pediatr Surg. 2017 Dec;52(12):1944–8.
158. Church JT, Mon R, Wright T, Coughlin MA, Ladino-Torres M, Tapley C, et al. Neurodevelopmental outcomes in CDH survivors: A single institution's experience. J Pediatr Surg. 2018 Jun;53(6):1087–91.
159. Hassan M, Patel D, LaRusso K, Koclas L, Smith-Morin Ot M, Shapiro AJ, et al. Risk stratification helps identify congenital diaphragmatic hernia (CDH) infants in need of formal neurodevelopmental assessment: Observations from a structured, interdisciplinary long-term follow-up clinic. J Pediatr Surg. 2022 May;57(5):846–50.
160. Bevilacqua F, Morini F, Zaccara A, Valfrè L, Capolupo I, Bagolan P, et al. Neurodevelopmental outcome in congenital diaphragmatic hernia survivors: role of ventilatory time. J Pediatr Surg. 2015 Mar;50(3):394–8.
161. Danzer E, Gerdes M, D'Agostino JA, Bernbaum J, Hoffman C, Herkert L, et al. Neurodevelopmental outcome at one year of age in congenital diaphragmatic hernia infants not treated with extracorporeal membrane oxygenation. J Pediatr Surg. 2015 Jun;50(6):898–903.
162. Danzer E, Gerdes M, D'Agostino JA, Bernbaum J, Hoffman C, Herkert LM, et al. Younger gestational age is associated with increased risk of adverse neurodevelopmental outcome during infancy in congenital diaphragmatic hernia. J Pediatr Surg. 2016 Jul;51(7):1084–90.
163. Alenazi A, Derraugh G, Levesque M, Morris MI, Shawyer AC, Lum Min SA, et al. The prevalence of hearing loss in children with congenital diaphragmatic hernia: A longitudinal population-based study. J Pediatr Surg. 2021 Feb;56(2):226–9.
164. Danzer E, Hoffman C, D'Agostino JA, Miller JS, Waqar LN, Gerdes M, et al. Rate and Risk Factors Associated with Autism Spectrum Disorder in Congenital Diaphragmatic Hernia. J Autism Dev Disord. 2018 Jun;48(6):2112–21.
165. Kutasy B, Skoglund C, Löf-Granström A, Öst E, Frenckner B, Mesas Burgos C. Increased risk of clinically relevant neurodevelopmental disorders in survivors of congenital diaphragmatic hernia: a population-based study. Pediatr Surg Int. 2024 Nov 11;40(1):304.
166. Van Ginderdeuren E, Allegaert K, Decaluwe H, Deprest J, Debeer A, Proesmans M. Clinical Outcome for Congenital Diaphragmatic Hernia at the Age of 1 Year in the Era of Fetal Intervention. Neonatology. 2017;112(4):365–71.
167. Marseglia L, Manti S, D'Angelo G, Gitto E, Salpietro C, Centorrino A, et al. Gastroesophageal reflux and congenital gastrointestinal malformations. World J Gastroenterol. 2015 Jul 28;21(28):8508–15.
168. Arcos-Machancoses JV, Ruiz Hernández C, Martin de Carpi J, Pinillos Pisón S. A systematic review with meta-analysis of the prevalence of gastroesophageal reflux in congenital diaphragmatic hernia pediatric survivors. Dis Esophagus. 2018 Jun 1;31(6).
169. Zanini A, Macchini F, Farris G, Morandi A, Festa I, Brisighelli G, et al. Follow-up of Congenital Diaphragmatic Hernia: Need for Routinary Assessment of Acid Gastroesophageal Reflux with pH-metry. Eur J Pediatr Surg. 2018 Dec;28(6):502–7.
170. Pulvirenti R, Sreeram II, van Wijk MP, IJsselstijn H, Kamphuis LS, Rottier RJ, et al. Prevalence of Gastroesophageal Reflux Disease in Congenital Diaphragmatic Hernia Survivors From Infancy to Adulthood. J Pediatr Surg. 2024 Oct;59(10):161593.
171. Ramaraj AB, Foster C, Stark RA. Epidemiology of swallow dysfunction in CDH patients. Am J Surg. 2021 Jun;221(6):1267–70.
172. Martusciello GR, Sullivan GA, Koo N, Pillai S, Madonna MB, Shah AN, et al. Reduced Long-Term Bowel Obstruction Risk With Minimally Invasive Diaphragmatic Hernia Repair. J Surg Res. 2024 Feb;294:144–9.
173. Haliburton B, Mouzaki M, Chiang M, Scaini V, Marcon M, Moraes TJ, et al. Long-term nutritional morbidity for congenital diaphragmatic hernia survivors: Failure to thrive extends well into childhood and adolescence. J Pediatr Surg. 2015 May;50(5):734–8.
174. Vu LT, McFarland C, Bratton B, Lee H. Closer Look at the Nutritional Outcomes of Patients After Primary Repair of Congenital Diaphragmatic Hernia. J Pediatr Gastroenterol Nutr. 2017 Aug;65(2):237–41.
175. Wong MKW, Haliburton B, Graham A, Lapidus-Krol E, Moraes TJ, Marcon MA, et al. Requirement and Duration of Tube Feed Supplementation among Congenital Diaphragmatic Hernia Patients. J Pediatr Surg. 2019 May;54(5):895–8.
176. Terui K, Nagata K, Hayakawa M, Okuyama H, Goishi K, Yokoi A, et al. Growth Assessment and the Risk of Growth Retardation in Congenital Diaphragmatic Hernia: A Long-Term Follow-Up Study from the Japanese Congenital Diaphragmatic Hernia Study Group. Eur J Pediatr Surg. 2016 Feb;26(1):60–6.
177. Leeuwen L, Mous DS, van Rosmalen J, Olieman JF, Andriessen L, Gischler SJ, et al. Congenital Diaphragmatic Hernia and Growth to 12 Years. Pediatrics. 2017 Aug;140(2):e20163659.
178. Kim SH, Park SH, Lee HN, Lee J, Jeong J, Kwon H, et al. Prevalence and perinatal risk factors of growth retardation in congenital diaphragmatic hernia survivors. BMC Pediatr. 2025 Apr 14;25(1):295.
179. Kassim Z, Moxham J, Davenport M, Nicolaides K, Greenough A, Rafferty GF. Respiratory muscle strength in healthy infants and those with surgically correctable anomalies. Pediatr Pulmonol. 2015 Jan;50(1):71–8.
180. Moawd SA, Azab AR, Ibrahim ZM, Verma A, Abdelbasset WK. Impacts of Respiratory Muscle Training on Respiratory Functions, Maximal Exercise Capacity, Functional Performance, and Quality of Life in School-Aged Children with Postoperative Congenital Diaphragmatic Hernia. Dis Markers. 2020 Sep 4;2020:8829373.
181. Velásquez AC, Supervia M, Mata DP, de Agustín Asensio JC, Riaño MA. Safety and efficacy of a therapeutic exercise program in young adults with repaired congenital diaphragmatic hernia. Rehabilitacion. 2024;58(4):100859.
182. Wong M, Reyes J, Lapidus-Krol E, Chiang M, Humpl T, Al-Faraj M, et al. Pulmonary hypertension in congenital diaphragmatic hernia patients: Prognostic markers and long-term outcomes. J Pediatr Surg. 2018 May;53(5):918–24.
183. Critser PJ, Buchmiller TL, Gauvreau K, Zalieckas JM, Sheils CA, Visner GA, et al. Exercise-Induced Pulmonary Hypertension in Long-Term Survivors of Congenital Diaphragmatic Hernia. J Pediatr. 2024 Aug;271:114034.
184. Levesque M, Lum Min SA, Morris MI, Shawyer AC, Keijzer R. Asthma Medication Use in Congenital Diaphragmatic Hernia Survivors: A Retrospective Population Level Data Analysis. Eur J Pediatr Surg. 2020 Feb;30(1):39–44.
185. Takayasu H, Masumoto K, Goishi K, Hayakawa M, Tazuke Y, Yokoi A, et al. Musculoskeletal abnormalities in congenital diaphragmatic hernia survivors: Patterns and risk factors: Report of a Japanese multicenter follow-up survey. Pediatr Int. 2016 Sep;58(9):877–80.
186. Koziara M, Irvine S, Wei N, Karpe P, Rushton P, Fender D, et al. The association of congenital diaphragmatic hernia with scoliosis. Spine Deform. 2025 May;13(3):845–50.
187. Riemer C, Lambsdorff LG, Hutflesz N, Mohr C, Weis M, Weiss C, et al. Children With CDH are at High Risk for Pectus Excavatum Deformity and Progressive Thoracic Asymmetry: Chest Wall Deformities in CDH Survivors. J Pediatr Surg. 2025 Jan;60(1):162057.
188. Darmaun L, Lejeune S, Drumez E, Mur S, de Langle-Chevalier F, Nève V, et al. Quality of life was similar in children with congenital diaphragmatic hernia and oesophageal atresia and related to respiratory morbidity. Acta Paediatr. 2021 Feb;110(2):695–703.
189. Bojanić K, Grizelj R, Vuković J, Omerza L, Grubić M, Ćaleta T, et al. Health-related quality of life in children and adolescents with congenital diaphragmatic hernia: a cross-sectional study. Health Qual Life Outcomes. 2018 Mar 14;16(1):50.
190. Larsen UL, Christensen SA, Herskind AM, Strøm T, Toft P, Halken S. Quality of life in congenital diaphragmatic hernia survivors treated at a non-ECMO centre from 1998 to 2015: a cross-sectional study. BMJ Paediatr Open. 2024 Jan 29;8(1):e002307.
191. Dimmer A, Meehan M, Beauseigle S, Koclas L, Paquette K, Michel Macias C, et al. Disease severity impacts perceived quality of life in congenital diaphragmatic hernia: a prospective observational study. Arch Dis Child. 2024 May 17;109(6):510–4.
192. Sreeram II, Schnater JM, van Rosmalen J, Cochius-den Otter SCM, Peters NCJ, Rottier RJ, et al. Longitudinal Health Status and Quality of Life in Congenital Diaphragmatic Hernia. Pediatrics. 2023 Jun 1;151(6):e2022060385.
193. Phillips R, Shahi N, Meier M, Niemiec S, Ogle S, Acker S, et al. The novel fetal MRI O/E CLV versus O/E LHR in predicting prognosis in congenital diaphragmatic hernias: can we teach an old dog new tricks? Pediatr Surg Int. 2021 Nov;37(11):1499–504.
194. Ali K, Aljuaid N, Algarni SS, Ghazwani A, Alshreedah S, Alghamdi N, et al., Congenital Diaphragmatic Hernia, Predictors of Survival and Adverse Outcomes. Global Pediatric Health. 2024;11.
195. Horn-Oudshoorn EJJ, Vermeulen MJ, Knol R, Te Pas AB, Cochius-den Otter SCM, Schnater JM, et al. The Oxygen Saturation Index as Early Predictor of Outcomes in Congenital Diaphragmatic Hernia. Neonatology. 2023;120(1):63–70.
196. Dassios T, Ali K, Makin E, Bhat R, Krokidis M, Greenough A. Prediction of Mortality in Newborn Infants With Severe Congenital Diaphragmatic Hernia Using the Chest Radiographic Thoracic Area. Pediatr Crit Care Med. 2019 Jun;20(6):534–9.
197. Cauley RP, Potanos K, Fullington N, Bairdain S, Sheils CA, Finkelstein JA, et al. Pulmonary support on day of life 30 is a strong predictor of increased 1 and 5-year morbidity in survivors of congenital diaphragmatic hernia. J Pediatr Surg. 2015 May;50(5):849–55.