Abstract
BIOMODULINA T® is a biological immunomodulator of natural origin, not blood derived, which promises to be a strategy for immune restoration in the elderly, in the midst of the worldwide epidemiological crisis due to the SARS-CoV-2 virus. This research aimed to determine the changes induced by BT in the distribution of lymphocyte compartments of institutionalized Cuban geriatric patients. The significant expansion of the naïve CD4+ and CD8+, CD19+, NK T cells and of the CD3+ HLA-DR+ and CD3+ CD25+ activation populations was observed; while the rest of the populations of T, B compartments, the T population that co-express CD4 and CD8, and the CD4-CD8-TCRαβ + T cells did not show significant changes. BT had an effect on the immune restoration of T and B lymphocyte populations of Cuban older adults. It modulates the immune response and inflammation, restores immunity, and can contribute to a strategy to prevent and treat COVID-19 in elderly.
Keywords
B lymphocytes, T lymphocytes, Naïve T cells, Lymphocyte activation, Elderly, Immunosnescence, BIOMODULINA T®
Highlights
• BT increases the population of naïve CD4+ and CD8+ T lymphocytes, thus contributing to immune restoration in senescence.
• Treatment with BT had a notable influence on the B lymphocyte subpopulation through the cooperative action of the T lymphocyte compartment.
• Increased populations of NK cells and T lymphocytes that co-express CD4+ and CD8+ support the contribution of BT treatment to the balance between tolerance and inflammation.
• BT promotes a regulated immunophenotype that places older adults in better conditions to face the coronavirus.
Introduction
Aging is accompanied by numerous changes that affect almost all components of the immune system, collectively called immunosenescence. Immunosenescence has long been considered detrimental because it is often accompanied by the subclinical accumulation of pro-inflammatory factors and inflammation. Together, it is suggested that immunosenescence and inflammation are at the origin of most diseases of the elderly, such as infections, cancer, autoimmune disorders and chronic inflammatory diseases. However, a large number of immunogerontologists have changed this negative interpretation of immunosenescence regarding its significance in age-related alterations of the immune system. Considering these changes from an evolutionary perspective, it is preferable to view it as adaptive or remodeling. Although it is conceivable that global immune changes can lead to various diseases, it is also obvious that these changes are necessary for extended survival or longevity. Recent accumulated data suggest that without the existence of the immunosenescence / inflammation duo (representing two faces of the same phenomenon), human longevity could be shortened [1].
Aging affects the components of the immune system and the processes that take place in it in a particular and different way. Important changes can be identified in:
i) the cells of the immune system: In innate immunity, the decrease in the phagocytic capacity of neutrophils, the cytotoxic functions and the secretion of cytokines of NK cells stand out; as well as the decrease in the migration capacity and the alteration of the diversity of receptors for activation and inhibition of these latter cells. On the other hand, in dendritic cells, the ability to recognize antigens and their “in vivo” maturation decrease, which is manifested in a lower migration and expression of co-stimulatory molecules and cytokines, keys for the activation of T lymphocytes [2-4].
T and B lymphocytes are the targets of cellular changes in the adaptive immune system. Some of these changes occur at an early stage of ontogenetic development, so they are common to the cell parent of both lineages. Highlights include: defective maintenance of virgin T and B lymphocytes in the periphery, accumulation of memory T and B cells due to lifelong antigenic stimulation, poor thymic performance, decreased diversity of receptors for T (TCR) and B (BCR) lymphocytes, the deterioration of the functional response of B cells, due to the low avidity of the antibodies and their limited protective capacity, among others [2-4].
ii) in the microenvironment and architecture of the lymphoid organs where these cells reside, including hematopoietic stem cells in the bone marrow. A number of non-negligible age-related changes have been discovered in the secondary lymphoid organs. Structural studies have revealed a profound loss of architecture between discrete areas of lymph nodes, whereby areas of T and B cells become blurred and overlap. In addition, this tissue shrinks significantly in older adults, in part due to the decrease in stromal cells. These changes affect not only the start of the immune response, but also the maintenance of “naive” lymphocytes and other key processes [2,4].
iii) and in the circulating factors that interact with both to ensure lymphocyte activation, differentiation and proliferation that initiates the immune response; the maintenance and cessation of response, as well as the homeostasis of the system [3,4].
In this sense, the most discussed change related to aging is the persistent and low-grade increase in inflammatory molecules, which has been called inflammasome (inflamm-aging). The sources of these proinflammatory changes with age are poorly understood. Some candidates point to increased adiposity, with altered secretion of proinflammatory cytokines into adipose tissue, accumulation of highly differentiated specific T cells, and accumulation of non-lymphoid cells that assume a senescence-associated secretory phenotype [3,4].
The understanding of aging of the immune system has progressed rapidly in recent decades, but some of the mechanisms underlying it remain unknown. The relationship between age-related changes affecting the immune system is incompletely elucidated [4].
Just as aging per se can be modified through nutrition and pharmacological interventions, immunosenescence is also a flexible process that can be approached from multiple angles. The improvement of the activation of the innate and adaptive immune system, the alteration of homeostasis through the manipulation of cytokines and the rejuvenation of the immune system with the aim of restarting the production of new naïve or virgin lymphocytes, are some of the strategies addressed by the specialists [4].
BIOMODULINA T® (BT) is a Cuban product [National Center for Biopreparations (BIOCEN)] registered in 1994 for the treatment of conditions that present immunological dysfunction mainly of the cellular type, such as recurrent infections in the elderly. BT is a a biological immunomodulator of natural origin, not blood-derived, composed of specific fractions of bovine thymus of polypeptide nature [5].
There are several reports of the benefits of this product in reducing lower respiratory tract infections in children [6] and also in older adults [7]. In addition, it has been used in chronic obstructive pulmonary disease (COPD) in geriatric patients [8] and in the treatment of a child with DiGeorge síndrome [9]. Its ability to increase the thymic area in children with thymic hypoplasia has been demonstrated [6]. More recently, Saavedra et al. [10] found findings that suggest that the administration of BT is a promising strategy for the immune restoration of the elderly and the improvement of the immunotherapeutic potential in cancer patients.
BT was recently used in a phase IV clinical trial to prevent COVID-19. It was administered to more than 10,000 older adults during this pandemic, mainly to SARS-CoV-2 positive patients, and to other people in nursing homes - as a preventive measure - taking into account that they have a greater risk of presenting the manifestations. The goal of the treatment was to improve the immune response and health in elderly individuals, and to help contain the transmission of COVID-19 and other infections, in these institutions [11].
Although the present study follows the aforementioned research line, the objective of this study was to determine the changes induced by BT in the distribution of lymphocyte compartments in institutionalized Cuban geriatric patients with compensated comorbidities, not infected by SARS CoV-2.
Materials and Methods
Patients and treatment
The research was developed within the framework of the Clinical Trial “Evaluation of the efficacy and safety of a new dosing scheme of BT for the prevention of infections, including COVID-19, in older adults in Cuba”, approved by the Center for the State Control of Medicines (CECMED), whose promoter institution is the National Center for Biopreparations (BioCen) http://registroclinico.sld.cu/ensayos/RPCEC00000319-Sp [12].
Inclusion criteria: 1. Patients who met the diagnostic criteria, 2. Any sex and skin color aged 60 years and over, 3. Patients who express their written consent, to participate in the study and in case they present major cognitive impairment, it would be signed by a family member, guardian or caregiver. The first patient was included on April 13, 2020.
Exclusion criteria: 1. Patients who have received treatment with BT in the previous two months, 2. Patients with known hypersensitivity to any component of the formulation, 3. Patients with acute allergic states or history of severe allergic reactions, 4. Patients with uncontrolled intercurrent illnesses which include, but are not limited to: acute infections with concomitant febrile symptoms, symptomatic congestive heart failure, unstable angina pectoris.
The patients were treated with two weekly doses of BT administered intramuscularly, for 6 weeks. The samples were obtained by venipuncture, in 30 patients from the “Alfredo Gómez Gendra” Nursing Home, in Havana, before starting the treatment (pre-treatment), one week after it ended (6 weeks) and 6 months later.
Multiparametric flow cytometry (MFC)
The sample from peripheral blood was deposited in a tube with ethylenediaminetetraacetate (EDTA). The identification and quantification of the antigens of interest was developed by the MFC technique, with the use of monoclonal antibodies (mAb) directed against the B and T lymphoid activation and differentiation antigens. The samples were processed and analyzed in the Immunology laboratory of the Institute of Hematology and Immunology (IHI). The mAb panel that was used was the following:
| Monoclonal antibodies | Fluorochromes |
|---|---|
| AntiCD4/CD8/CD3 | FITC/RPE/PC5*** |
| Anti-CD3 | FITC**/ PerCP*** |
| Anti-CD4 | FITC*** |
| Anti-CD8 | FITC*** |
| Anti-CD19 | PC5** |
| Anti-CD25 | APC** |
| Anti-CD27 | RPE**/APC*** |
| Anti-CD45 | PC5**/PerCP** |
| Anti-CD45RA | APC** |
| Anti-IgD | FITC*** |
| Anti-IgM | RPE*** |
| Anti-HLA-DR | RPE** |
| Anti-TCRαβ | APC*** |
| FITC: Fluorescein Isothiocyanate; PE: Phycoerythrin; APC: Allophycocyanin; PC5: Cyanine 5 Phycoerythrin; PerCP: Peridinin Chlorophyll Protein; *: DAKO; **: Milteny; ***: Beckman Coulter. | |
Extracellular staining was performed following two protocols, one for T lymphocytes and the other for B. In the case of T lymphocytes, 50 μL of sample was incubated with mAbs for 20 minutes, protected from light at room temperature. The red blood cells were subsequently lysed using Miltenyi Biotec’s Red Blood Cell Lysis Solution (10X), according to their recommendations, for 10 minutes at room temperature. Two washes were carried out with 0.9% physiological saline solution, 10 minutes at 1500 rpm, to eliminate the lyse and obtain a clean cell button. Cells were fixed with 300 μL of 1% formaldehyde and stored at 4°C.
For B lymphocytes, 100 μL of sample were first lysed and washed in the manner described above, in order to eliminate immunoglobulins (Ig) present in plasma, which could cause nonspecific binding when quantifying membrane (mIg), and were performed the incubation with mAbs for 30 minutes. After this time, a washing and fixing was carried out.
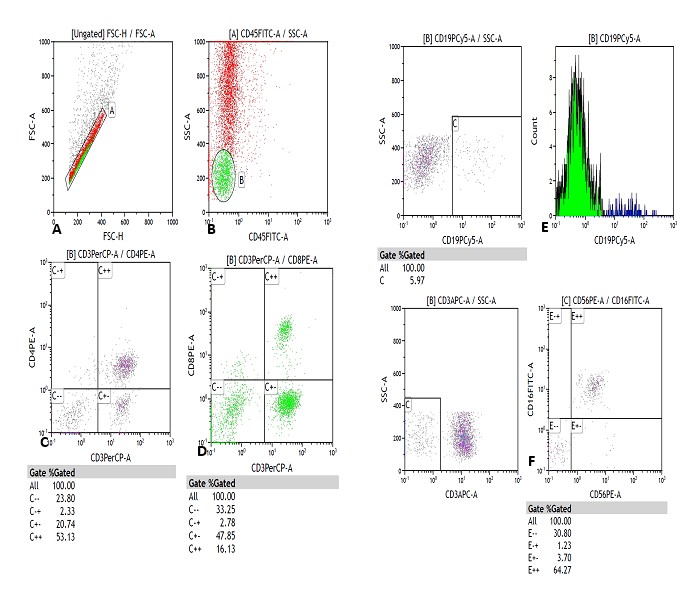
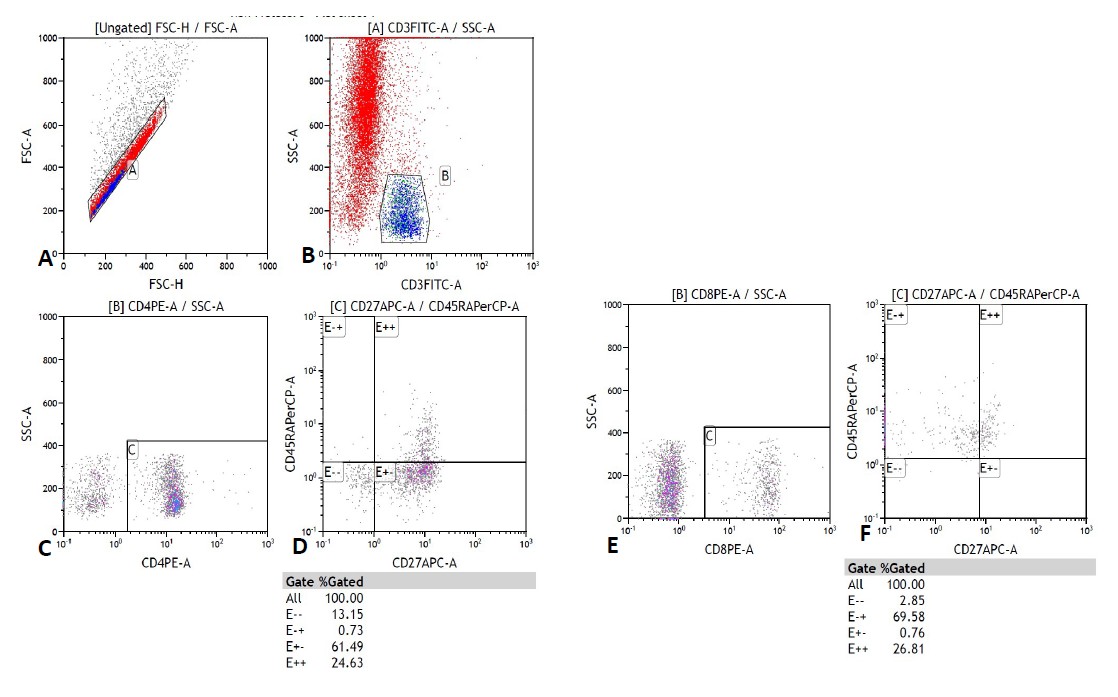
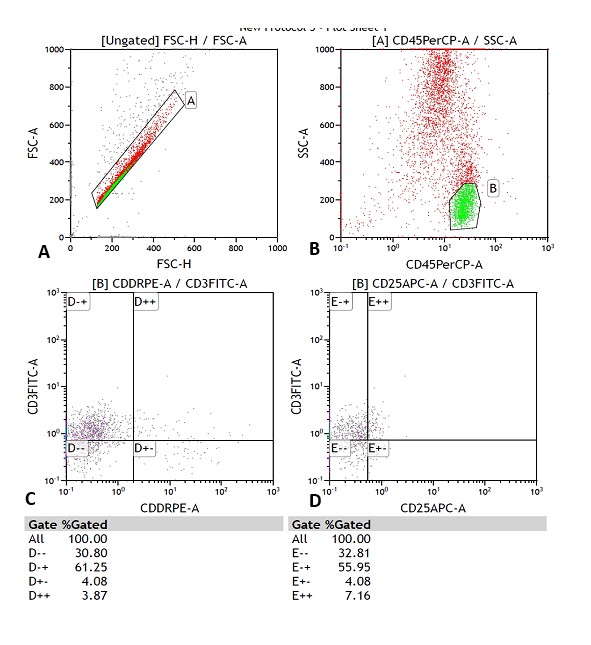
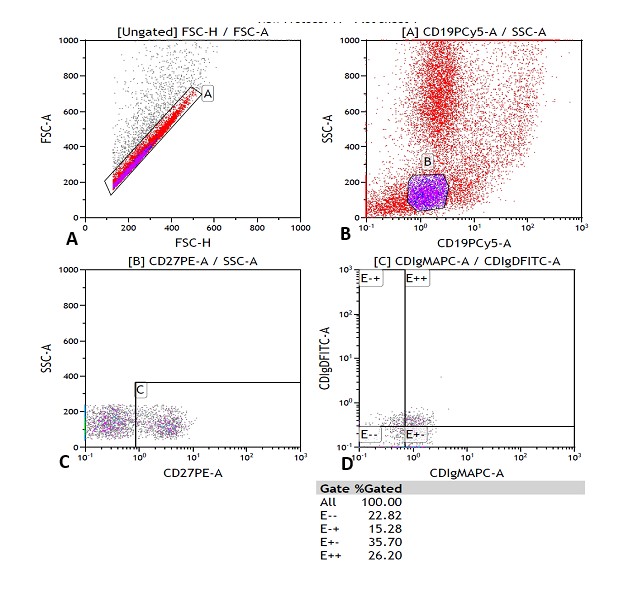
The reading was performed on a MACSQuant® flow cytometer from MiltenyiBiotec, Germany. 100,000 cells were acquired per tube. The analysis was carried out using the Kaluza computer program, version 1.2, of the GALLIOS cytometer, BeckmanCoulter. The analysis strategy is shown in Figures 1-4.
The results were expressed in relative values (% of cells) and in absolute values (cells / μL). The latter were calculated according to the following formula: Absolute count (cells / μL) = Lymphocyte count (number of cells / μL) in blood count x% of the cell subpopulation of interest ÷ 100.
Statistical analysis
The Shapiro-Wilk normality test was used. Statistical significance between the times studied was evaluated using the Wilcoxon Matched Pairs Signed Frank Test (Nonparametric Test) and Paired t test (Parametric Test). Statistical analysis was performed with the “compare Groups” package [13]. Odds ratios (OR) and their associated confidence intervals (CI) were calculated.
The categorical variables were defined as follows: TRUE (percentage or absolute values within the reference range for each population studied) and FALSE (percentage or absolute values outside the reference range for each population studied).
In all analyses, p<0.05 was considered statistically significant.
Ethical considerations
The Research Center on Longevity, Aging and Health (CITED) and IHI Research Ethics Committees approved this study. The Declaration of Helsinki was applied in all processes.
Results
The sample under study in this article was characterized by the presence of comorbidities such as: cardiovascular disease (83.3%), Diabetes mellitus (30.0%), dementia syndrome (53.3%), neoplasia (13.3%), asthma (6.6%), chronic obstructive pulmonary disease (26.6%) and cerebrovascular disease (26.6%). This reinforces the complexity of public health interventions in this age group and negatively influences the analyzes that are made of them.
When evaluating the distribution of the CD4+ and CD8+ T cell compartments (naïve [CD27+ CD45RA+], central memory [CD27+ CD45RA-], effector memory [CD27-CD45RA-] and effector [CD27-CD45RA+]), a significant change was noted for these variables after BT treatment.
As expected, in the elderly studied, decreased values of “naïve” T cells were found, both for CD4+ and CD8+, with a predominance of central and effector memory, before treatment (data not revealed).
The analysis showed a statistically significant increase in naïve CD4+ T cells at 6 weeks (OR 1.03 [1.00; 1.07], p 0.023) and at 6 months (OR 1.04 [1.00; 1.08], p 0.040) but only in the values percentage. (Table A and B).
| (A) | ||||
| Immunophenotypes [%] (RRV [37-39]) | Timeline | Mean Before treatment ± SD / Mean After treatment ± SD | OR [95 %CI] | P value |
| CD3+/CD4+/CD27+/CD45RA+ (Naive) [34.8 - 70.3] | 6 Weeks Post- Treatment | 17.48 ± 15.33 / 25.46 ± 17.13 | 1.03 [1.00;1.07] | 0.023 * |
| 6 Months Post-Treatment | 17.48 ± 15.33 / 25.13 ± 13.97 | 1.04 [1.00;1.08] | 0.040 * | |
| CD3+/CD4+/CD27+/CD45RA-(Memoria Central) [24.3 - 42.7] |
6 Weeks Post- Treatment | 30.65 ± 21.73 / 36.71 ± 18.29 | 1.02 [0.99;1.04] | 0.248 |
| 6 Months Post-Treatment | 30.65 ± 21.73 / 49.24 ± 16.64 | 1.05 [1.02;1.08] | 0.002 * | |
| CD3+/CD8+/CD27+/CD45RA+ (Naive) [48.6 - 87.5] | 6 Weeks Post- Treatment | 14.32 ± 12.93 / 21.58 ± 15.80 | 1.04 [1.00;1.09] | 0.069 |
| 6 Months Post-Treatment | 14.32 ± 12.93 / 22.35 ± 15.15 | 1.04 [1.00;1.08] | 0.031 * | |
| CD3+/CD8+/CD27+/CD45RA-(Memoria Central) [9.8 - 37.6] |
6 Weeks Post- Treatment | 19.23 ± 17.77 / 24.54 ± 17.58 | 1.02 [0.99;1.05] | 0.208 |
| 6 Months Post-Treatment | 19.23 ± 17.77 / 29.54 ± 15.84 | 1.04 [1.00;1.07] | 0.029 * | |
| CD3-/CD16+/CD56+ [3.7 - 28.0] | 6 Weeks Post- Treatment | 14.42 ± 7.551 / 17.84 ± 13.63 | 1.03 [0.98;1.08] | 0.473 |
| 6 Months Post-Treatment | 14.42 ± 7.551 / 40.74 ± 23.05 | 1.11 [1.05;1.17] | <0.001 * | |
| CD3+/HLA-/DR+ [ - ] | 6 Weeks Post- Treatment | 2.899 ± 2.737 / 8.117 ± 5.166 | 1.61 [1.24;2.09] | <0.001 * |
| 6 Months Post-Treatment | 2.899 ± 2.737 / 5.333 ± 7.467 | 1.12 [0.95;1.32] | 0.102 | |
| CD3+/CD25+ [ - ] | 6 Weeks Post- Treatment | 1.407 ± 0.945 / 7.199 ± 7.765 | 2.51 [1.46;4.31] | <0.001 * |
| 6 Months Post-Treatment | 1.407 ± 0.9447/1.357 ± 6.158 | 1.00 [0.89;1.12] | <0.001 * | |
| (B) | ||||
| Immunophenotypes [cells per μL] (RRV [37-39]) |
Timeline | Mean Before treatment ± SD / Mean After treatment ± SD | OR [95 %CI] | P value |
| CD3+/CD4+/CD27+/CD45RA+ (Naive) [ 335-725] | 6 Weeks Post- Treatment | 294,2 ± 293.8 / 413,1 ± 268.6 | 0.99 [0.94;1.04] | 0.367 |
| 6 Months Post-Treatment | 294,2 ± 293.8 / 433,3 ± 296,3 | 1.26 [1.02;1.56] | 0.940 | |
| CD3+/CD4+/CD27+/CD45RA-(Memoria Central) [201-419] |
6 Weeks Post- Treatment | 538,5 ± 518.3 / 634,9 ± 352.4 | 1.00 [1.00;1.00] | 0.147 |
| 6 Months Post-Treatment | 538,5 ± 518.3 / 825,2 ± 525,4 | 1.00 [1.00;1.00] | 0.017 * | |
| CD3+/CD8+/CD27+/CD45RA+ (Naive) [118-312] | 6 Weeks Post- Treatment | 230,5 ± 265.7 / 336,9 ± 229,1 | 1.04 [1.00;1.09] | 0.069 |
| 6 Months Post-Treatment | 230,5 ± 265.7 / 409,4 ± 398.4 | 1.04 [1.00;1.08] | 0.031 * | |
| CD3+/CD8+/CD27+/CD45RA-(Memoria Central) [30-166] |
6 Weeks Post- Treatment | 296,5 ± 289.7 / 418,1 ± 341 | 1.00 [1.00;1.00] | 0.329 |
| 6 Months Post-Treatment | 296,5 ± 289.7 / 492,3 ± 295,7 | 1.00 [1.00;1.00] | 0.024 * | |
| CD3-/CD16+/CD56+ [70-652] | 6 Weeks Post- Treatment | 253,6 ± 211.0 / 305,2 ± 268.3 | 1.00 [1.00;1.01] | 0.469 |
| 6 Months Post-Treatment | 253,6 ± 211.0 / 715,7 ± 651,7 | 1.00 [1.00;1.01] | 0.001 * | |
| CD3+/HLA-/DR+ [ - ] | 6 Weeks Post- Treatment | 48,02 ± 44,17/ 144,5 ± 115.1 | 1.03 [1.01;1.04] | <0.001 * |
| 6 Months Post-Treatment | 48,02 ± 44,17/ 70,7 ± 85.40 | 1.01 [1.00;1.01] | 0.281 | |
| CD3+/CD25+ [ - ] | 6 Weeks Post- Treatment | 26,61 ± 28,15/ 127,2 ± 155.1 | 1.03 [1.01;1.05] | <0.001 * |
| 6 Months Post-Treatment | 26,61 ± 28,15/ 22,3 ± 97,51 | 1.00 [0.99;1.01] | <0.001 * | |
| RRV: Range of normal Reference Values; SD: Standard Deviation; CI: Confidence Interval; *: Statistically significant differences between the groups before and after treatment. | ||||
Meanwhile, naive CD8+ lymphocytes showed a significant p value in the evaluation carried out at 6 months, both for the percentage values (OR 1.04 [1.00; 1.08], p 0.031) and the absolute counts (OR 1.00 [1.00; 1.00], p 0.091), after treatment with BT (Table A and B).
In the case of central memory cells, both for CD4+ and CD8+ lymphocytes, a significant increase was found 6 months after treatment; and this effect was observed for both the percentage values (CD3+ CD4+ OR 1.05 [1.02; 1.08], p 0.002; CD3+ CD8+ OR 1.04 [1.00; 1.07], p 0.02) (Table A) and for absolute counts (CD3+ CD4+ OR 1.00 [1.00; 1.00], p 0.017; CD3+ CD8+ OR 1.00 [1.00; 1.00], p 0.024). (Table B) The rest of the compartments did not show statistically significant differences.
Also, treatment with BT did not show statistically significant changes on the CD4+ and CD8+ T subpopulation, when comparing the evaluation times (pre-treatment, 6 weeks and 6 months); taking into account the reference values (data not revealed).
For the CD19+ subpopulation, at the beginning of treatment 43% of the patients showed values below the reference, and at the end of 6 months only 3 patients (10%) showed low values of this lymphocytic population (Table C). A more exhaustive analysis confirmed that this effect was indeed significant and maintained over time. For the percentage values, the dependence of the increase in the CD19+ population with the BT treatment -measured by the effect size- at 6 weeks (OR 1.21 [1.06; 1.39], p 0.002) and at 6 months (OR 1.29 [1.11;1.50], p 0.001). Although the same behavior was not observed for the absolute counts, the significant values of p suggest that it was not caused by chance (Table D).
| % of patients outside the RRV/number of patients (n=30) | |||
| CD19+ | pre treatment | 6 weeks | 6months |
| 43/13 | 20/6 | 10/3 | |
| RRV: Range of normal Reference Values. | |||
| Immunophenotypes (RRV [37-39] | Timeline | Mean Before treatment ± SD / Mean After treatment ± SD | OR [95 %CI] | P value |
|---|---|---|---|---|
| CD19+ [5.4 – 49.5] (%) | 6 Weeks Post- Treatment | 6,46 ± 3.438 / 11,07± 7.140 | 1.21 [1.06;1.39] | 0.002 * |
| 6 Months Post-Treatment | 6,46 ± 3.438 / 14,36 ± 9.054 | 1.29 [1.11;1.50] | <0.001 * | |
| CD19+ [114 - 1491] (cells per μL) | 6 Weeks Post- Treatment | 111,5 ± 80.52 / 184,8 ± 125.4 | 1.01 [1.00;1.01] | 0.005 * |
| 6 Months Post-Treatment | 111,5 ± 80.52 / 234 ± 162,6 | 1.01 [1.00;1.01] | 0.003 * | |
| RRV: Range of normal Reference Values; *: Statistically significant differences between the groups before and after treatment; SD: Standard Deviation; CI: Confidence Interval. | ||||
In the B compartment, the naïve (IgD+ IgM+ CD27-), memory IgM (IgD-IgM+ CD27+) and postswitched memory (IgD-IgMCD27+) populations were analyzed [14].
The statistical analysis did not show significance when the pre-treatment and post-treatment status with BT were compared in each of these populations. However, it was observed that after 6 weeks of BT treatment, 36.6% of subjects increased their naïve B cells, 55.1% of them decreased their IgM memory B population and 30% the postswitched one (data not revealed). Although this observation was not made 6 months after the end of treatment, it constitutes a sample button for future projections in the study of the age-related effects of this important compartment, in relation to BT treatment.
Other populations in which it was evaluated the effect of BT treatment in elderly were NK cells (CD3-CD16+ CD56+) and those related to the T lymphocyte activation process (CD3+ HLA-DR+ and CD3+ CD25+). Treatment with BT showed statistical significance on the population of natural killer (NK) cells at 6 months, as shown by the percentage values (OR 1.11 [1.05; 1.17], p 0.001) (Table A), whereas the absolute counts only showed a significant increase as a continuos variable (p<0.05) and not for the categorical variable (OR 1.00 [1.00; 1.01], p 0.001). On the other hand, the activation of T lymphocytes measured through the expression of the HLA-DR antigen, and expressed in percent, increased significantly in the evaluation carried out at the 6th week after treatment (OR 1.61 [1.24; 2.09], p 0.001), showing an association to the effect size as measured bt the categorical OR; but it was not observed its persistence over time, since the effect was not significant at 6 months (Table A). Meanwhile, the absolute counts showed significance at this same time of evaluation, but only for the continous variable (p <0.001) (Table B).
This same function, evaluated through the expression of CD25, showed a sustained effect over time. The analysis of the percentage reference values yielded at 6 weeks (OR 1.21 [1.06; 1.39], p 0.002) and 6 months (OR 1.29 [1.11; 1.50], p 0.001) after the treatment with BT was performed, which in addition to the significant change of the continous variable made possible to verify a direct influence of BT treatment on said population, as measured through OR (Table A). On the other hand, when the absolute reference values were obtained, statistically significant p values were observed at both times of evaluation (6 weeks p <0.005 and 6 months p <0.003), ruling out the possibility of random events in relation to the proposed treatment (Table B).
In addition, the CD3+ CD4+ CD8+ populations (co-expression) and the CD4-CD8- T lymphocytes expressing TCRαβ (double negative T lymphocytes, DNT- TCRαβ) were explored. Statistically significant differences were not found for neither of these two cell populations (data not revealed).
However, the analysis of CD4+ CD8+ co-expression showed that at the beginning of treatment with BT 9 older adults did not show normal values, of which 66% recovered their count after 6 weeks of treatment. Only 4 elderly did not achieve adequate counts (data not revealed).
On the other hand, 25 patients had normal values of the double negative population of T-lymphocytes expressing TCRαβ before treatment with BT (83.3%) and the remaining 16% (five patients) recovered their normal values after 6 weeks of it (data not revealed).
Discussion
The main findings found in this study can be summarized for better understanding in immunophenotype T, B and in other populations of interest. Regarding the T-lymphocytic phenotype, a significant and treatment-dependent increase in naïve and central memory cells, CD4+ and CD8+, was confirmed.
Compartment B immunophenotype, although it did not show statistically significant differences when comparing the pre-treatment state and the treatments at 6 weeks and 6 months, did show a tendency to restore homeostasis, which should be evaluated in more detail and using another design. experimental. However, a significant increase in the CD19+ lymphocyte population was observed.
The evaluation of other populations of interest was equally interesting. The activation of T lymphocytes measured through the expression of CD25 and HLA-DR antigens was significant. The expansion of the population of double negative T lymphocytes with TCRαβ+ and of the T population that co-expresses CD4 and CD8, although it did not show statistical significance, had a tendency towards normalization. This evidence is discussed in this section.
Decreased values of the “naïve” populations were found, both for CD4+ and CD8+ in the elderly studied, before treatment with BT. The reduction of naïve cells is physiologically related to aging, while the composition of the CD4+ and CD8+ subpopulations reflects the individual immunological situation [15,16].
Just as the ability to recognize various antigens is central to immune competence, the reduction of the lymphocyte repertoire affects immunoefficiency and creates an imbalance in peripheral lymphocyte homeostasis, with aging. These changes are accompanied by the progressive involution of the thymus [17]; which, added to the antigenic challenge and the infections that occur during life, contributes to the differentiation of these “naïve” cells into more differentiated T subtypes [15]. While the production of new cells is dependent on the thymus, the maintenance of the repertoire is independent of it [17].
Although the aging of the adaptive immune system is often described as a change from “naïve” to memory lymphocytes, this change is only relative. Old age by itself does not lead to an absolute accumulation of memory T and B cells. The survival and expansion of memory T cells is favored by the increase in IL-7 and IL-15 cytokines, and by infection by cytomegalovirus (CMV) [4,16,18]. Farber et al. [19] divided human life span into three phases: memory generation (0-20 years), memory homeostasis (20-65 years) and immunosenescence (>65 years), based on changes in memory T cell frequency, pathogen susceptibility, and mortality. Taking this classification into account, Li et al. [15] studied the changes in the evolution of T populations and immunosenescence for the Chinese population. They found that there was an accumulation of memory cells for CD4+ and CD8+, with statistically significant differences for the effector or TEMRA CD8+ and for the effector memory CD4+ and CD8+.
In our study, no similar results were found; however, in the central memory CD4+ and CD8+ population statistical analysis led us to think that the immunomodulation of these cells is directly related to the application of the BT. The thymic extract would contribute to the restoration of the proliferative response and generation of effector cells, the main factor in the success of vaccination and in the antitumor response. It would also affect lymphocyte proliferation, partially verified in this study with the use of activation markers CD25 and HLA-DR; and would intervene in the inflammatory response through the stimulation of the generation of essential cytokines in this response. If we understand the latter as part of an adaptive response to aging, we would assume that this effect is very beneficial. These hypotheses could be the object of study and contrast for future research by this work team.
The main evidence of the effect of aging on the B cell compartment is the dramatic decline in circulating B lymphocytes, which is part of a group of immunological parameters collectively known as “Immune Risk Phenotype” [3,20,21].
In addition to the effect on T cells, the treatment with BT had a marked influence on the CD19+ B lymphocyte population. The effect is higly significant for % values and not significant for absolute counts, meaning a relative increase of CD19+ cells.
A recent study that evaluated the lymphocyte subpopulations of Cuban older adults under treatment with BT and the VAMENGOC- BC® vaccine (individual and combined) found also that BT induced a statistically significant increase in CD19+ observed through the effect size OR [22].
The fact that the CD19+ lymphocyte population has shown a relative increase under the influence of BT treatment, and not the rest of the B compartments analyzed in this study, suggests that the mechanism by which this effect is achieved is dependent of the B-T cooperation. Saavedra et al. found that treatment with BT increases the number of CD4+ T cells that produce IFN-γ and hypothesized that this treatment involves the recovery of the Th1 pattern in the elderly [10].
In aged individuals, modifications occur in the “naïve” / memory subpopulations and in the production of immunoglobulins, accompanied by the deficient ability to produce protective antibodies against new antigens, which corresponds to the elderly with a poor health status [3,20,21].
The decrease in circulating B cells is primarily due to the decrease in the formation of new cells from the bone marrow, as a direct impact of inflammation on B lymphopoiesis. In turn, the reduction in these naive B cells is accompanied by the expansion of memory B cells showing a phenotype associated with senescence. And finally, the defective ability of these memory cells to differentiate into plasma cells is shown. In this way, it is possible to affirm that inflammation is the cause that guides the imbalance of the B compartment related to age and plays a key role in the development of age-related diseases [21].
For these reasons, the observation that under BT treatment there is a trend toward achieving B-compartment homeostasis (increase in naïve cells and decrease in memory cells) even without demonstrated statistical significance, is an encouraging finding. To complete the understanding of the effect of BT on this compartment, it would be necessary to carry out new research that includes a larger sample size and allows further insight into what type of memory B cells is influenced and whether double negative B cells are involved. (IgD-CD27-), which are accompanied by high levels of markers of an aging-associated secretory phenotype (SASP).
Changes associated with age in NK cells have been the most studied in innate immunity and it is considered that their functional alterations can contribute decisively to the higher incidence of infectious and neoplastic diseases in aging. Although these cells increase in immunosenescence, they suffer a decline in their tumor cytotoxic capacity and in the production of cytokines [23].
From the expansion of this population after treatment with BT, a restoration of its protective functions is expected. This could not be verified in this work, and should be further investigated.
As part of its potentially immunoregulatory functions, BT must promote a regulatory environment for the balance between inflammation and tolerance, hence, although it stimulates cell proliferation, it must contribute to the control of clonal proliferation.In this research, the effect of this product on the CD4+CD8+ double positive (DP) T population was evaluated. The functions of TDP lymphocytes are not yet fully understood. They seem to play an important role in peripheral sites as potent immunosuppressants or with high cytotoxic potential, which is also related to lymphoproliferation [24,25]. They have an effector and memory role, and react with specific antigens of a wide variety of pathogens, in particular, against self-limited and latent viral infections, such as Epstein Barr virus (EBV) and cytomegalovirus (CMV) [18,26]. They increase in healthy individuals, particularly in the elderly, and in neoplasms and autoimmune diseases [26]. The expansion of this cell population occurs under conditions of immune inflammation, specifically inflammation related to aging [24,27].
Taking into account the above, and supported by the idea that in this research there was a predominance of older adults with comorbidities - chronic non-communicable diseases, neoplasms and infections -, in which there is an inflammatory microenvironment, even more so in the context of immunosenescence; BT had an immunoregulatory role, since it was able to restore the normal values of this population in 86% of the elderly in the study. These findings constitute a starting point for subsequent cellular and molecular studies that demonstrate this potentiality and its mechanism of action.
In this sense, the establishment of the thymic microenvironment could contribute to the preservation or restoration of immune protection and enhance the quality of the immune response against infections and cancer. [28]
A recent article published showed that most of the Cuban older adults evaluated who exhibited high percentage and absolute values of TDP lymphocytes, had some associated comorbidity, among them infections and cancer [29]. This supports the claimed idea about its role as an immunosuppressant or potentially cytotoxic cell. More indepth studies of this population will be required to elucidate the events that lead to immunesenescence and, especially, to interpret their role in it [18].
The expression of markers that reflect cell function is important to assess the degree of activation of the T compartment; CD25 and HLA-DR have proven to be very helpful in this regard. In some cases, they can be interpreted as a non-pathogenic factor and in others as a physiological or pathological consequence of a disease or condition. For example, increased HLA-DR expression is found in patients with chronic spinal cord damage, possibly contributing to persistence of chronic inflammation or decreased resistance to infection [24].
The altered clonal expansion and the decrease in the production of cytokines, especially IL-2, have been shown to be affected in early T signaling events and are the main properties affected with aging [30].
Most viral infections induce proliferation and activation of CD8+ T cells. In recently evaluated COVID-19 patients it was determined that there is an expanded CD8+ T activation phenotype, confirmed by the co-expression of HLA-DR and CD38, which formed a clinical immunophenotype characterized by populations of non-naive, activated and proliferating T cells, in a subgroup of individuals [31].
Following these pieces of evidence, the fact that this study has yielded significant increase in lymphocyte activation upon BT treatment is more relevant, since it puts the subject in a better condition to respond to infection. It is known that there is a common profile of dysregulation in critically ill patients [31].
On the other hand, the decrease in IL-2 production is a feature of immunosenescence, with the consequent implication in cell activation and proliferation [1,32,33]. The very significant increase in the subpopulations involved in T activation and proliferation is interpreted as the physiological consequence of BT-induced immune modulation.
Finally, DNT- TCRαβ are assigned pro-inflammatory and regulatory roles, suggesting that they are a heterogeneous cell subtype. Its importance is exemplified in certain autoimmune diseases such as systemic lupus erythematosus (SLE), Sjögren’s syndrome and autoimmune lymphoproliferative syndrome (APLS), where this population is widespread and it has been suggested that they play a leading role [34]. The fact that these cells are associated with autoimmunity and are increased in conditions where there are failures in the tolerance mechanisms and an increase in autoreactive T cells, raises the possibility that this lymphocyte population includes cells reactive against self antigens [35].
Scientific evidence suggests the important role of the “naive” T subpopulation in the origin and maintenance of autoreactive effectors in the periphery. It is even known that cell cycle arrested memory T cells are capable of responding to epitopes previously recognized by naïve lymphocytes [36]. Thus, it is expected that after the administration of BT treatment the values of the DNT- TCRαβ population will be recovered in all patients, largely due to the quantitative recovery of the “virgin” compartment. The fact that the majority of the elderly showed normal values of this lymphocyte population (83.3%), alludes to a state of relative homeostasis in the middle of the immunosenescence process.
This study has some limitations. For more in-depth evaluations of the T and B cell compartments, new markers should be included that allow us to better characterize the lymphocyte populations; as well as the quantification of proinflammatory cytokines. New BT therapeutic schemes should also be tested, which, in combination with the one already tested, would allow obtaining results applicable to different clinical situations in the elderly.
Conclusions
BT has an effect on the immune restoration of the T and B lymphocyte populations of Cuban older adults. The main contributions to the achievement of homeostasis in aging were the significant expansion of naive and central memory CD4+ and CD8+ T cells, CD19+, NK cells and of the activation populations CD3+ HLA-DR+ and CD3+ CD25+ cells; while the trends observed for the remaining lymphocyte populations should be evaluated with a larger sample size. Due to its influence on the restoration of immunity, Biomodulin T® can also become a Cuban strategy to prevent and treat COVID-19 in vulnerable groups.
Abbrevations
BT: Biomodulina T®; COVID-19: Coronavirus Disease; MFC: Multiparametric Flow Cytometry; EDTA: Ethylenediaminetetraacetate; COPD: Chronic Obstructive Pulmonary Disease; mAb: Monoclonal Antibodies; IHI: Institute of Hematology and Immunology; FITC: Fluorescein Isothiocyanate; PE: Phycoerythrin; APC: Allophycocyanin; PC5: Cyanine 5 Phycoerythrin; PerCP: Peridinin Chlorophyll Protein; OR: Odds Ratios; CI: Confidence Intervals; IL: Interleukine; CMV: Cytomegalovirus; NK: Natural Killer Cell; TDP: Double Positive T Lymphocytes; EBV: Epstein Barr Virus; IFN-γ: Gamma Interferon; DNT- TCRαβ: Double Negative T lymphocytes; TEMRA: Effector Memory T cells re-expressing RA; SLE: Systemic Lupus Rrythematosus; ALPS: Autoimmune Lymphoproliferative Syndrome; Ig: Immunoglobulins; mIg: Membrane Immunoglobulins
Competing Interests
No conflicts of interest are declared between the participating institutions and researchers.
Declarations of Interest
None.
CRediT Author Statement
Imilla Casado Hernandez: Methodology, Investigation, Writing - Original Draft. Consuelo Macías Abraham: Validation, Data Curation, Writing - Review & Editing, Supervision. Vianed Marsán Suárez: Conceptualization, Investigation, Writing - Review & Editing. Mary Carmen Reyes Zamora: Resources, Data Curation. Elizabeth Ramos Hernández: Investigation. Sahily Estradé Fernandez: Formal analysis. Yenisey Triana Marrero: Investigation. Gabriela Diaz Dominguez: Investigation. Yaneisy Duarte Pérez: Investigation. Dr. Luis Felipe Heredia Guerra: Resources.
References
2. Tabibian-Keissar H, Hazanov L, Schiby G, Rosenthal N, Rakovsky A, Michaeli M, et al. Aging affects B-cell antigen receptor repertoire diversity in primary and secondary lymphoid tissues. European Journal of Immunology. 2016 Feb;46(2):480-92.
3. Gibson KL, Wu YC, Barnett Y, Duggan O, Vaughan R, Kondeatis E, et al. B-cell diversity decreases in old age and is correlated with poor health status. Aging Cell. 2009 Feb;8(1):18-25.
4. Nikolich-Žugich J. The twilight of immunity: emerging concepts in aging of the immune system. Nature Immunology. 2018 Jan;19(1):10- 9.
5. Centro para el control estatal de medicamentos, e. y. d. m. C. Biomodulina t®. RESUMEN de Las CARACTERISTICAS DEL PRODUCTO. 2015
6. Christian López LD, Rodríguez Marín RR, Rabassa Pérez J, Santamaría Lafargue M, Romero del Sol JM, González Ross E. Efecto de la biomodulina T 1000 sobre el timo en niños con infecciones recurrentes. Revista cubana de Pediatría. 2000 Mar;72(1):3-9.
7. García Orihuela M, Capdevila V, Suárez Martínez R, Rodríguez Rivera L, Castro González I. Efecto de la Biomodulina T sobre las infecciones respiratorias altas y la polifarmacia del anciano. Revista Habanera de Ciencias Médicas. 2014 Jun;13(3):425-36.
8. Devesa E, García R, Rodríguez R. La Biomodulina T como inmunomodulador en Geriatría. Ensayo clínico controlado, Fase II. La Habana. 1994.
9. Jauma AJ, Macías C, Padilla M, González C. Uso de Biomodulina T en el Síndrome DiGeorge. Presentación de un caso. VacciMonitor. 2011; 20 (Supplement 1).
10. Saavedra D, Fuertes SA, Suárez GM, González A, Lorenzo-Luaces P, García B, et al. Biomodulina T partially restores immunosenescent CD4 and CD8 T cell compartments in the elderly. Experimental Gerontology. 2019 Sep 1;124:110633.
11. Rodriguez Martin RR, Gonzalez Gonzalez O, Rodriguez Gonzalez C, Rodriguez Gonzalez RR. Biomodulina T (InmunyVital®) restores T cells and helps contain COVID-19. Frontiers in Immunology. 2020 Dec 9;11:3275.
12. Registro Público Cubano de Ensayos Clínicos. 2020. Disponible en: http://www.rpcec.sld.cu
13. Isaac Subirana JV, Sanz HC. 4.0: Descriptives by Groups. htttps:// cran.rproject.org/web/packages/compareGroups/vignettes/ compareGroups_vignette.html
14. Warnatz K, Schlesier M. Flowcytometric phenotyping of common variable immunodeficiency. Cytometry Part B: Clinical Cytometry: The Journal of the International Society for Analytical Cytology. 2008 Sep;74(5):261-71.
15. Li M, Yao D, Zeng X, Kasakovski D, Zhang Y, Chen S, et al. Age related human T cell subset evolution and senescence. Immunity & Ageing. 2019 Dec;16(1):1-7.
16. Alves AS, Bueno V. Immunosenescence: participation of T lymphocytes and myeloid-derived suppressor cells in aging-related immune response changes. Einstein (Sao Paulo). 2019 May 2;17.
17. Bektas A, Schurman SH, Sen R, Ferrucci L. Human T cell immunosenescence and inflammation in aging. Journal of Leukocyte Biology. 2017 Oct;102(4):977-88.
18. Ghia P, Prato G, Stella S, Scielzo C, Geuna M, Caligaris-Cappio F. Age-dependent accumulation of monoclonal CD4+ CD8+ double positive T lymphocytes in the peripheral blood of the elderly. British Journal of Haematology. 2007 Dec;139(5):780-90.
19. Farber DL, Yudanin NA, Restifo NP. Human memory T cells: generation, compartmentalization and homeostasis. Nature Reviews Immunology. 2014 Jan;14(1):24-35.
20. Tabibian-Keissar H, Hazanov L, Schiby G, Rosenthal N, Rakovsky A, Michaeli M, et al. Aging affects B-cell antigen receptor repertoire diversity in primary and secondary lymphoid tissues. European Journal of Immunology. 2016 Feb;46(2):480-92.
21. Bulati M, Caruso C, Colonna-Romano G. From lymphopoiesis to plasma cells differentiation, the age-related modifications of B cell compartment are influenced by “inflamm-ageing”. Ageing Research Reviews. 2017 Jul 1;36:125-36.
22. Ramos EH, Suárez VM, Hernández IC, Gomez RP, Rivera DG, Zamora MC, et al. Effect of Biomodulina-T® and VA-MENGOC-BC® on lymphocyte subpopulations in older adults. Experimental Gerontology. 2021 Oct 1;153:111497.
23. Salas LA. Mecanismos de inmunosenescencia y longevidad: posibles estrategias para mejorar la calidad de vida en el envejecimiento (Doctoral dissertation, Universidad Complutense de Madrid).
24. Villegas-Valverde CA, Kokuina E, Breff-Fonseca MC. Estimating normal Values of rare T-Lymphocyte populations in peripheral blood of healthy Cuban adults. MEDICC Review. 2018;20:20-6.
25. Overgaard NH, Jung JW, Steptoe RJ, Wells JW. CD4+/CD8+ double-positive T cells: more than just a developmental stage?. Journal of Leukocyte Biology. 2015 Jan;97(1):31-8.
26. Eljaafari A, Yuruker O, Ferrand C, Farre A, Addey C, Tartelin ML, et al. Isolation of Human CD4/CD8 Double-Positive, Graft-Versus- Host Disease–Protective, Minor Histocompatibility Antigen–Specific Regulatory T Cells and of a Novel HLA-DR7–Restricted HY-Specific CD4 Clone. The Journal of Immunology. 2013 Jan 1;190(1):184-94.
27. Parel Y, Chizzolini C. CD4+ CD8+ double positive (DP) T cells in health and disease. Autoimmunity reviews. 2004 Mar 1;3(3):215-20.
28. Saavedra Hernández D, García Verdecia B, González Morera A, Lorenzo-Luaces Álvarez P, Lage Dávila A. Marcadores de inmunosenescencia y su relación con el cáncer de pulmón. Anales de la Academia de Ciencias de Cuba. 2021 Apr;11(1).
29. Marrero YT, Suárez VM, Abraham CM, Hernández IC, Ramos EH, Domínguez GD, et al. Immunophenotypic characterization of double positive T lymphocytes in Cuban older adults. Experimental Gerontology. 2021 Sep 1;152:111450.
30. Le Page A, Dupuis G, Larbi A, Witkowski JM, Fülöp T. Signal transduction changes in CD4+ and CD8+ T cell subpopulations with aging. Experimental Gerontology. 2018 May 1;105:128-39.
31. Mathew D, Giles JR, Baxter AE, Oldridge DA, Greenplate AR, Wu JE, et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science. 2020 Sep 4;369(6508):eabc8511.
32. Villarrubia VG, Navarro SR. Inmunopatogenia del envejecimiento: el deterioro de la inmunidad innata y su repercusión sobre la inmunidad específica. Restauración por AM3. Rev Esp Geriatr Gerontol. 2000;35(1):30-42.
33. Hernández DS, Verdecia BG. Immunosenescence: effects of aging process on immune system. Revista Cubana de Hematología, Inmunología y Hemoterapia. 2014;30(4):332-45.
34. Rodríguez-Rodríguez N, Apostolidis SA, Penaloza-MacMaster P, Villa JM, Barouch DH, Tsokos GC, et al. Programmed cell death 1 and Helios distinguish TCR-αβ+ double-negative (CD4− CD8−) T cells that derive from self-reactive CD8 T cells. The Journal of Immunology. 2015 May 1;194(9):4207-14.
35. Rodríguez NR. Origen y caracterización de los linfocitos TCR- αβ+ CD4-CD8-doble negativos en relación a su posible naturaleza autorreactiva (Doctoral dissertation, Universidad Complutense de Madrid) 2018.
36. Casado Hernández I, Marsán Suárez V, Díaz Domínguez G, Macías Abraham C. Utilidad diagnóstica de la evaluación de linfocitos T CD4-CD8-TCRαβ+ en el Sindrome Linfoproliferativo Autoinmune. Revista Cubana de Hematología, Inmunología y Hemoterapia. 2017 Jun;33(2):1-4.
37. Arango Prado MD, Villegas Valverde CA, Soto Pardeiro P, Torres López G, Morejón Morales A, Faxas García ME. Caracterización de los inmunofenotipos linfocitarios de sangre periférica en pacientes con cáncer. Revista Cubana de Hematología, Inmunología y Hemoterapia. 2020 Jun;36(2).
38. Kokuina E, Breff-Fonseca MC, Villegas-Valverde CA, Mora- Díaz I. Normal values of T, B and NK lymphocyte subpopulations in peripheral blood of healthy Cuban adults. MEDICC Review. 2019 Nov 11;21:16-21.
39. van Gent R, van Tilburg CM, Nibbelke EE, Otto SA, Gaiser JF, Janssens-Korpela PL, Sanders EA, Borghans JA, Wulffraat NM, Bierings MB, Bloem AC. Refined characterization and reference values of the pediatric T-and B-cell compartments. Clinical Immunology. 2009 Oct 1;133(1):95-107.