Abstract
Miscanthus capensis is a hardy, evergreen, medium-high, clump-forming grass that belongs to the Poaceae family. It is used in South Africa for the treatment of pimples, wounds, eczema, and acne. The present study was investigated to examine the biological activity of Miscanthus capensis roots extract. The effect of the plant extract on protein glycation and collagen production was investigated using in vitro assays. The plant demonstrated strong inhibition of protein glycation and also exhibited an increase in collagen production in human dermal fibroblast (MRHF) cells at all the concentrations tested. The result observed in this study further complements what was reported in our previous study with respect to the understudy plant in eradicating skin related diseases.
Introduction
Miscanthus capensis (Nees) Andersson, a hardy, evergreen, medium-high, clump-forming grass that belongs to the Poaceae family. It is commonly called Daba Grass (English); Ruigtegras (Afrikaans) and Umpumelelo (Xhosa) [1]. The plant’s leaves clumps are about 1 m tall and the flowering stalks are up to 2 m tall. M. capensis is extensively dispersed in South Africa provinces including Free State, KwaZulu-Natal, Eastern Cape, Northern Cape and Western Cape. Traditionally, the root decoction of the plant has been reported to treat pimples, wounds, eczema, and acne [2,3]. However, previous studies on M. capensis root methanol extract demonstrated that the plant extract showed strong antioxidant and non-genotoxic activities [4]. In addition, the extract was also reported to possessing a maximum amount of bioactive components [4]. In this study, we further investigate its biological activity thereby examining its anti-glycation and collagen production activities using in vitro assays. This present study further complements what has been reported on the understudy plant.
Materials and Methods
Chemical used
All reagents used in this study were of analytical grade and purchased from Sigma or Merck Chemicals.
Collection of plant materials and extraction procedure.
The Fresh whole plant material together with the roots of M. capensis was collected in the Eastern Cape Province, precisely November 2019. The plant materials were identified and authenticated at the Giffen Herbarium, University of Fort Hare, South Africa, where voucher specimens (MC3876) are kept. The roots of the plant were detached from the rest of the plant after which they were washed with clean tap water and later oven-dried to a constant weight at 30°C for 10 days. The dried roots were milled to a homogenous powder using an electric blender (Polymix PX-MFC90D Switzerland). About 30 g of the powdered plant was soaked in 500 mL of methanol extract and then shake using an orbital shaker for 24 h after which the extract was filtered and the resulting filtrate was concentrated by using an evaporator (Heidolph Laborota 4000, Heidolph Instruments, GmbH & Co, Germany).
Anti-glycation Assay
The plant extract at varying concentrations of 25-200 μg/mL or the positive control, aminoguanidine (100 μM) was mixed with 500 μL of glucose solution and gelatin substrate (500 μL) in a black-96 well plate. The resulting mixture was incubated for 24 h at 55°C after which 200 μL of the mixture was removed and then transferred into a new black 96-well plate. Then, the absorbance of the fluorescent intensity was measured at 370 nm (excitation) and 440 nm (emission).
Maintenance of cell cultures
The Human foreskin fibroblast (MRHF) cells were cultured in 10 cm culture dishes in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with fetal calf serum (10%) and incubated in a humidified incubator with 5% CO2.
Collagen production
The collagen production assay for M. capensis methanol root extract was carried as described previously by Otang-Mbeng and Sagbo [5].
Statistical analysis
Three replicates of each test sample were used. The data were analysed statistically by the Student’s t-test (two-tailed paired).
Results and Discussion
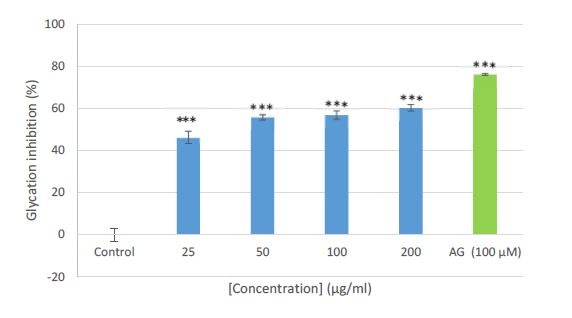
Glycation is the non-enzymatic process responsible for many complications in diabetes mellitus and is implicated in some diseases and aging [6,7]. It is a reaction between the carbonyl groups of reducing sugars and the amino groups of proteins thereby initiating a complex cascade of repeated condensations and oxidative modifications to form a structure called Schiff’s bases and over a long period produces glycation products [8,9]. In this study, the extract demonstrated a strong inhibition against protein glycation at all the tested concentrations (Figure 1). In comparison, the extract also exhibited strong effects as compared to the control, but less marked when compared to the positive control, aminoguanidine (100 μM). This observed anti-glycation effect of this plant extract could be due to the presence of several phytochemicals constituents earlier reported in the extract [3]. However, it is imperative to note that, this present study represents the first attempt to investigate anti-glycation of the extract of M. capaensis. Therefore, it can be deduced from this study that the methanol root extract of M. capaensis may be a good therapeutic agent in alleviating some diseases including aging.
Figure 1. The effect of methanol extract from M. capensis on protein glycation. Data are expressed as the mean ± SD, n = 3. *** p<0.001 compared to control.
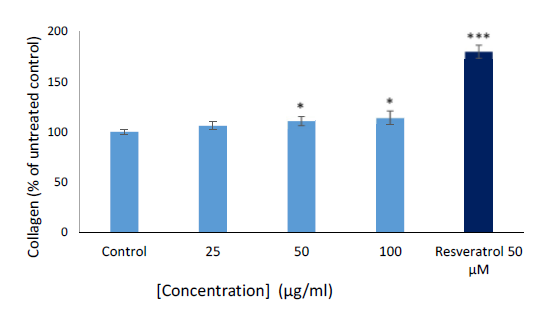
Collagen production
Collagen is the most abundant protein in the body and it plays a significant role in providing firmness to the skin [10]. In this study, the M. capensis methanol root extract was tested for its effects on collagen production in MRHF cells. The result (Figure 2) revealed a slight increase of collagen production in MRHF cells when treated with the tested concentrations (25–100 μg/mL) of the plant extract. This effect was significantly higher when compared to the untreated control. However, the positive control (resveratrol), a well-known collagen booster significantly increased the collagen production in MRHF cells compared to both the extract and the control. It is imperative to note that the slight increase in collagen production in MRHF as exhibited by the extract is not as a result of cytotoxicity. This supported what was reported in our previous study with respect to the extract exhibited no meaningful cytotoxicity and cell death towards MRHF cells [3]. Thus, the literature survey revealed that no previous studies have reported the effect of collagen production of any type of extracts from the M. capensis. Therefore, it could be deduced from this study that the stimulation of collagen production as exhibited by the M. capensis extract might be beneficial in eradicating or slow down the process of skin aging.
Conclusion
In summary, the findings in this study indicated that M. capensis extract could be a potential candidate in alleviating the process of skin antiaging and other skin related diseases as confirmed by inhibiting protein glycation activity and stimulation of collagen production without toxic to the cells. The result observed in this study support the ethnobotanical usage of this plant in the treatment of skin disease. The findings in this study further complement what was reported in our previous study with respect to the understudy plant in eradicating skin related diseases.
References
2. Sagbo I, Mbeng W. Plants used for cosmetics in the Eastern Cape Province of South Africa: A case study of skin care. Pharmacognosy Reviews. 2018 Jul 1;12(24):139-156.
3. Josia M. Medicinal Properties of some Plants used for the Treatment of Skin Disorders in the OR Tambo and Amathole Municipalities of the Eastern Cape Province. Masters Dissertation Department Botany Walter Sisulu University. 2013 Sep.
4. Sagbo IJ, Otang-Mbeng. Chemical Constituents, Antioxidative, Cytotoxic, And Genotoxic Effects of Miscanthus Capensis Roots Extract. Asian Journal Pharmerceutical Clinical Research. 2019;12(12):277-232.
5. Duffy CJ, Tetewsky SJ, O’Brien H. Cortical motion blindness in visuospatial AD. Neurobiology of Aging. 2000 Nov 1;21(6):867-9.
6. Sagbo IJ, van de Venter M, Koekemoer T, Bradley G. In vitro antidiabetic activity and mechanism of action of Brachylaena elliptica (Thunb.) DC. Evidence-Based Complementary and Alternative Medicine. 2018 Jan 1;2018.
7. Kim CS, Park S, Kim J. The role of glycation in the pathogenesis of aging and its prevention through herbal products and physical exercise. Journal of Exercise Nutrition & Biochemistry. 2017 Sep 30;21(3):55.
8. Cepas V, Collino M, Mayo JC, Sainz RM. Redox Signaling and Advanced Glycation Endproducts (AGEs) in Diet-Related Diseases. Antioxidants. 2020 Feb;9(2):142.
9. Iannuzzi C, Irace G, Sirangelo I. Role of Glycation in Amyloid: Effect on the Aggregation Process and Cytotoxicity, Exploring New Findings on Amyloidosis, Ana-Maria Fernandez-Escamilla, IntechOpen. 2016 Aug 24:167-85.
10. Shoulders MD, Raines RT. Collagen structure and stability. Annual Review of Biochemistry. 2009 Jul 7;78:929-58.