Abstract
Cancer is one of the leading causes of death worldwide. The immune system plays an important role in all steps of cancer development, growth and spreading as was repeatedly proven in a variety of experimental and clinical studies. Among the immune cells, Natural Killer cells, or NK cells, are critical to the innate immunity as one of the key effector cells of cancer immunosurveillance. NK cells’ role in the immunity against cancer is mediated by exerting cytotoxicity and secreting a variety of cytokines to inhibit tumor growth and progression. Therefore, one of essential pathways of cancerogenesis and tumor escape from immune recognition and elimination may be associated with NK cell defects. In fact, the abnormal NK cells and their functional impairment in patients with cancer may contribute to the progression of different cancer types. In particular, the exhaustion of NK cells that represents lower cytotoxicity and impaired cytokine production may serve as a predictor for the occurrence of several cancers. The authors have evaluated potential molecular defects in NK cells isolated from healthy donors and cancer patients by assessing expression of key molecular regulators of NK cells, including c-Myc, Notch1, Notch2, p-53, Cdk6 and Rb. The results revealed a reduced expression of c-myc proto-oncogene and Notch1 signaling in NK cells in cancer patients. It was also uncovered that molecular abnormalities were not limited to NK cells but could be also seen in other lymphocytes, particularly T cells. It is conceivable that correction of NK cell molecular defects and functional rescue of NK cells will be great promise for cancer treatment.
Keywords
NK cells, c-Myc, Notch1.2, Lung cancer, Gastric cancer, STAT3, Cdk6
Commentary
Natural Killer (NK) cells play a key role in the immune responses against infection and cancer as powerful cytotoxic effector cells and regulators of both innate and adaptive immunity [1,2]. Therefore, defects in NK cell functions are important mechanisms for immune evasion of malignant cells [3]. For instance, the ability of tumor cells and tumor-associated stromal/infiltrating cells to inhibit NK cell activity, which results in preventing NK cells from recognizing and killing tumor cells, has been reported for melanoma, neuroblastoma, gastrointestinal sarcoma, hepatocellular cancer (HCC), pancreatic cancer, colorectal carcinoma and other types of cancer [4-9]. A potential loss of NK cell numbers and function at preneoplastic stages of tumorigenesis as a possible mechanism for cancer induction and progression has been also recently proposed [5,10]. However, molecular mechanisms regulating NK cell dysfunction and exhaustion in cancer are largely unclear.
Furthermore, the link between NK cell deficiency and cancer risk has been also suggested. For instance, the abnormal NK cells and their functional impairment in patients with chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infection, which contribute to the progression of HCC have been documented [11]. Moreover, comparative mRNA gene expression profiling by wholehuman- genome microarrays combined with phenotypic and functional characterization of circulating NK cells from patients with HCV-related liver cirrhosis and HCC identified energy metabolism and cell motility defects of NK cells as main mechanisms responsible for functional NK cell damage in patients with cancer [12]. Published analysis of the ‘omic’ data (i.e., tumor RNA-seq and wholeexome sequences of germline genomes) representing 13 common types of cancer showed that people with inherited defects in NK cells have a higher risk of developing cancer [13]. The number of inheritably defected genes in NK cells was irreversibly correlated with patients’ survival, level of tumor-infiltrating lymphocytes and response to immunotherapy. The authors proposed that “cancer was a disease of NK cell inherited deficiencies” [13]. They concluded that their results suggest that high cancer risk individuals may be identified based on germline genomes and that cancer development may be prevented by adoptive transfer of healthy NK cells. But the exact mechanisms and origin of NK cell defects that might predispose to cancer development are unknown [14-16].
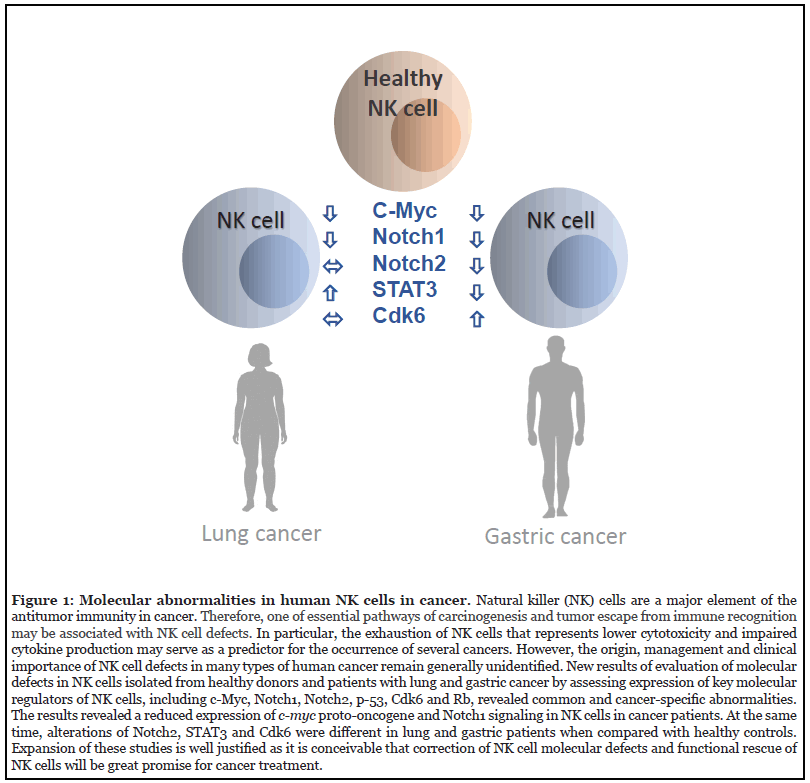
Proto-oncogenes in nonmalignant cells are often involved in signal transduction and performance of mitogenic signals and this regulates cell growth and proliferation or inhibition of apoptosis. They could become oncogenes through mutations or overexpression predisposing the cell to cancer, i.e., unregulated cell division, a slower rate of cell differentiation and increased inhibition of cell death [17]. For example, overexpression of the c-myc proto-oncogene features prominently in most human cancers. Since proto-oncogenes control cell functions that are pivotal for multiple cell development and normal physiologic function, it was hypothesized that proto-oncogenes in immune cells may also be associated with immune defects in cancer [18]. Analyzing peripheral blood lymphocytes harvested from healthy donor and patients with cancer, the authors revealed a significant reduction of c-myc proto-oncogene expression in both circulating NK and T cells in cancer [19]. Of particular importance of these unexpected results was the fact that a decrease in c-myc expression was observed in all cancer patients regardless of the stage of the disease and the presence of metastases. Thus, these results suggest that cancer patients may have a common mechanism of lymphocyte abnormalities in proto-oncogene expression. Next logical step was to evaluate signaling pathways that are functionally associated with c-Myc. Therefore, Notch1, Notch2, p53, Cdk6 and retinoblastoma (Rb) protein expression was evaluated.
Analysis of tumor suppressor protein p53 expression was important for two reasons: it regulates c-Myc transcriptional repression and monitors NK cell maturation [20,21]. And defects in the maturation of NK cells in cancer have been reported [22,23]. However, no significant changes in p53 expression in cancer patients have been detected in the study [19]. Thus, the observed decrease in c-Myc transcription factor was not associated with tumor suppressor p53 abnormality in NK cells isolated from patients with cancer. This is in contrast to tumor cells where a combined defect of c-myc protooncogene and p53 oncosuppressor in the same cells are commonly observed [20,24]. Therefore, it is likely that the immune cells, particularly NK cells, have a different nature of the proto-oncogene defects than seen in malignant cells.
Next, the Notch signaling pathways play an important role in NK cell development and function [25,26], and among four known Notch receptors, only Notch1 and Notch2 have been shown to be expressed on the peripheral blood NK cells [27]. Interestingly, c-myc may serve as a direct target of Notch1signaling in leukocytes [27,28]. In this study, the authors revealed a significantly downregulated expression of Notch1 receptor on NK cells in all cancer patients regardless of the stage of the disease and the presence of metastases. In contrast to Notch1, the expression of Notch2 was not suppressed in all tested patients: Notch2 expression was downregulated only in patients with gastric cancer but not in patients with lung cancer [19]. Another parameter which was determined in this study was the expression of cyclin-dependent kinase Cdk6, an activator of cell proliferation. Interestingly, its expression was changed only in NK cells from in patients with gastric cancer [19]. Cdk6 is known to regulate the progression of the cell cycle via controlling activity of tumor suppressor retinoblastoma protein Rb: after interactions with cyclins, the C-CDK6 enzymatic complex phosphorylates the protein pRb [29,30]. The authors have observed significantly upregulated Cdk6 in NK cells in gastric cancer group, while the expression of total and activated Rb was not changed. It is not known whether there is a connection between an increase in Cdk6 and a decrease in Notch2 levels in NK cells in patients with gastric cancer, but it is apparent that lung and gastric cancer have a similar c-Myc-Notch1 signaling axis in terms of NK cell regulation. Interestingly, the reduction of Notch1expression in NK cells in patients with gastric cancer was more significant than in NK cells in patients with lung cancer.
Thus, while lung cancer patients show a defect in Notch1 expression, gastric cancer patients displayed a significant decrease in both Notch1 and Notch2 expression in NK cells [19]. These results are consistent with other studies where Notch2 signaling was assessed in patients with other cancers. For instance, the central role of Notch2 but not Notch1 in pancreatic intraepithelial neoplasia progression and malignant transformation has been recently demonstrated [31]. Similarly, Notch2 but not Notch1 has been shown to play a key role in pancreatic carcinogenesis [32]. In addition, unlike Notch1, Notch2 is considered to be important for both hepatogenesis and hepatocarcinogenesis [33,34]. Furthermore, elevated expression of Notch2 is associated with gastric cancer formation and a poor prognosis in patients with intestinal and diffuse-type gastric cancers [35,36]. Notch2 may also act as an oncogene that promotes bladder cancer growth and metastasis [37].
Thus, the results of this study revealed the combined defect in both Notch2 and Cdk6 in NK cells in patients with gastric but not lung cancer [19]. Interestingly, previous analysis of NK cells in these patients has demonstrated a highly visible mitotic arrest of NK cells harvested from patients with both types of cancer in G0/G1 phase of the cell cycle [38]. It is possible that Notch2 and Cdk6 are responsible for the mitotic arrest of NK cells in patients with gastric cancer, as both Notch2 and Cdk6 can regulate the cell cycle [39-41]. Despite the common defects of the NK cell cycle in cancer patients, the mechanism of this abnormality in lung cancer seems differ from one in gastric cancer and probably depends on the localization of the malignant process.
Furthermore, CDK6 activation plays an important role in the onset and progression of many types of cancer including lymphoma, leukemia, glioma, glioblastoma, medulloblastoma, squamous cell carcinoma, HCC, bladder, pancreatic, prostate, gastric and lung cancers [42-45]. However, the results of this study suggest that the defect of the NK cell cycle in cancer patients is not associated with the expression or activation of Cdk6. C-Cdk6 enzymatic complex phosphorylates Rb resulting in release of a transcriptional activator E2F, which activates DNA replication, but in this study no significant differences in the appearance of total and activated Rb in NK cells of cancer patients has been detected [38]. Although Cdk6 is important for the control of G1 to S phase transition, new evidence showed that the presence of Cdk6 is not necessary for proliferation in all types of cells [46]. Genetic analysis confirms that many cell types can proliferate in the absence of Cdk4/6 or D cyclins [47]. It has been also reported that Cdk6, in addition to being a cell-cycle kinase, has important kinase-independent functions as a transcriptional regulator and can exert its full tumorpromoting function by enhancing proliferation and stimulating angiogenesis [48]. Therefore, the significance of increased Cdk6 expression in immune cells in cancer is still unclear.
Previous analysis of NK cells in patients with gastric and lung cancer revealed differential changes in STAT3 expression: a significant upregulation of STAT3 expression in lung cancer and a decrease in STAT3 expression in gastric cancer [49]. These changes may be associated with the mitotic disruption of NK cells as STAT3 also plays a role in the transition of the cell cycle from G1 to S and Cdk6 activation depends on STAT3 activity [50]. Thus, observed association between STAT3 and Cdk6 in NK cells in patients with gastric cancer but not in lung cancer may suggest that STAT3 is relevant to the mitotic arrest of NK cells in patients with cancer, while the involvement of Cdk6 requires further investigation.
Finally, studies aiming at identification of potentially inherent or extrinsic tumor-induced immune cell abnormalities in cancer should comprehensively analyze different subsets of lymphocytes and their precursors in patients with different types of cancer and in high cancer risk populations. The studies discussed here [18,19,38,49] represent an example of Notch1/c-Myc signaling evaluation in NK and T cells in cancer. These signaling molecules among other molecules may represent a proper target for immunotherapeutic approach aimed at NK-cell functional repair. This opens the opportunity to test and verify various approaches to restore NK cell response in cancer patients.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
2. Marcenaro E, Notarangelo LD, Orange JS, Vivier E. NK cell subsets in health and disease: New developments. Frontiers in Immunology. 2017 Oct 18;8:1363.
3. Vitale M, Cantoni C, Pietra G, Mingari MC, Moretta L. Effect of tumor cells and tumor microenvironment on NK-cell function. European Journal of Immunology. 2014 Jun;44(6):1582-92.
4. Pietra G, Manzini C, Rivara S, Vitale M, Cantoni C, Petretto A, et al. Melanoma cells inhibit natural killer cell function by modulating the expression of activating receptors and cytolytic activity. Cancer Research. 2012 Mar 15;72(6):1407-15.
5. Jewett A, Kos J, Kaur K, Turnsek TL, Breznik B, Senjor E, et al. Multiple Defects of Natural Killer Cells in Cancer Patients: Anarchy, Dysregulated Systemic Immunity, and Immunosuppression in Metastatic Cancer. Critical Reviews™ in Immunology. 2020;40(2):93-133.
6. Castriconi R, Dondero A, Bellora F, Moretta L, Castellano A, Locatelli F, et al. Neuroblastoma-derived TGF-β1 modulates the chemokine receptor repertoire of human resting NK cells. The Journal of Immunology. 2013 May 15;190(10):5321-8.
7. Ghiringhelli F, Ménard C, Martin F, Zitvogel L. The role of regulatory T cells in the control of natural killer cells: relevance during tumor progression. Immunological Reviews. 2006 Dec;214(1):229-38.
8. Hoechst B, Voigtlaender T, Ormandy L, Gamrekelashvili J, Zhao F, Wedemeyer H, et al. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology. 2009 Sep;50(3):799-807.
9. Li T, Yi S, Liu W, Jia C, Wang G, Hua X, et al. Colorectal carcinoma-derived fibroblasts modulate natural killer cell phenotype and antitumor cytotoxicity. Medical Oncology. 2013 Sep 1;30(3):663.
10. Jewett A, Kos J, Kaur K, Safaei T, Sutanto C, Chen W, et al. Natural killer cells: diverse functions in tumor immunity and defects in pre-neoplastic and neoplastic stages of tumorigenesis. Molecular Therapy-Oncolytics. 2020 Mar 27;16:41-52.
11. Sun C, Sun HY, Xiao WH, Zhang C, Tian ZG. Natural killer cell dysfunction in hepatocellular carcinoma and NK cell-based immunotherapy. Acta Pharmacologica Sinica. 2015 Oct;36(10):1191-9.
12. Zecca A, Barili V, Canetti D, Regina V, Olivani A, Carone C, et al. Energy metabolism and cell motility defect in NK-cells from patients with hepatocellular carcinoma. Cancer Immunology, Immunotherapy. 2020 Aug;69(8):1589-603.
13. Xu X, Li J, Zou J, Feng X, Zhang C, Zheng R, et al. Inherited defects in natural killer cells shape tumor immune microenvironment, clinical outcome and immunotherapy response. BioRxiv. 2018 Jan 1:471912.
14. Messaoudene M, Frazao A, Gavlovsky PJ, Toubert A, Dulphy N, Caignard A. Patient's natural killer cells in the era of targeted therapies: role for tumor killers. Frontiers in Immunology. 2017 Jun 12;8:683.
15. Jaeger BN, Vivier E. Natural killer cell tolerance: control by self or self-control?. Cold Spring Harbor Perspectives in Biology. 2012 Mar 1;4(3):a007229.
16. Vivier E, Ugolini S, Blaise D, Chabannon C, Brossay L. Targeting natural killer cells and natural killer T cells in cancer. Nature Reviews Immunology. 2012 Apr;12(4):239- 52.
17. Felsher DW. MYC inactivation elicits oncogene addiction through both tumor cell–intrinsic and host-dependent mechanisms. Genes & Cancer. 2010 Jun;1(6):597-604.
18. Zakiryanova GK, Wheeler S, Shurin MR. Oncogenes in immune cells as potential therapeutic targets. ImmunoTargets and Therapy. 2018;7:21-28.
19. Zakiryanova GK, Kustova E, Urazalieva NT, Baimukhametov ET, Makarov VA, Turaly GM, et al. Notch signaling defects in NK cells in patients with cancer. Cancer Immunology, Immunotherapy. 2021 Apr;70(4):981-8.
20. Sachdeva M, Mo YY. p53 and c-myc: how does the cell balance “yin” and “yang”? Cell Cycle. 2009; 8:(9):1303.
21. Collin R, St-Pierre C, Guilbault L, Mullins-Dansereau V, Policheni A, Guimont-Desrochers F, et al. An unbiased linkage approach reveals that the p53 pathway is coupled to NK cell maturation. The Journal of Immunology. 2017 Aug 15;199(4):1490-504.
22. Mamessier E, Pradel LC, Thibult ML, Drevet C, Zouine A, Jacquemier J, et al. Peripheral blood NK cells from breast cancer patients are tumor-induced composite subsets. The Journal of Immunology. 2013 Mar 1;190(5):2424-36.
23. Caras I, Grigorescu A, Stavaru C, Radu DL, Mogos I, Szegli G, et al. Evidence for immune defects in breast and lung cancer patients. Cancer Immunology, Immunotherapy. 2004 Dec 1;53(12):1146-52.
24. Dai MS, Jin Y, Gallegos JR, Lu H. Balance of Yin and Yang: ubiquitylation-mediated regulation of p53 and c-Myc. Neoplasia. 2006 Aug 1;8(8):630-44.
25. Manaster I, Gazit R, Goldman-Wohl D, Stern- Ginossar N, Mizrahi S, Yagel S, et al. Notch activation enhances IFNγ secretion by human peripheral blood and decidual NK cells. Journal of Reproductive Immunology. 2010 Jan 1;84(1):1-7.
26. Felices M, Ankarlo DE, Lenvik TR, Nelson HH, Blazar BR, Verneris MR, et al. Notch signaling at later stages of NK cell development enhances KIR expression and functional maturation. The Journal of Immunology. 2014 Oct 1;193(7):3344-54.
27. Palomero T, Lim WK, Odom DT, Sulis ML, Real PJ, Margolin A, et al. NOTCH1 directly regulates c-MYC and activates a feed-forward-loop transcriptional network promoting leukemic cell growth. Proceedings of the National Academy of Sciences. 2006 Nov 28;103(48):18261-6.
28. Weng AP, Millholland JM, Yashiro-Ohtani Y, Arcangeli ML, Lau A, Wai C, et al. c-Myc is an important direct target of Notch1 in T-cell acute lymphoblastic leukemia/lymphoma. Genes & Development. 2006 Aug 1;20(15):2096-109.
29. Malumbres M, Barbacid M. Mammalian cyclindependent kinases. Trends in Biochemical Sciences. 2005 Nov 1;30(11):630-41.
30. Ezhevsky SA, Ho A, Becker-Hapak M, Davis PK, Dowdy SF. Differential regulation of retinoblastoma tumor suppressor protein by G1 cyclin-dependent kinase complexes in vivo. Molecular and Cellular Biology. 2001 Jul 15;21(14):4773-84.
31. Mazur PK, Einwächter H, Lee M, Sipos B, Nakhai H, Rad R, et al. Notch2 is required for progression of pancreatic intraepithelial neoplasia and development of pancreatic ductal adenocarcinoma. Proceedings of the National Academy of Sciences. 2010 Jul 27;107(30):13438- 43.
32. Liu H, Zhou P, Lan H, Chen J, Zhang YX. Comparative analysis of Notch1 and Notch2 binding sites in the genome of BxPC3 pancreatic cancer cells. Journal of Cancer. 2017;8(1):65.
33. Geisler F, Nagl F, Mazur PK, Lee M, Zimber-Strobl U, Strobl LJ, et al. Liver-specific inactivation of Notch2, but not Notch1, compromises intrahepatic bile duct development in mice. Hepatology. 2008 Aug;48(2):607- 16.
34. Jeliazkova P, Jörs S, Lee M, Zimber-Strobl U, Ferrer J, Schmid RM, et al. Canonical Notch2 signaling determines biliary cell fates of embryonic hepatoblasts and adult hepatocytes independent of Hes1. Hepatology. 2013 Jun;57(6):2469-79.
35. Sun Y, Gao X, Liu J, Kong QY, Wang XW, Chen XY, Wang Q, Cheng YF, Qu XX, Li H. Differential Notch1 and Notch2 expression and frequent activation of Notch signaling in gastric cancers. Archives of Pathology & Laboratory Medicine. 2011 Apr;135(4):451-8.
36. Wu X, Liu W, Tang D, Xiao H, Wu Z, Chen C, et al. Prognostic values of four Notch receptor mRNA expression in gastric cancer. Scientific Reports. 2016 Jul 1;6(1):1-9.
37. Hayashi T, Gust KM, Wyatt AW, Goriki A, Jäger W, Awrey S, et al. Not all NOTCH is created equal: the oncogenic role of NOTCH2 in bladder cancer and its implications for targeted therapy. Clinical Cancer Research. 2016 Jun 15;22(12):2981-92.
38. Zakiryanova GK, Kustova E, Urazalieva NT, Baimuchametov ET, Nakisbekov NN, Shurin MR. Abnormal expression of c-Myc oncogene in NK cells in patients with cancer. International Journal of Molecular Sciences. 2019 Jan;20(3):756.
39. Yu HP, Qi ST, Feng WF, Zhang GZ, Zhang HP, Tian JJ. Interference of Notch 2 inhibits the progression of gliomas and induces cell apoptosis by induction of the cell cycle at the G0/G1 phase. Molecular Medicine Reports. 2015 Jan 1;11(1):734-8.
40. Li X, He X, Tian W, Wang J. Short hairpin RNA targeting Notch2 inhibits U87 human glioma cell proliferation by inducing cell cycle arrest and apoptosis in vitro and in vivo. Molecular Medicine Reports. 2014 Dec 1;10(6):2843-50.
41. Tadesse S, Yu M, Kumarasiri M, Le BT, Wang S. Targeting CDK6 in cancer: State of the art and new insights. Cell Cycle. 2015 Oct 18;14(20):3220-30.
42. Timmermann S, Hinds PW, Munger K. Elevated activity of cyclin-dependent kinase 6 in human squamous cell carcinoma lines. Cell Growth and Differentiation- Publication American Association for Cancer Research. 1997 Apr 1;8(4):361-70.
43. Wang G, Zheng L, Yu Z, Liao G, Lu L, Xu R, et al. Increased cyclin-dependent kinase 6 expression in bladder cancer. Oncology Letters. 2012;4(1):43-46.
44. Yoon JH, Kim SA, Kwon SM, Park JH, Park HS, Kim YC, et al. 5'-Nitro-indirubinoxime induces G1 cell cycle arrest and apoptosis in salivary gland adenocarcinoma cells through the inhibition of Notch-1 signaling. Biochimica et Biophysica Acta (BBA)-General Subjects. 2010 Mar 1;1800(3):352-8.
45. Gong W, Wang L, Zheng Z, Chen W, Du P, Zhao H. Cyclin-dependent kinase 6 (CDK6) is a candidate diagnostic biomarker for early non-small cell lung cancer. Translational Cancer Research. 2020 Jan 1;9(1):95-103.
46. Kozar K, Sicinski P. Cell cycle progression without cyclin D-CDK4 and cyclin D-CDK6 complexes. Cell Cycle. 2005 Mar 1;4(3):388-91.
47. Malumbres M, Sotillo R, Santamaría D, Galán J, Cerezo A, Ortega S, et al. Mammalian cells cycle without the D-type cyclin-dependent kinases Cdk4 and Cdk6. Cell. 2004 Aug 20;118(4):493-504.
48. Kollmann K, Heller G, Schneckenleithner C, Warsch W, Scheicher R, Ott RG, et al. A kinase-independent function of CDK6 links the cell cycle to tumor angiogenesis. Cancer Cell. 2013 Aug 12;24(2):167-81.
49. Zakiryanova GK, Kustova E, Urazalieva NT, Amirbekov A, Baimuchametov ET, Nakisbekov NN, et al. Alterations of oncogenes expression in NK cells in patients with cancer. Immunity, Inflammation and Disease. 2017 Dec;5(4):493-502.
50. Fukada T, Ohtani T, Yoshida Y, Shirogane T, Nishida K, Nakajima K, et al. STAT3 orchestrates contradictory signals in cytokine-induced G1 to S cell-cycle transition. The EMBO Journal. 1998 Nov 16;17(22):6670-7.
