Abstract
Background: Cancer remains a leading cause of mortality with modest declines, highlighting the need for more efficacious prevention strategies like early immunological intervention against premalignant disease.
Main body of abstract: Oncogenic viruses demonstrate prophylactic vaccines can successfully reduce malignancy by blocking precipitating infections. However, most cancers lack viral etiology, requiring novel approaches targeting sporadic precancerous states to enable early immunoprevention. Preneoplastic tissues exhibit biological changes making them appealing targets for stimulating immune surveillance before additional mutations cause unconstrained proliferation. High-risk precancers also provide sources of dysregulated self-antigens. Yet challenges exist in lesion identification, overcoming tolerance, and avoiding inflammation potentially worsening progression. Multidisciplinary insights into precancer immunology, predictive biomarkers, antigen discovery, and combinatorial vaccination strategies are illuminating rational vaccine design. Despite obstacles, prophylactic immunization against early dysplastic changes holds disruptive potential if key steps advance this approach. Elucidating preneoplasia immunobiology and progression risk modeling will be critical to guide productive immune targeting while mitigating immunotherapy hazards. Thoughtful translation could eventually shift paradigms by priming immunosurveillance against peak vulnerability lesions.
Short Conclusion: Advancements in precancer vaccines may profoundly expand prevention horizons. Cautious immune targeting of premalignant states could intercept progression toward widely disseminated malignancies. This warrants methodical efforts to unravel the promise of thwarting lethal cancers before they start.
Keywords
Prophylactic Vaccines, Cancer prevention, Preneoplastic lesions, Immune microenvironment, HPV and HBV vaccine
Background
Cancer remains one of the foremost public health challenges worldwide, responsible for nearly 10 million deaths in 2020. While treatment advances have improved survival for certain malignancies, overall cancer mortality rates have only decreased modestly over the past several decades. This underscores the urgent need for more efficacious preventive strategies to reduce cancer incidence on the front end, rather than solely relying on better therapies after diagnosis occurs. Cancer arises from accumulative genetic and epigenetic alterations that allow cells to gradually acquire the hallmark capabilities of malignant growth. This multi-step process provides unique windows of opportunity for early intervention against precancerous conditions before they evolve into invasive disease. Prophylactic vaccination represents one promising but challenging preventive approach, aiming to stimulate immune surveillance against antigenic targets expressed by precancerous lesions in order to halt further progression to cancer [1].
Oncogenic viruses have provided proof-of-principle that prophylactic immunization can successfully reduce cancer incidence by preventing infections associated with malignancy development. Vaccines against hepatitis B virus (HBV) and human papillomavirus (HPV) have demonstrated remarkable efficacy in decreasing hepatocellular carcinoma and cervical cancer rates where vaccination coverage is widespread [2]. However, viral tumors represent only a small minority of the overall cancer burden. The vast majority of human malignancies are not caused by viruses, highlighting the need for novel vaccination strategies targeting non-viral precancerous states. This pioneering frontier in cancer immunoprevention faces considerable obstacles, including uncertainties around defining premalignant stages most likely to progress, technical hurdles in identifying appropriate antigenic targets, challenges in optimizing precancer screening coupled with early vaccination, and concerns regarding potentially aggravating lesion growth. Nevertheless, prophylactic vaccination against precancerous conditions remains a compelling possibility for expanding the horizons of cancer prevention [3]. In recent years, scholarly efforts have made notable progress in optimizing prophylactic vaccination approaches against precancerous conditions prior to malignant transformation. Studies have provided insights into immune tolerance mechanisms within dysplastic lesions, like immunosuppressive cell networks, that enable early evasion of immune clearance; reversing these defects could enhance vaccine efficacy [4]. Advancing biomarkers now allow stratifying individuals’ risk of progression to invasive cancer to precisely time early vaccination for maximal immune prevention efficacy. Applying high-dimensional immune profiling techniques to map the transitional microenvironment of precancerous tissues has expanded understanding of rational combination vaccine/immunomodulator regimens. Genomic, proteomic, and immunopeptidomic approaches have identified candidate antigens differentially expressed in advanced versus normal tissues to focus vaccine targeting [5]. Developing novel vaccine platforms and adjuvants aims to potently trigger cytotoxic T cell responses against typically tolerated dysplastic self-antigens. Probabilistic modeling continues to delineate high-risk transitional lesion stages to optimize early vaccination timing. Investigating prime-boost schedules and combinatorial vaccination seeks sustained immune responses through lengthy preneoplastic phases [6]. While challenges persist, these advances elucidate pathways for continued enhancement of cancer immunoprevention through vaccination strategies informed by emerging preneoplasia immunobiology insights. Prudent translation of these findings may eventually make vaccination a disruptive approach to intercept cancer progression before invasive disease, contingent on rigorous safety assessment.
Cancer prevention through prophylactic vaccination
For decades, the primary strategies for cancer prevention have involved behavioral modification to avoid environmental carcinogens in tobacco, diet, sun exposure, and other modifiable lifestyle factors. While public health campaigns have reduced exposures to hazards known to increase cancer risk, behavioral prevention has limits and therapeutic interventions are still required after malignancy develops. The remarkable success of vaccines for infectious disease prevention has spurred new thinking around applying prophylactic immunization against human cancers as well. Rather than solely treating late-stage malignancies, emerging understanding supports intervening earlier during drawn-out carcinogenesis processes to prevent benign or precancerous lesions from progressing to invasive disease. This could provide the most proximate opportunity for cancer interception before significant morbidity and mortality occur [7]. Recent updates on the development and implementation of H. pylori vaccines highlight the potential for these vaccines to prevent H. pylori infections and associated diseases, including gastritis, peptic ulcers, and gastric cancer [8].
Carcinogenesis is now recognized as an evolutionary process arising from accumulated DNA mutations and epigenetic instability over months to decades, gradually leading cells to acquire hallmark capabilities of malignancy including sustained proliferation, resistance to growth suppression, replicative immortality, angiogenesis, invasion, and metastasis. This step-wise model of cancer development provides discrete preinvasive phases that could serve as rational targets for preventive immunization. High-risk precancerous lesions often already harbor some of the cellular alterations promoting eventual cancer, making them appealing targets for vaccines aimed at initiating immune surveillance and clearance before further progression. Progression risk correlates with the degree of underlying cellular and molecular aberrations, supporting early vaccination against premalignant changes while they remain intrinsically more vulnerable to immune targeting, before additional mutations confer greater resistance [9].
Rationale for focusing on high-risk preneoplastic lesions
Premalignant tissues by definition have already acquired some concerning cancer-associated changes, putting them on a trajectory for likely progression to malignancy without timely intervention. This makes preneoplastic lesions attractive targets for immunopreventive strategies including prophylactic vaccination given their intermediate risk profile. Low-grade dysplastic and hyperplastic epithelial proliferations with minimal cellular atypia have relatively lower inherent risk for progression and can often revert spontaneously, making them less compelling vaccination targets with higher risk of overtreatment or adverse effects. In contrast, more advanced intraepithelial neoplasias classified as high-grade dysplasias or carcinoma in situ are far less likely to regress and have significantly elevated risk for progression, warranting early intervention [10].
Certain chronic inflammatory conditions also predispose cells to malignant transformation, including gastritis leading to stomach cancer, inflammatory bowel disease increasing colon cancer risk, and chronic hepatitis progressing to hepatocellular carcinoma. These represent informative model systems to study premalignancy evolution and opportunities for immunoprevention. Persistent inflammation sustains proliferation, mutagenesis, and tissue damage, allowing initiated cells to accumulate oncogenic alterations until transforming into malignant clones. Targeting precancerous states arising in chronically inflamed tissues via immunotherapy may help intercept this process [11].
Optimally identifying lesions at highest risk for progression using molecular markers or imaging remains a key challenge. Several types of cancer such as hepatocellular carcinoma can be prevented by noninvasive biomarkers. Without biomarkers to precisely identify incipient progression, prevention could be applied too broadly, resulting in overtreatment of indolent lesions unlikely to advance. But focusing only on late-stage precancers risks missing the ideal window for immune-mediated clearance. Defining premalignant phases with the greatest hazard for progression is critical to guide early preventive interventions like vaccination against the lesions most likely to become cancerous if left untreated [12].
Key challenges and promise prophylactic vaccination
Prophylactic vaccination against viral-associated cancers like HPV and HBV has already proven highly successful, lending credibility to the premise of precancer immunization for non-viral tumors. However, over 150 human cancer types lack a viral etiology. This presents challenges for vaccine development including target antigen selection and technical barriers to effective early immunization against slowly evolving, heterogeneous precancerous lesions. No single magic bullet antigen exists for most sporadic cancers. Premalignant progression involves complex emerging phenotypes with considerable diversity across patients, tissues, and cancer types. Defining optimal target antigens associated with high-risk lesions is an essential obstacle. For viral tumors, antiviral vaccines are administered before initial oncogenic infection. Precancer vaccines will need to immunize against endogenous antigens in patients with established risk factors, requiring priming immunity against antigens likely already encountered as self. Administering prophylactic vaccination early enough to intercept progression, while avoiding overtreatment of indolent lesions, will also be logistically challenging. Premalignant progression can occur over many years, demanding sustained immunization through initial priming, boosting, and repeated doses. Intensive screening will be essential to identify subjects with high-risk lesions in time for successful vaccination. Adjuvants and delivery systems will need to overcome tolerance to self antigens. Close immune monitoring is imperative both for maximizing efficacy and assessing concerning adverse events like hyperprogression [13].
Current Prophylactic Vaccines against Viral Cancers
One of the most transformative cancer prevention advances has been the development of prophylactic vaccines against viral infections that can lead to malignancy. By blocking oncogenic viruses before they take hold, immunization provides a means to intercept viral carcinogenesis and reduce cancer incidence. The success of viral cancer vaccines provides a compelling proof-of-principle and model system to inform novel vaccine strategies against non-viral precancerous states.
Human papillomavirus and hepatitis B virus vaccines
Human papillomavirus (HPV) and hepatitis B virus (HBV) are two oncogenic viruses targeted by current prophylactic vaccines aimed at cancer prevention. Persistent HPV infection is responsible for nearly all cases of cervical cancer and also causes a substantial proportion of anal, oropharyngeal, vaginal, vulvar and penile cancers. Meanwhile, chronic HBV infection is the leading cause of hepatocellular carcinoma worldwide. By immunizing against HPV and HBV to prevent these viral infections from ever occurring, vaccines provide an upstream strategy to reduce multiple cancer types associated with each virus.
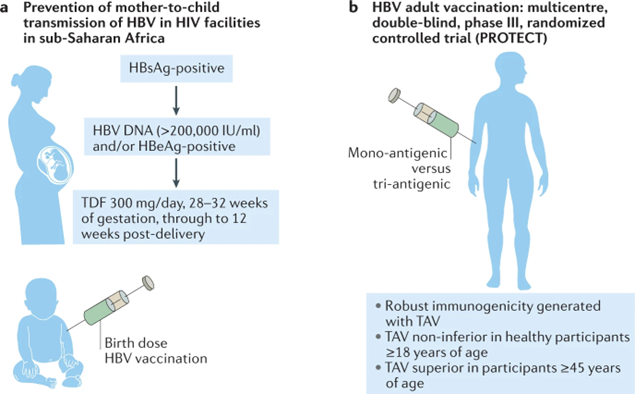
Figure 1 highlights two key vaccination strategies at the forefront of efforts to combat hepatitis B virus (HBV) infection. Part A of the figure depicts integration of HBV screening and management into existing mother-to-child transmission prevention programs for human immunodeficiency virus (HIV). This integrated approach involves screening pregnant women for HBV, providing antiviral treatment if indicated, and administering HBV vaccine immediately after birth to protect infants from vertical transmission. Part B summarizes the PROTECT trial design, a multisite randomized controlled trial comparing the efficacy of a tri-antigenic recombinant HBV vaccine (TAV) to a mono-antigenic alum-adjuvanted yeast-derived vaccine (MAV). By assessing rates of HBV surface antigen, hepatitis B e antigen, and antibody seroconversion, this seminal trial aimed to optimize vaccination strategies to increase protection against chronic HBV infection. Together, these cutting-edge programs exemplify how maximizing HBV vaccination coverage and efficacy through integration and optimization can make vaccination a cornerstone of hepatitis control [14].
Figure 1. Moving towards hepatitis B eradication through vaccination advances [14].
HPV vaccines utilize virus-like particles (VLPs) produced from recombinant expression of major HPV capsid proteins L1 and L2 in yeast to mimic the viral shell structure. Leading HPV vaccines include the bivalent Cervarix targeting oncogenic HPV types 16 and 18, the quadrivalent Gardasil targeting HPV 16/18 along with genital wart-causing types 6 and 11, and the 9-valent Gardasil-9 covering seven high-risk HPV types including 16/18/31/33/45/52/58. Gardasil-9 confers nearly 90% protection against HPV-associated malignancies and has become the dominant global HPV vaccine. For HBV, modern recombinant subunit vaccines contain viral surface antigen HBsAg to elicit high-titer neutralizing antibodies and sterilizing immunity to prevent HBV infection. The first HBV vaccines were plasma-derived, but recombinant vaccines including Engerix-B and Recombivax HB are now standard [15].
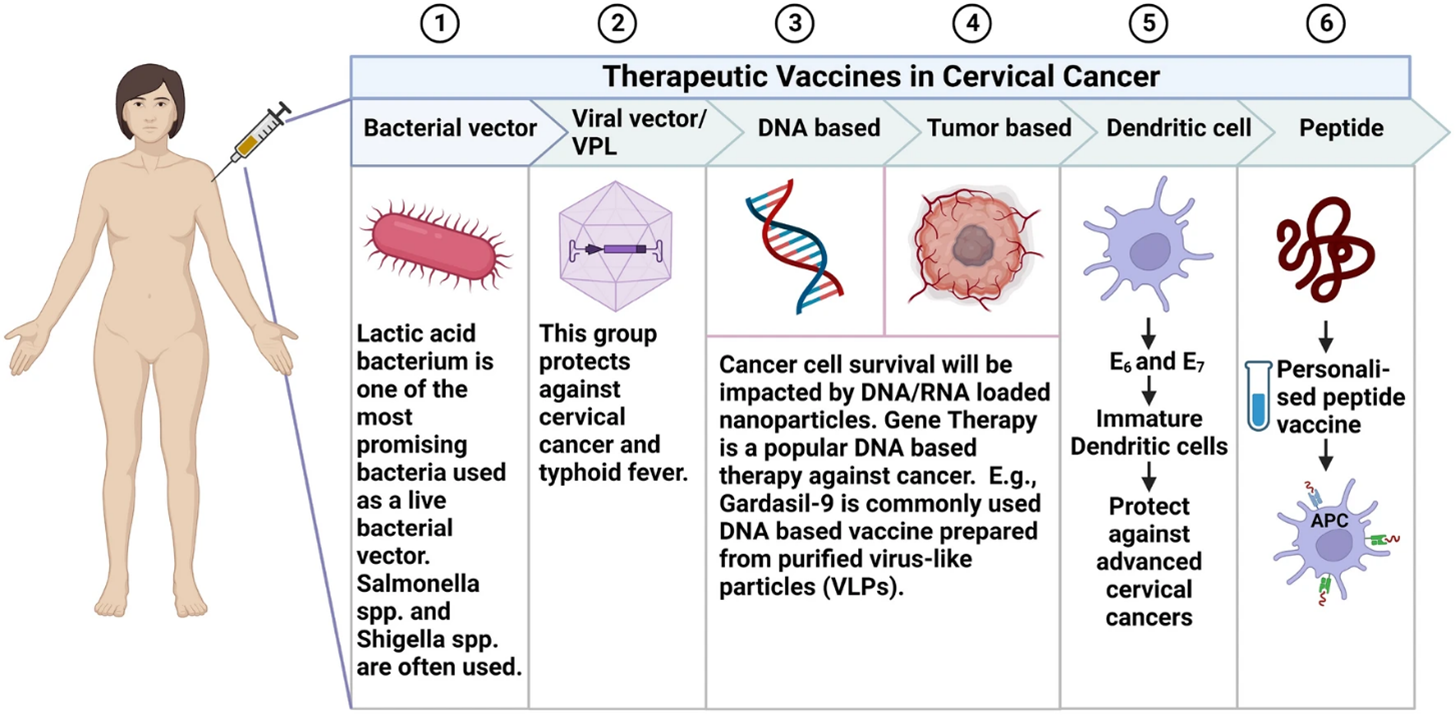
An overview of the various therapeutic vaccine approaches under investigation for cervical cancer is provided in Figure 2. This figure summarizes the diverse strategies being explored to develop therapeutic cervical cancer vaccines. As depicted, these emerging vaccine modalities include peptide/protein vaccines, DNA vaccines, tumor cell vaccines, and dendritic cell vaccines. Each vaccine type is designed to induce tumor-specific immune responses against cervical cancer by targeting cervical cancer-associated antigens through different immunogenic formulations and delivery methods. Together, the therapies highlighted in the figure represent the range of innovative therapeutic vaccination approaches currently under evaluation for the treatment of existing cervical cancer [16].
Figure 2. Types of therapeutic vaccines used against cervical cancer [16].
Practical application of prophylactic precancer vaccination concepts
Prophylactic vaccination against human papillomavirus (HPV) provides a compelling real world example of successful cancer prevention through early immunization. Population-level vaccination of preadolescent girls against cancer-causing HPV strains, before sexual debut and viral exposure, has already led to profound reductions in HPV infection rates and associated precancerous cervical lesions in multiple countries. For instance, Australia was one of the first countries to introduce nationwide HPV vaccination starting in 2007. Routine immunization of 12-13 year old girls led to HPV 16/18 prevalence decreasing from 22.7% to 1.1% over a decade, along with cervical high-grade abnormalities declining by over 90% in vaccinated young women. This dramatic impact demonstrates the potential of prophylactic vaccination in curbing virally-induced malignancies when applied at the optimal pre-exposure time point [17].
An ongoing phase II clinical trial highlights the potential of prophylactic precancer vaccines by targeting Barrett’s esophagus, an established premalignant lesion preceding esophageal adenocarcinoma (NCT04665258). This vaccine comprises antigens associated with progression risk coupled with an adjuvant to stimulate T cell responses against Barrett’s dysplastic lesions. Patients with confirmed high-risk Barrett’s receive the candidate vaccine before invasive malignancy develops, aiming to elicit immune-mediated clearance of precancerous cells and halt further progression. If proven safe and effective, this approach could provide an early interception strategy to prevent the lethal outcome of esophageal cancer. Carefully designed trials like this, applying emerging insights around precancer immunobiology, will continue advancing the disruptive potential of strategic vaccination against early precursor states to prevent cancer before it starts [18].
Efficacy in preventing cancer by blocking infection
Robust data across large clinical trials and population vaccination programs have demonstrated profound efficacy of HPV and HBV vaccines in reducing viral infection incidence and subsequent virus-associated cancer rates. HPV vaccines exceed 95% prophylactic efficacy in preventing persistent HPV infection when administered prior to viral exposure through sexual contact. Consequently, HPV vaccination elicits high-grade cervical lesion reductions upwards of 90% in real-world screening studies if coverage is widespread before girls become sexually active. Universal childhood HPV vaccination has already led to 65% decreases in cervical pre-cancers in young women in the US and UK. Similar reductions are emerging for HPV-linked cancers like oropharyngeal and anal cancers as vaccine coverage expands. Likewise, HBV vaccination provides 90-95% protection against chronic HBV infection when administered in infancy or early childhood. In Taiwan, implementation of universal infant HBV vaccination since 1984 led liver cancer rates to drop from over 300 to less than 100 per 100,000 over 25 years as vaccinated children reached adulthood protected from chronic HBV infection risks. Similar dramatic declines in hepatocellular carcinoma incidence have been observed after initiation of HBV newborn vaccination programs globally from Gambia to Thailand. These data clearly demonstrate the population-level cancer prevention benefits achievable when effective viral vaccines are administered early and broadly to intercept oncogenic infection [19].
Importance of widespread vaccination for success
Maximizing the cancer prevention impact from HPV and HBV vaccines depends critically on achieving high population coverage through routine childhood vaccination. Catch-up vaccination for adolescents and adults does provide direct protection but has less effect on transmission dynamics and infection rates. However, the full benefits are only realized when immunization occurs before viral exposure – emphasizing the need to incorporate both vaccines into widespread pediatric schedules globally. Unfortunately, coverage remains below targets in many countries, particularly for HPV vaccine which faces more hesitancy and access barriers. Improving early vaccination rates will be key to harnessing the prophylactic promise of these vaccines to dramatically lower HPV- and HBV-associated malignancies over decades to come through primary cancer prevention starting at the root [20].
The remarkable success of viral cancer vaccine exemplifies the preventive potential when immunization occurs early against precipitating agents before cancer-driving infection ever occurs. This lends credibility to proposals for expanding vaccine strategies to target non-viral precancers before progression to invasive disease. However, many key differences exist between blocking exogenous oncogenic viruses versus developing prophylaxis against endogenous precancerous conditions. Effectively translating lessons from viral cancer prevention will require overcoming new challenges including target selection, tolerance, delivery timing, and more. While cancer vaccines against HPV and HBV have paved the way, developing safe and effective immunopreventive vaccines for complex sporadic preneoplasias remains a formidable but promising frontier in need of robust multidisciplinary science to move from theory to clinical benefit [21].
Novel Prophylactic Vaccines against Non-viral Cancers
While prophylactic vaccination against oncogenic viruses has proven invaluable, the vast majority of human cancers do not have a viral etiology. Developing preventive vaccines against precancerous states not caused by pathogens represents an intriguing but challenging frontier. Premalignant lesions themselves may provide ideal antigen targets and sources for generating novel immunogens. However, significant knowledge gaps exist around defining premalignant stages most likely to progress and pose the greatest risk. Research to elucidate the probabilistic natural history of preneoplastic progression and distinguish relatively indolent lesions from those destined to become invasive malignancies will be critical to guide targeted vaccine development against the highest risk precancers.
Targeting premalignant lesions directly
Rather than aiming to prevent cancer by blocking upstream infectious triggers, next-generation cancer vaccines will need to target endogenous precancerous lesions directly to stimulate immune clearance [22]. Premalignant tissues exhibit cancer-associated biological changes short of full malignancy, making them intrinsically rational targets for prophylactic vaccines to prevent further progression. Dysplastic and hyperproliferative lesions often already display some degree of uncontrolled growth, resisting normal cell cycle regulation. These early stages of pathogenesis arising from accumulated mutations provide accessible targets for immunological intervention and clearance of abnormal cells before additional genetic events promote unconstrained proliferation and invasion [23].
Preneoplastic cells upregulate a range of cancer-associated antigens compared to their normal counterparts, reflecting activities such as increased proliferation, angiogenesis, apoptosis evasion, and genomic instability [24]. These antigens tend to be overexpressed self molecules rather than foreign antigens. Given intrinsic similarities between normal and precancerous tissues, a key challenge is eliciting effective immune responses against dysplastic lesions while avoiding autoimmunity against healthy cells expressing shared antigens, which may be tolerated at lower levels [25]. Risk stratification to identify precancers at highest risk for progression will help guide vaccination against lesions with greatest potential to become malignant. Optimally leveraging next-generation sequencing, radiomics, and other emerging technologies to molecularly characterize precancers could illuminate biomarkers associated with progression risk and inform target antigen selection [26].
Precancerous lesions as sources of antigens
In addition to serving as targets, premalignant lesions themselves may provide valuable sources of antigenic material to formulate novel precancer vaccines. Their intermediate stage of progression positions preneoplasias to express tumor-associated antigenic changes while remaining more intrinsically vulnerable to immune attack compared to established cancers, making their antigens appealing triggers to prime immunization against lesions before additional progression. Dysplastic cells exhibit upregulated or mutated self-proteins that elicit immune responses which could be harnessed for precancer vaccines [27].
Antigens selectively expressed or overexpressed in premalignant tissues compared to normal cells represent high priority targets, including growth factors, oncofetal proteins, chromosome instability markers, signaling molecules, differentiation regulators, and cancer-testis antigens associated with progression risk. Beyond proteins, accumulating mutations and genomic instability in precancers produce neoantigens recognizable as non-self immunologic triggers. Premalignant lesion cells pruned by immune surveillance could provide an endogenous supply of antigens for vaccine production. Some evidence also supports directly utilizing irradiated precancerous cells or lysates as vaccine ingredients able to stimulate responses against antigens naturally presented in their native conformations. Further genomic and proteomic profiling of preneoplastic states could elucidate additional antigens differentially expressed in lesions prone to progress [28].
Challenges in identifying highest risk lesions destined to progress
While premalignant lesions present enticing antigen sources and targets, a formidable challenge is identifying which preneoplastic states are most likely to progress if left untreated. Low-grade squamous intraepithelial lesions, glandular dysplasias, and early polyps often harbor some degree of genetic instability but limited additional alterations, conferring relatively lower inherent risk of developing into cancers. These may regress or remain indolent without intervention. Conversely, higher grade intraepithelial neoplasias exhibit greater degrees of architectural and cytological atypia, signaling significantly elevated risk for progression if not removed. Yet our probabilistic understanding of the natural history of many precancerous states remains limited [29].
Without reliably distinguishing precancers likely to relentlessly advance from more indolent lesions likely to plateau, interventions carry risks of overtreatment if applied too broadly. But waiting until late-stage carcinomas in situ misses opportunities for early vaccination to stimulate clearance before invasive capability develops. Defining rational risk thresholds to guide vaccination timing remains challenging. Improved biomarkers, imaging, and molecular characterization of preneoplasias could help stratify lesions demonstrating sustained driver mutations or epigenetic alterations most clearly charting a trajectory toward malignancy from more equivocal hyperplasias that could resolve spontaneously. Progress in identifying antigens differentially expressed in advanced precancers and early malignancies shows promise to help prioritize vaccination against lesions already exhibiting a genome-wide cancer-like expression profile [30].
Immune Monitoring to Optimize Early Immunological Intervention against Precancerous Lesions
Advances in immune monitoring and precision biomarkers offer growing opportunities to optimize early immunological intervention against high-risk precancerous states before progression to malignancy. Defining biomarkers to identify lesions at greatest risk of advancement enables targeted vaccination against premalignancies on a trajectory toward cancer. Novel techniques to deeply characterize the local immune microenvironment of dysplastic tissues can elucidate mechanisms of immune tolerance and guide strategies to enhance immune recognition of aberrant cells. Ultimately, leveraging emerging insights on preneoplasia immunology aims to prime immune surveillance selectively against early lesions poised to become invasive cancers absent immunological intervention [31].
Need for biomarkers to identify lesions at highest risk of progression
A formidable challenge confronting precancer vaccination is determining which premalignant changes warrant early immunological intervention versus expectant monitoring. Low-grade epithelial dysplasias often harbor some genetic instability but limited additional driver mutations, posing relatively lower inherent risk for advancement. These may regress or remain indolent without intervention. Conversely, higher grade intraepithelial neoplasias exhibit greater architectural and cytological atypia, signaling substantially elevated risk of progression if unaddressed. Our probabilistic understanding of the natural history of preneoplastic states remains constrained for many lesions [32].
Without reliable biomarkers to distinguish precancers likely to relentlessly advance from more static lesions unlikely to progress further, interventions carry risks of overtreatment if applied too broadly. But waiting until late-stage carcinomas in situ misses opportunities for early vaccination at the precancer’s peak window of immune vulnerability before acquiring additional mutations enabling invasion and dissemination. Defining rational risk thresholds to guide vaccination timing remains challenging but critical. Improved molecular characterization and omics profiling of preneoplasias could help risk-stratify lesions exhibiting clonal driver mutations most indicative of progression potential from equivocal hyperplasias more likely to spontaneously resolve. For example, DNA methylation markers and mutation signatures hold promise to predict risk of progression from Barrett’s esophagus to esophageal adenocarcinoma. Continued advances in precision biomarkers aim to illuminate the precancerous states most clearly charting a trajectory toward malignancy for timely immunological intervention [33].
Novel techniques to characterize local immune microenvironment
In addition to identifying lesions at highest risk for advancement, progress in characterizing the local immune microenvironment within dysplastic tissues provides growing opportunities to elucidate mechanisms of immune tolerance. Premalignant lesions exhibit a complex immunobiology reflecting their intermediate position between normal and invasive cancer. Relative to established malignancies, preneoplasias tend to be more immunogenic due to higher antigenicity combined with less immune suppression. Yet they display some degree of “acquired immune privilege” escaping initial immune recognition through localized immunosuppressive networks [34].
Novel multiplex imaging and cytometry techniques allow deep profiling of immune cell subsets, functional states, checkpoint expression, and architectural relationships within the tumor microenvironment. Applying these high-dimensional tools to characterize precancerous lesion immunology could illuminate transitional states of dysregulation amenable to reprogramming [35]. For example, dense infiltrates of pro-tumorigenic myeloid cells appear to promote immune tolerance even at early stages, representing potential targets for reversal. Checkpoint inhibition may also reinvigorate initially robust but exhausted T cell responses against premalignant antigens. Deciphering the immunological contexture of the precancer microenvironment is critical to counteract acquired immune privilege and inform rational combinatorial strategies for early immunological intervention including synergistic vaccination regimens [36].
Priming immune surveillance of high-risk tissues
A seminal goal of precancer vaccination is preventing the immune system’s “blind spot” against slowly evolving dysplastic changes by actively stimulating immunosurveillance within susceptible tissues. Chronic inflammation predisposing to malignancy induces counterregulatory mechanisms limiting collateral tissue damage, but which also impair immune recognition of precancerous cells. Neoplasia-associated immunosuppression further enables “tumor immune privilege” as premalignant clones expand. Breaching these tolerogenic barriers through prophylactic vaccination could potentially prime cytotoxic targeting of abnormal cells otherwise ignored, especially in concert with checkpoint inhibitors [37].
Certain groups at elevated risk for environmentally driven cancers could be primed by prophylactic vaccination against precipitating antigens or probable early dysplastic changes. For example, women with BRCA1/2 mutations placing them at high lifetime risk for breast/ovarian cancer could be vaccinated against likely occult premalignant lesions before their progression to cancer becomes clinically detectable. Similarly, lung cancer screening participants with heavy smoking exposure could be precancer-vaccinated based on risk for premalignant changes. As probabilistic and mechanistic insights into early immunobiology expand, prime-boost vaccination against tissue-specific antigens upregulated during early carcinogenesis may awaken dormant immunity to precancerous self-proteins otherwise regarded as self [38].
Challenges Ahead for Turning the Promise of Precancer Vaccines into Reality
While prophylactic vaccination against precancerous states holds exciting theoretical promise, turning this potential into clinical reality faces considerable challenges. The heterogeneous and probabilistic nature of preneoplasia progression hampers vaccine development absent defined viral oncogenic triggers. Logistical obstacles also exist around early patient identification, optimal timing of delivery, and challenges of maintaining boosting through lengthy premalignant phases. Perhaps most concerning, immunotherapy carries intrinsic risks of aggravating dysplasia or eliciting malignant evolution which must be cautiously assessed. Nevertheless, concerted efforts to overcome these hurdles could pave the way for precancer vaccines to eventually redefine possibilities for cancer prevention [39].
Difficulty of advancing vaccines without defined precancerous stages
Oncogenic viruses provide clear viral antigens and at-risk populations to target for achieving successful cancer prevention through prophylactic vaccination. In contrast, most human malignancies lack defined infectious triggers, creating uncertainty around optimal targets and timing for precancer vaccines in the absence of known virally-induced precursor stages. While theoretically appealing to vaccinate against various dysplastic and hyperproliferative states to prevent their progression, substantial heterogeneity exists in the probabilistic risk and longitudinal evolution of any given premalignant lesion. Whereas viral carcinogenesis follows a largely deterministic trajectory, sporadic preneoplasia progression is subject to considerable stochastic influences based on complex emergent interactions between cells and their microenvironment. The inherently probabilistic nature of most premalignant states creates challenges in precisely defining transitional stages to guide vaccination windows. Without viral shortcuts, advances in risk-stratification biomarkers, probabilistic modeling, and antigen discovery will be imperative to delineate precancer subgroups most likely to advance absent timely intervention [40].
Logistical issues with early immunization and boosters
Another formidable logistical obstacle is identifying at-risk patients during opportune windows for successful precancer intervention and providing sustained boosting to maintain protective immunity through often slow preneoplasia progression over months to years. Public health vaccination depends on widespread population coverage, whereas precancer vaccination will require intensive personalized screening to identify patients with emergent dysplastic changes. Early targeted immunization against progressive lesions would face the challenge of boosting on an ongoing basis even without signs of malignant progression. Precancer vaccination would benefit from advances in biomarkers, proteogenomics, and longitudinal imaging to guide delivery specifically during high-risk lesion development. Patient compliance will be critical for maintaining boosting through lengthy subclinical phases, unlike single-course childhood vaccines. Improved understanding of immunological memory and adjuvants may support prudent boosting schedules. Logistical solutions for intensive longitudinal monitoring, coupled with technological improvements supporting rational antigen selection, will be instrumental in overcoming delivery barriers inherent to this new vaccination paradigm [41].
Potential risk of promoting rather than suppressing progression
Perhaps the most concerning challenge inherent to precancer vaccination is the possibility of unintentionally aggravating dysplasia or even eliciting adverse malignant evolution rather than preventing it. Immunotherapy carries intrinsic risks of fueling inflammation, disrupting tissue homeostasis, and promoting progression. While next-generation vaccines aim to stimulate productive anti-tumor immunity, suboptimal responses could potentially drive deleterious inflammation and autoimmunity. Vigilant safety monitoring for neurological, cardiovascular, hepatic, renal and other toxicities will be imperative during clinical translation. Preclinical studies also raise warnings about the double-edged nature of immunotherapy, demonstrating that improperly activated T cells, myeloid cells, or cytokines can enhance precancerous outgrowth instead of controlling it, especially with incomplete blockade of immunosuppressive mechanisms. Adjuvant selection and optimizing combinatorial regimens to avoid pro-tumorigenic effects will be critical. Vaccine antigens will need careful selection to minimize risks of precipitating autoimmunity against off-target antigens cross-reactive with healthy tissue. Ultimately, the promise of preventing cancer through prophylactic vaccination will only be realized if the perils of improperly promoting it are prudently avoided. This demands comprehensive preclinical modeling and vigilant clinical safety monitoring to ensure emerging immunopreventive strategies stimulate productive anti-precancer immunity without fueling counterproductive inflammation or autoimmunity [42].
Ethical and Societal Implications that Warrant Consideration Regarding Pre-Cancer Vaccination
While prophylactic precancer vaccines offer promising opportunities to expand cancer prevention horizons, their clinical translation demands prudence to navigate complex ethical quandaries. A foremost consideration is equitable access - early privilege to interventions conferring "cancer immunity" could exacerbate disparities if optimal vaccination is unaffordable or preferentially allocated. Preventive precedence must balance individual risk-benefit with population health gains [43]. Precancer screening prerequisites may disadvantage underserved groups lacking healthcare access. Public attitudes assessing vaccination tradeoffs will shape uptake, requiring transparent education on realistic expectations. Invasive cancers directly threaten life, yet pre-cancers pose probabilistic risks, so defining appropriate risk thresholds for early intervention is ethically ambiguous [44]. Vaccinating against progressive precancers prevents human suffering, but overtreatment of indolent lesions that may not advance could cause undue harm. Rare adverse events including potential promotion of progression demand precautions when manipulating high-risk tissues. While cancer immunoprevention promises profound benefits, delivering equitable, optimal and safe early vaccination necessitates navigating complex clinical, ethical, and societal considerations [45]. Open, evidence-based dialogue including diverse stakeholder voices will help guide policies maximizing benefit while minimizing preventive harms as this disruptive paradigm evolves.
Effects and drawbacks of prophylactic precancer vaccination
While precancer immunization offers disruptive possibilities, realizing benefits without aggravating harm demands prudent assessment of potential adverse outcomes. Vaccines stimulating productive anti-tumor immunity could also incur autoimmune toxicity if responses cross-react against healthy tissue antigens. Adjuvants and cytokines intended to boost efficacy may fuel counterproductive inflammation worsening progression if improperly calibrated [46]. Lesions surviving initial vaccination may evolve more aggressive phenotypes resistant to subsequent immune targeting. Suboptimal vaccine design risks generating dysregulated inflammation and incomplete cytokine signaling which enhance rather than suppress tumorigenesis. Repeated antigen stimulation could exhaust T cells into dysfunctional anergy rather than sustained surveillance [47]. Inadequate precancer screening coverage may miss early vaccination windows for many patients. Difficulties maintaining compliance with multi-dose boosting through lengthy premalignant phases could limit enduring protection. While the premise warrants continued optimization, realizing lasting benefit without inadvertently aggravating pathology will require mitigating this potential, if surmountable, adverse effects through comprehensive translational study and vigilant safety monitoring to prudently harness precancer vaccination for maximal cancer prevention impact [48].
Future Directions and Outlook
Premise and promise of preneoplasia vaccines
Prophylactic vaccination against precancerous stages represents an emerging strategy to intercept cancer progression earlier by targeting dysplastic lesions before additional mutations result in metastatic malignancy. Premalignant tissues exhibit inherent vulnerabilities including apoptosis sensitivity and immunogenic antigens that are lost as cancers evolve resistance. Vaccines stimulating cytotoxic clearance or sustained cytostasis of lesions during transient windows of susceptibility before terminal progression could disruptively expand cancer prevention horizons [49].
Realizing this potential requires optimally timed delivery against validated antigens to engage immunity when preneoplasias are intrinsically vulnerable yet before progression to complex resistant phenotypes. Despite hurdles including defining precise transitional windows, modeling risk thresholds, and avoiding promotion of progression, strategic vaccination against precancer vulnerabilities highlights opportunities to intercept progression trajectories pre-malignancy. But careful orchestration of precancer immunobiology will be imperative to successfully leverage prophylactic vaccines for early immunological interception before insurmountable oncogenesis [50].
Key next steps needed to move field forward
Realizing the full potential of preneoplasia vaccines to prevent cancer will require concerted efforts to advance a number of pivotal steps in this emerging field. Deepening on our probabilistic understanding of precancer natural history through mathematical modeling approaches will be critical to better predict progression risk trajectories for diverse lesion types and identify key transitional states that warrant early immunological intervention [51]. Elucidating candidate target antigens selectively expressed in advanced precancerous states at highest risk for advancement will guide vaccine design, leveraging new genomics, proteomics and immunopeptidomics technologies to systematically characterize dysregulated antigens. Combinatorial vaccination regimens can then be optimized along with innovative delivery strategies tailored to the distinct immunobiology and kinetics of precancerous lesion progression. In parallel, developing and validating robust biomarkers to identify emerging lesions with high probability of progression will enable precise early vaccination targeting during peak windows of immune susceptibility prior to malignant transformation. Advances in vaccine vectors, carriers, and adjuvants to potently stimulate adaptive immunity to typically tolerated self-antigens are needed. Telemedicine, point-of-care diagnostics, and leveraging existing screening infrastructure could aid access along with centralized precancer vaccine hubs serving communities at elevated risk for certain neoplasms. Real-time integration of multilayered longitudinal diagnostics with vaccination schedules tailored to an individual’s phase of progression will be pivotal for therapeutically optimizing early immunization [52]. Longitudinal screening and imaging modalities to closely monitor precancer evolution must progress in step to align interception with vulnerable transitional states. Comprehensive preclinical modeling and clinical safety monitoring will remain imperative at each step to avoid fueling deleterious inflammation or autoimmunity through precancer vaccination. Navigating these multifaceted challenges will require cross-disciplinary collaboration to turn the promise of preneoplasia vaccines into patient benefit through strategic immune-mediated prevention. Illuminating mechanisms of immune tolerance, privilege, and evasion in the preneoplasia microenvironment to inform rational countermeasures and immune conditioning regimens [53].
Current Clinical Vaccine Studies Targeting Non-Viral Cancers
While prophylactic vaccines against viral malignancies have proven highly successful, development of vaccines targeting non-viral sporadic cancers remains in earlier phases [54]. However, pioneering clinical trials provide early evidence supporting the feasibility and potential efficacy of this approach against non-viral neoplasms. For example, a phase 2 trial investigating a personalized neoantigen vaccine in melanoma patients revealed immune responses against vaccine neoantigens and increased progression-free survival compared to controls [55].
A dendritic cell vaccine pulsed with glioblastoma lysate demonstrated vaccine-induced immune activation and favorable median overall survival versus historical controls in a pilot study [56]. A phase 1 trial of a five shared melanoma antigen vaccine showed detectable immune responses in most patients along with early clinical benefit signals [57]. A phase 1b study of a vaccine targeting aberrantly glycosylated MUC1 in advanced breast cancer elicited antibody responses and stable disease in multiple participants. Though early, these and other emerging clinical studies demonstrate promising immunogenicity, immune activation, and hints of clinical efficacy for vaccines targeting non-viral tumor antigens [58]. While challenges remain, pioneering translation efforts provide grounds for continued optimization of prophylactic vaccination against sporadic preneoplastic states based on early feasibility data. Advancing these platforms through further rigorous testing could eventually make vaccination a disruptive approach against a broader array of non-virally driven malignancies. Table 1 concisely summarizes major categories of target antigens being investigated for prophylactic precancer vaccines, along with representative examples and brief descriptions.
|
Antigen Category |
Examples |
Description |
|
Viral oncoproteins |
HPV E6/E7, HBV HBx |
Oncogenic viral proteins; target to prevent infection and malignancy |
|
Cancer-testis antigens |
MAGE, NY-ESO-1 |
Restricted expression in germ & cancer cells; immunogenic |
|
Overexpressed cell cycle proteins |
Cyclin B1, CDC20 |
Drivers of uncontrolled proliferation; upregulated in precancers |
|
Mutated tumor suppressors |
p53, Rb, VHL |
Frequent loss of function mutations promote progression |
|
Oncofetal antigens |
CEA, AFP |
Normally restricted to embryo; aberrant expression in cancers |
|
Tissue/lineage antigens |
PSA, tyrosinase |
Tissue-restricted proteins; potential targets for tissue-specific vaccines |
|
Cancer stem cell antigens |
CD44, CD133 |
Expressed on self-renewing cancer stem cells; key progression drivers |
|
Neoantigens |
Mutant peptides |
Novel antigens from somatic mutations; foreign so immunogenic |
Advanced Computational Methodologies to Identify Biomarkers for Developing Precancer Vaccines
Emerging big data analysis, machine learning, and artificial intelligence technologies hold immense promise to accelerate biomarker discovery efforts that will be critical for developing effective prophylactic vaccines against sporadic preneoplastic states [59]. Advanced algorithms applied to multidimensional omics datasets of precancerous lesions could help unravel signatures distinguishing progressive versus indolent disease, illuminating profiles to guide productive immune targeting. High-throughput sequencing paired with sophisticated bioinformatics pipelines may elucidate panels of genes, transcripts, or mutations indicative of progression risk [60].
Artificial intelligence applied to medical imaging data of premalignant states could enable earlier lesion risk stratification based on radiomic features associated with advancement. Deep learning approaches integrating diverse multilayered data types may derive integrative signatures forecasting progression more accurately than any single biomarker [61]. Furthermore, computational methods like immune repertoire analysis and neoantigen prediction can help systematically prioritize antigens likely to elicit productive anti-precancer immunity. While human validation remains essential, applying these cutting-edge techniques to mine expediting datasets could significantly accelerate identification of predictive biomarkers and immunogenic targets to overcome key obstacles in developing efficacious prophylactic precancer vaccines.
Conclusions
Prophylactic vaccination against high-risk precancerous lesions represents an emerging frontier with disruptive potential to profoundly expand cancer prevention horizons if key challenges can be prudently navigated. Oncogenic viruses have demonstrated proof-of-concept that prophylactic immunization can successfully intercept malignancy by blocking precipitating infections before they take hold. The vision now emerges to extend this success by leveraging targeted vaccination against sporadic preneoplastic states to stimulate selective immune clearance of dysplastic cells before additional mutations enable invasion and metastasis.
While alluring in theory, realizing this potential faces considerable obstacles including elucidating definitive premalignant antigens and biomarkers, overcoming tolerance to self-antigens, logistical barriers in early screening and delivery, and serious risks that improper inflammatory stimulation could worsen rather than halt progression. Tremendous research opportunities exist across cancer immunology, longitudinal omics, probabilistic modeling, antigen discovery, and combinatorial immunotherapy to address these complexities. However, thoughtful and comprehensive translation of emerging strategies will be imperative, as the hazards inherent in manipulating precancerous states demand prudent design and vigilant safety monitoring to avoid fueling deleterious outcomes.
If key steps in unlocking precancer immunobiology can pave the way for rational vaccination regimens selectively targeting lesions approaching peak transitional vulnerability, prophylactic precancer vaccines hold disruptive potential to fundamentally change the cancer prevention paradigm. By awakening dormant immunity against early dysplastic changes poised to become invasive malignancies, the dream emerges of thwarting lethal cancers before they even have a chance to start by targeting neoplastic risk at its most proximate and receptive point of intervention. Though formidable obstacles remain, this vision highlights profound opportunities on the horizon for strategic vaccination to finally move beyond solely early detection toward true cancer immunoprevention.
List of Abbreviations
HBV: Hepatitis B Virus; HPV: Human Papillomavirus; DNA: Deoxyribonucleic Acid; BRCA1/2: Breast Cancer gene ½
Declarations
Ethics approval and consent to participate
Not Applicable
Consent for publication
Not Applicable
Availability of data and materials
All data are available, and sharing is available as well as publication.
Competing interests
The authors hereby say that they have no competing interests.
Funding
Corresponding author supplied all study materials. There was no further funding for this study.
Authors' contributions
The authors completed the study protocol and were the primary organizers of data collection and the manuscript's draft and revision process. Tamer A. Addissouky wrote the article and ensured its accuracy. All authors contributed to the discussion, assisted in designing the study and protocol and engaged in critical discussions of the draft manuscript. Lastly, the authors (TA, IE, MA, YW, AE, AK, and NE) reviewed and confirmed the final version of the manuscript.
Acknowledgements
The authors thank all the researchers who have made great efforts on their studies. Acknowledgement: The authors would like to thank the Deanships of all the participated Universities for supporting this work. Moreover, we are grateful to the editors, reviewers, and readers of this journal.
References
2. Vraga EK, Brady SS, Gansen C, Khan EM, Bennis SL, Nones M, et al. A review of HPV and HBV vaccine hesitancy, intention, and uptake in the era of social media and COVID-19. Elife. 2023 Aug 18;12:e85743.
3. Tian Y, Hu D, Li Y, Yang L. Development of therapeutic vaccines for the treatment of diseases. Molecular Biomedicine. 2022 Dec 8;3(1):40.
4. Zhou X, Sun L, Yao X, Li G, Wang Y, Lin Y. Progress in vaccination of prophylactic human papillomavirus vaccine. Frontiers in Immunology. 2020 Jul 10;11:1434.
5. Pan C, Yue H, Zhu L, Ma GH, Wang HL. Prophylactic vaccine delivery systems against epidemic infectious diseases. Advanced Drug Delivery Reviews. 2021 Sep 1;176:113867.
6. Kumari M, Lu RM, Li MC, Huang JL, Hsu FF, Ko SH, et al. A critical overview of current progress for COVID-19: development of vaccines, antiviral drugs, and therapeutic antibodies. Journal of Biomedical Science. 2022 Sep 12;29(1):68.
7. Addissouky TA, Wang Y, El Sayed IE, Baz AE, Ali MM, Khalil AA. Recent trends in Helicobacter pylori management: harnessing the power of AI and other advanced approaches. Beni-Suef University Journal of Basic and Applied Sciences. 2023 Sep 2;12(1):80.
8. Ashouri A, Zhang C, Gaiti F. Decoding Cancer Evolution: Integrating Genetic and Non-Genetic Insights. Genes. 2023 Sep 24;14(10):1856.
9. López-Gil L, Pascual-Ahuir A, Proft M. Genomic Instability and Epigenetic Changes during Aging. International Journal of Molecular Sciences. 2023 Sep 19;24(18):14279.
10. Martinis E, Ricci C, Trevisan C, Tomadini G, Tonon S. Cancer Vaccines: From the State of the Art to the Most Promising Frontiers in the Treatment of Colorectal Cancer. Pharmaceutics. 2023 Jul 17;15(7):1969.
11. Restrepo JC, Dueñas D, Corredor Z, Liscano Y. Advances in Genomic Data and Biomarkers: Revolutionizing NSCLC Diagnosis and Treatment. Cancers. 2023 Jul 3;15(13):3474.
12. Addissouky TA, Wang Y, Megahed FA, El Agroudy AE, El Sayed IE, El-Torgoman AM. Novel biomarkers assist in detection of liver fibrosis in HCV patients. Egyptian Liver Journal. 2021 Dec;11(1):1-5.
13. de Oliveira NR, Santos FD, dos Santos VA, Maia MA, Oliveira TL, Dellagostin OA. Challenges and Strategies for Developing Recombinant Vaccines against Leptospirosis: Role of Expression Platforms and Adjuvants in Achieving Protective Efficacy. Pathogens. 2023 May 31;12(6):787.
14. El-Sayed MH, Feld JJ. Vaccination at the forefront of the fight against hepatitis B and C. Nature Reviews Gastroenterology & Hepatology. 2022 Feb;19(2):87-8.
15. Srivastava V, Nand KN, Ahmad A, Kumar R. Yeast-Based Virus-like Particles as an Emerging Platform for Vaccine Development and Delivery. Vaccines. 2023 Feb 18;11(2):479.
16. Das S, Babu A, Medha T, Ramanathan G, Mukherjee AG, Wanjari UR, et al. Molecular mechanisms augmenting resistance to current therapies in clinics among cervical cancer patients. Medical Oncology. 2023 May;40(5):1-6.
17. Rancic NK, Miljkovic PM, Deljanin ZM, Marinkov-Zivkovic EM, Stamenkovic BN, Bojanovic MR, et al. Knowledge about HPV Infection and the HPV Vaccine among Parents in Southeastern Serbia. Medicina. 2022 Nov 22;58(12):1697.
18. Enokida T, Moreira A, Bhardwaj N. Vaccines for immunoprevention of cancer. The Journal of Clinical Investigation. 2021 May 3;131(9).
19. Li C, Hall TG, Hall JJ, He WQ. Effectiveness of quadrivalent HPV vaccination in reducing vaccine-type and nonvaccine-type high risk HPV infection. Epidemiology & Infection. 2023;151:e37.
20. Laganà AS, Chiantera V, Gerli S, Proietti S, Lepore E, Unfer V, et al. Preventing Persistence of HPV Infection with Natural Molecules. Pathogens. 2023 Mar 6;12(3):416.
21. Tojjari A, Saeed A, Singh M, Cavalcante L, Sahin IH, Saeed A. A Comprehensive Review on Cancer Vaccines and Vaccine Strategies in Hepatocellular Carcinoma. Vaccines. 2023 Aug 12;11(8):1357.
22. Faggioli F, Velarde MC, Wiley CD. Cellular senescence, a novel area of investigation for metastatic diseases. Cells. 2023 Mar 10;12(6):860.
23. Kirk NA, Kim KB, Park KS. Effect of chromatin modifiers on the plasticity and immunogenicity of small-cell lung cancer. Experimental & Molecular Medicine. 2022 Dec;54(12):2118-27.
24. Zheng C, Snow BE, Elia AJ, Nechanitzky R, Dominguez-Brauer C, Liu S, et al. Tumor-specific cholinergic CD4+ T lymphocytes guide immunosurveillance of hepatocellular carcinoma. Nature Cancer. 2023 Aug 28:1-8.
25. Polverini PJ, Nör F, Nör JE. Crosstalk between cancer stem cells and the tumor microenvironment drives progression of premalignant oral epithelium. Frontiers in Oral Health. 2023 Jan 10;3:1095842.
26. Arena GO, Forte S, Abdouh M, Vanier C, Corbeil D, Lorico A. Horizontal Transfer of Malignant Traits and the Involvement of Extracellular Vesicles in Metastasis. Cells. 2023 Jun 6;12(12):1566..
27. Koyande NP, Srivastava R, Padmakumar A, Rengan AK. Advances in Nanotechnology for Cancer Immunoprevention and Immunotherapy: A Review. Vaccines. 2022 Oct 16;10(10):1727.
28. Zach M, Greslehner GP. Understanding immunity: an alternative framework beyond defense and strength. Biology & Philosophy. 2023 Feb;38(1):7.
29. Suryawanshi YN, Biswas DA, Biswas D. Herd Immunity to Fight Against COVID-19: A Narrative Review. Cureus. 2023 Jan 9;15(1).
30. Fang J, Singh S, Cheng C, Natarajan S, Sheppard H, Abu-Zaid A, et al. Genome-wide mapping of cancer dependency genes and genetic modifiers of chemotherapy in high-risk hepatoblastoma. Nature Communications. 2023 Jul 6;14(1):4003.
31. Liu S, Sun Q, Ren X. Novel strategies for cancer immunotherapy: counter-immunoediting therapy. Journal of Hematology & Oncology. 2023 Apr 13;16(1):38.
32. M. Naeini M, Newell F, Aoude LG, Bonazzi VF, Patel K, Lampe G, et al. Multi-omic features of oesophageal adenocarcinoma in patients treated with preoperative neoadjuvant therapy. Nature Communications. 2023 May 31;14(1):3155.
33. Ergun P, Kipcak S, Bor S. Epigenetic Alterations from Barrett's Esophagus to Esophageal Adenocarcinoma. International Journal of Molecular Sciences. 2023 Apr 25;24(9):7817.
34. Xiao X, Guo Q, Cui C, Lin Y, Zhang L, Ding X, et al. Multiplexed imaging mass cytometry reveals distinct tumor-immune microenvironments linked to immunotherapy responses in melanoma. Communications Medicine. 2022 Oct 21;2(1):131.
35. Rochwarger A, Mattern S, Gaudillière B, Schürch CM. High-multiplex tissue imaging in routine pathology—are we there yet?. Virchows Archiv. 2023 Feb 9:1-2.
36. Lin JR, Wang S, Coy S, Chen YA, Yapp C, Tyler M, et al. Multiplexed 3D atlas of state transitions and immune interaction in colorectal cancer. Cell. 2023 Jan 19;186(2):363-81.
37. Cosenza M, Sacchi S, Pozzi S. Cytokine release syndrome associated with T-cell-based therapies for hematological malignancies: pathophysiology, clinical presentation, and treatment. International Journal of Molecular Sciences. 2021 Jul 17;22(14):7652.
38. Saad AA. Targeting cancer-associated glycans as a therapeutic strategy in leukemia. All Life. 2022 Dec 31;15(1):378-433.
39. Gupta M, Wahi A, Sharma P, Nagpal R, Raina N, Kaurav M, et al. Recent advances in cancer vaccines: challenges, achievements, and futuristic prospects. Vaccines. 2022 Nov 25;10(12):2011.
40. Liu J, Fu M, Wang M, Wan D, Wei Y, Wei X. Cancer vaccines as promising immuno-therapeutics: platforms and current progress. Journal of Hematology & Oncology. 2022 Mar 18;15(1):28.
41. Priyanka, Choudhary OP, Singh I. Adjudicating the logistics of COVID-19 vaccine boosters from a global perspective. Human Vaccines & Immunotherapeutics. 2022 Jan 31;18(1):2020572.
42. Anderson NM, Simon MC. The tumor microenvironment. Current Biology. 2020 Aug 17;30(16):R921-5.
43. Schiappacasse GV. Ethical Considerations in Chemotherapy and Vaccines in Cancer Patients in Times of the COVID-19 Pandemic. Current Oncology. 2021 May 26;28(3):2007-13.
44. Dykens JA, Peterson CE, Holt HK, Harper DM. Gender neutral HPV vaccination programs: Reconsidering policies to expand cancer prevention globally. Frontiers in Public Health. 2023 Feb 21;11:1067299.
45. Lubeya MK, Chibwesha CJ, Mwanahamuntu M, Mukosha M, Frank S, Kawonga M. “When you get the HPV vaccine, it will prevent cervical cancer; it will act as a shield”: adolescent girls’ knowledge and perceptions regarding the human papillomavirus vaccine in Zambia. Frontiers in Health Services. 2023;3.
46. Reuschenbach M, Doorbar J, Del Pino M, Joura EA, Walker C, Drury R, et al. Prophylactic HPV vaccines in patients with HPV-associated diseases and cancer. Vaccine. 2023 Sep 12;45: 6194-6205.
47. Yuill S, Velentzis LS, Smith M, Egger S, Wrede CD, Bateson D, et al. The impact of HPV vaccination beyond cancer prevention: effect on pregnancy outcomes. Human vaccines & immunotherapeutics. 2021 Oct 3;17(10):3562-76.
48. Mo Y, Ma J, Zhang H, Shen J, Chen J, Hong J, et al. Prophylactic and therapeutic HPV vaccines: current scenario and perspectives. Frontiers in Cellular and Infection Microbiology. 2022 Jul 4;12:909223.
49. Liu Y, Pagacz J, Wolfgeher DJ, Bromerg KD, Gorman JV, Kron SJ. Senescent cancer cell vaccines induce cytotoxic T cell responses targeting primary tumors and disseminated tumor cells. Journal for Immunotherapy of Cancer. 2023;11(2):e005862.
50. Tirziu A, Paunescu V. Cytotoxic T-Cell-Based Vaccine against SARS-CoV-2: A Hybrid Immunoinformatic Approach. Vaccines. 2022 Jan 30;10(2):218..
51. Arias-Hidalgo C, Juanes-Velasco P, Landeira-Viñuela A, García-Vaquero ML, Montalvillo E, Góngora R, et al. Single-Cell Proteomics: The Critical Role of Nanotechnology. International Journal of Molecular Sciences. 2022 Jun 16;23(12):6707.
52. Abelin JG, Bergstrom EJ, Rivera KD, Taylor HB, Klaeger S, Xu C, et al. Workflow enabling deepscale immunopeptidome, proteome, ubiquitylome, phosphoproteome, and acetylome analyses of sample-limited tissues. Nature Communications. 2023 Apr 3;14(1):1851.
53. Xie N, Shen G, Gao W, Huang Z, Huang C, Fu L. Neoantigens: promising targets for cancer therapy. Signal Transduction and Targeted Therapy. 2023 Jan 6;8(1):9.
54. D’Alise AM, Scarselli E. Getting personal in metastatic melanoma: neoantigen-based vaccines as a new therapeutic strategy. Current Opinion in Oncology. 2023 Mar;35(2):94.
55. Blass E, Ott PA. Advances in the development of personalized neoantigen-based therapeutic cancer vaccines. Nature Reviews Clinical Oncology. 2021 Apr;18(4):215-29.
56. Zhou J, Li L, Jia M, Liao Q, Peng G, Luo G, et al. Dendritic cell vaccines improve the glioma microenvironment: Influence, challenges, and future directions. Cancer Medicine. 2023 Mar;12(6):7207-21.
57. Li Z, Yang D, Guo T, Lin M. Advances in MUC1-Mediated Breast Cancer Immunotherapy. Biomolecules. 2022 Jul 6;12(7):952.
58. Liu Y, Hu Y, Xue J, Li J, Yi J, Bu J, et al. Advances in immunotherapy for triple-negative breast cancer. Molecular cancer. 2023 Sep 2;22(1):145.
59. Nygård M, Nygård S. The Future of Cervical Cancer Prevention: From “One-Size-Fits-All” to Personalized Screening. Journal of Personalized Medicine. 2023 Jan 17;13(2):161.
60. Pulumati A, Pulumati A, Dwarakanath BS, Verma A, Papineni RV. Technological advancements in cancer diagnostics: Improvements and limitations. Cancer Reports. 2023 Feb;6(2):e1764.
61. Mansur A, Saleem Z, Elhakim T, Daye D. Role of artificial intelligence in risk prediction, prognostication, and therapy response assessment in colorectal cancer: current state and future directions. Frontiers in Oncology. 2023 Jan 25;13:1065402.